Abstract
Background and objectives: Hemodialysis (HD)-induced regional wall motion abnormalities (RWMAs) are common in HD patients and driven by ischemia. In nondialysis patients, repeated ischemia leads to chronic reduction in left ventricular (LV) function. HD-induced myocardial ischemia may initiate the same process. We examined the effect of HD-induced repetitive myocardial stunning on global and regional LV function.
Design, setting, participants & measurements: We analyzed data from 30 patients, previously identified as developing HD-induced myocardial ischemia. Serial echocardiographic assessments of global and regional LV performance were performed at baseline and repeated after 12 mo.
Results: Several patients developed segments with a fixed reduction in systolic function of >60% after 1 yr. In this patient group, there was a significant reduction in resting LV ejection fraction (EF) from 61.5 ± 10.1% to 52.9 ± 8.6% (P < 0.007). Peak LV EF in response to dialysis also decreased from 59.5 ± 10% versus 49.9 ± 6.5% (P < 0.003), with a consequent increase in HD-induced hypotension (P < 0.0001).
Conclusions: HD-induced myocardial stunning may progress over 12 mo to the development of regional fixed systolic dysfunction, consistent with underlying myocardial hibernation and fibrosis. This may be an important and potentially modifiable process in the development of heart failure in HD patients.
Dialysis patients display hugely elevated rates of cardiac mortality (1), and this rate of cardiovascular decline is not driven by the same traditional risk factors that are important in the general population (2). Classical complicated atherosclerotic disease appears not to be the predominant mode of death in hemodialysis (HD) patients. Records from the U.S. Renal Data System have shown that HD is an independent risk factor for the development of de novo and recurrent heart failure with a 2-yr mortality after a diagnosis of congestive heart failure as high as 51% (3), making it the one of the most common causes of cardiovascular mortality in this patient group.
Several studies have described the phenomenon of HD-induced myocardial stunning (4,5) which is now known to be common and associated with reductions in myocardial contractile function and patient survival (6). Measurement of myocardial blood flow (MBF) during dialysis demonstrated that HD can precipitate myocardial ischemia (7). The exact mechanisms involved in HD-induced myocardial stunning remain to be fully elucidated.
However, what is known is that during HD patients are particularly susceptible to demand myocardial ischemia for several reasons (8–11). In the nondialysis population, repeated episodes of demand ischemia and stunning can result in chronic reduction in left ventricular (LV) function (12). This observation led to the hypothesis that repeated episodes of myocardial ischemia leads to a spectrum of disease-encompassing myocardial stunning through to myocardial hibernation (13) and ending in myocardial remodeling and scarring (14). Standard conventional thrice-weekly HD as a cause of repetitive myocardial stunning may lead to such a process, resulting in chronic LV dysfunction. Myocardial hibernation may represent a functional adaptation to chronic hypoperfusion that can be reversed with restoration of regional MBF (the “smart heart” hypothesis) (15). There is evidence to suggest that hibernating myocardium is still highly vulnerable to increases in demand or reductions in oxygen supply (16), such as further hemodynamic stress during HD. Therefore, ongoing recurrent episodes of ischemia precipitated by HD may have negative consequences on this adaptive balance, leading to further myocardial injury and eventual nonviable myocardium with irreversible reduction in LV function.
The aim of this study was to look for evidence of chronic resting myocardial dysfunction in HD patients precipitated by repeated episodes of HD-induced myocardial stunning over a 12-mo period.
Materials and Methods
Patient Population
Fifty prevalent HD patients were recruited for a 12-mo observational cohort study from a single hospital-based HD unit. These patients had previously been identified as developing HD-induced myocardial stunning (6) and dialyzed thrice-weekly for 4 h at 37°C using a standard dialysate as described previously (7). All studies were conducted after the first 2-d interdialytic period because arrhythmias and cardiac events are known to be increased after the 3-d interdialytic break. Nineteen patients were censored from the final analysis [patients who died (n = 9), kidney transplant (n = 6), changed dialysis modality (n = 3), and withdrawal of consent (n = 1)].
Definitions of HD-Induced Cardiac Dysfunction and Rationale
On the basis of segmental performance, the following definitions of HD-induced cardiac dysfunction were applied.
Myocardial stunning.
Regions that showed a functional decline of >20% during HD compared with rest with evidence of functional recovery in the postdialysis period were classified as stunned segments. Previous work using positron emission tomography demonstrated a corresponding reduction in MBF with poststress recovery in function and perfusion in those areas (7).
Myocardial hibernation/fibrosis.
A reduction in resting systolic function of >60% within previously stunned myocardial segments that remained fixed during HD was taken to represent chronically dysfunctional myocardium (within the disease spectrum of myocardial hibernation/fibrosis). A value of 60% was used based on animal studies showing a similar reduction in wall motion score between chronically stunned and hibernating myocardial segments (17).
Initial Echocardiographic Assessment
Patients assessment at baseline reevaluated the presence and extent of HD-induced regional wall motion abnormalities (RWMAs). Two-dimensional echocardiography was performed before commencement (pre-HD), during HD at 4 h (peak-HD), and 30 min into the recovery period (post-HD) (1.5- to 3.6-MHz 3S probe, GE medical systems, Germany). Standard apical two- and four-chamber views were digitally recorded for subsequent analysis (Echo-CMS; MEDIS, The Netherlands), as described previously (6,18). Three consecutive heartbeats were analyzed for each time point and endocardial borders traced semiautomatically for each frame of the three-beat sequence. Any anomalies were manually corrected. Maximal displacement of the endocardial border from a center point was measured over 100 chords around the LV wall, corrected for end-diastolic LV circumference, and expressed as percentage of shortening fraction (SF).
Each apical view was divided into five segments, and SF for the chords in each segment averaged so that 10 regions of the left ventricle were assessed at each time. New RWMAs were classified as segments that showed a decline in SF >20% from baseline as previously defined. Ejection fraction (EF) was calculated using the biplane disc method.
Follow-Up Assessment of LV Function
An identical study session was performed 12-mo later. Changes in global EF at rest and during peak stress on HD were re-assessed. SF for each time point in all myocardial regions was recalculated and compared with initial values. Functional changes over 12-mo in segments that were identified as developing RWMAs were compared with those that did not develop such abnormalities.
Patients were further stratified at 12 mo into two groups: those who developed LV regions with a fixed reduction in function of >60% versus those who had no such regions. Hemodynamic parameters were compared in both groups at rest and in response to the stress of HD to observe the clinical consequences of such segments.
Objectives
The primary objectives of this study were to assess whether HD-induced myocardial stunning progressed over time to the development of fixed systolic dysfunction, consistent with the continuum of myocardial hibernation, and to identify the hemodynamic consequences of this with respect to blood pressure (BP) during dialysis. Secondary objectives were investigating the potential role of hematologic and biochemical variables and the use of cardiac troponin-T (cTnT) as a risk marker for the progression of regional myocardial dysfunction.
Statistical Analyses
Power calculation utilizing the widest SD identified for EF (measured with echocardiography) in HD patients (SD 10.1%) indicated a study of 50 patients was required to detect a 4% reduction in EF at 80% power. Results are presented as the mean ± SD or the median and interquartile range unless otherwise stated. BP data were analyzed using two-way ANOVA with Bonferroni's tests for multiple comparisons. Categorical variables between the two groups were analyzed using Fisher's exact test. All other data were analyzed using the paired or unpaired t tests and the Mann–Whitney or Wilcoxon matched-pair tests.
Results
Patient Characteristics
Baseline clinical characteristics are shown in Table 1. Patients were divided into two groups: patients with evidence of fixed segmental reduction after 12 mo in previously stunned myocardial regions and those without. There were no statistically significant differences between the 2 groups with respect to underlying etiology, comorbid conditions [including diabetes mellitus and ischemic heart disease (IHD)], antihypertensive use, or dialysis vintage.
Table 1.
Demographic characteristics and dialysis-related factors based on the presence (with) or absence (without) of new fixed segmental systolic reduction of >60% after 12 months
| Parameters | Patients without Fixed Reduction in Segmental Systolic Reduction | Patients with Fixed Reduction in Segmental Systolic Reduction | P |
|---|---|---|---|
| Age (mean, yr) | 65 ± 14.5 | 64.8 ± 11.8 | >0.9 |
| Men:women (n) | 6:5 | 13:6 | >0.6 |
| Dialysis vintage (mean, mo) | 31.9 ± 21.6 | 41.6 ± 28.5 | >0.9 |
| Etiologies (n) | |||
| diabetes mellitus | 4 (36%) | 10 (53%) | >0.4 |
| glomerular disease | 2 (18%) | 2 (11%) | >0.5 |
| APKD | 1 (9%) | 3 (16%) | >0.9 |
| other | 3 (27%) | 1 (5%) | >0.2 |
| unknown | 1 (9%) | 3 (16%) | >0.1 |
| IHD (n) | 6 (45%) | 5 (25%) | >0.2 |
| Smoker (n) | 3 (27%) | 5 (25%) | >0.9 |
| Intradialytic weight gain (mean, kg) | 1.6 ± 0.7 | 1.84 ± 0.7 | >0.9 |
| Ultrafiltration volume (mean, L) | 2.03 ± 0.6 | 2.3 ± 0.9 | >0.6 |
| Antihypertensive medication (mean, n) | 1.1 ± 2 | 1.3 ± 1.3 | >0.7 |
| calcium-channel blocker (n) | 4 (36%) | 6 (32%) | >0.9 |
| beta blocker (n) | 3 (27%) | 4 (21%) | >0.9 |
| ACE inhibitor/ARB (n) | 4 (36%) | 4 (21%) | >0.4 |
| other (n) | 4 (36%) | 4 (21%) | >0.4 |
APKD, adult polycystic kidney disease; HT, hypertension; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker.
Progression of HD-Induced Myocardial Stunning into Fixed Segmental Systolic Dysfunction
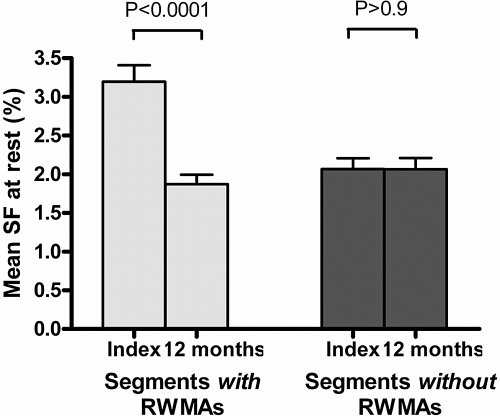
At baseline, a total of 146 regions (48.7%) developed myocardial stunning out of a maximum of 300 in 30 patients. The mean resting SF of those regions that developed myocardial stunning on HD was significantly higher than those that did not (3.19 ± 1.18% versus 2.07 ± 0.75%, P < 0.01). At follow-up, 47 of the 146 stunned regions (32.2%) had developed a fixed reduction in systolic function of >60% that did not show an increase on HD. There was a significant decline in resting SF over 12 mo in all regions that developed HD-induced myocardial stunning at baseline (3.19 ± 1.18% versus 1.87 ± 0.7%, P < 0.0001). However, there was no significant change over 12 mo in those regions that were unaffected by HD at baseline (2.07 ± 0.75% versus 2.06 ± 0.78%, P = 0.99; Figure 1).
Figure 1.
Change in regional SF over time. There was a significant reduction in SF over 12 mo in those segments that developed RWMAs at baseline (index) compared with those that did not. Values are expressed as mean ± SEM.
Effect on EF at Rest and on HD
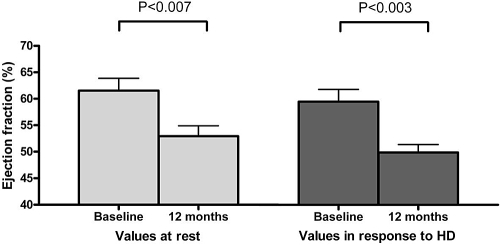
Patients who developed myocardial segments with fixed systolic reduction of >60% (n = 19) showed a significant decline in EF over 12 mo at rest and at peak stress during HD (61.5 ± 10.1% versus 52.9 ± 8.6%, P < 0.007 and 59.5 ± 10% versus 49.9 ± 6.5%, P < 0.003, respectively; Figure 2). In comparison, patients who did not develop such abnormalities showed no significant reduction in these values at rest or during HD (63.3 ± 14.8% versus 56.2 ± 13.1%, P > 0.1 and 53.9 ± 10.4% versus 52.4 ± 12.4% P > 0.7, respectively).
Figure 2.
Change in EF at rest and during HD over 12 mo in patients with fixed reductions in segmental function of >60%. The development of fixed segmental reduction in previously stunned myocardial segments was associated with a significant reduction in LV EF at rest and during HD. Values are mean ± SEM.
Intradialytic Changes in BP
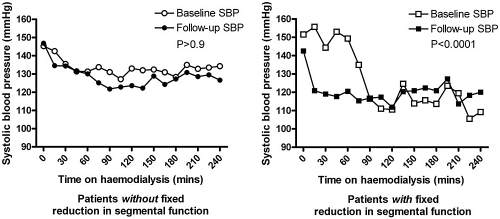
Using a two-way ANOVA, there was a significant reduction in systolic BP (SBP) during HD and over 12 mo in the patient group that developed fixed segmental systolic dysfunction of >60% [F (16,336) = 4.71; mean square error = 227; P < 0.0001]. This was not true in the other group who had no significant change in their SBP during HD over 12 mo (F (16,820) = 0.25; mean square error = 655; P > 0.9); Figure 3).
Figure 3.
Changes in SBP during HD over 12 mo. After 12 mo there was a significant difference in SBP during HD in those patients with new fixed reductions of >60% in previously stunned myocardial segments.
Hematologic, Biochemical, and Dialysis Details
At baseline and follow-up, 83% of patients had a significantly raised plasma cTnT concentration (≥0.03 μg/L), consistent with the presence of repetitive acute myocardial injury. However, there were no significant differences among any of the hematologic or biochemical measurements (including cTnT) within or between either of the groups at baseline or over the 12-mo follow-up period. There were also no significant differences between the groups with respect to dialysis adequacy (Kt/Vurea) or ultrafiltration volumes. These data are summarized in Table 2.
Table 2.
Pre- and post-HD hematological and biochemical values for both groups at baseline and follow-upa
| Parameter | Patients without Fixed Reduction in Segmental Systolic Reduction |
Patients with Fixed Reduction in Segmental Systolic Reduction |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Follow-Up |
Baseline |
Follow-Up |
|||||
| Pre-HD | Post-HD | Pre-HD | Post-HD | Pre-HD | Post-HD | Pre-HD | Post-HD | |
| Hemoglobin (g/dl) | 10.8 ± 1.1 | 11.3 ± 1.0 | 11.8 ± 1.7 | 12.2 ± 1.8 | 11.2 ± 1.5 | 11.7 ± 1.6 | 11.9 ± 1.6 | 12.2 ± 1.7 |
| Sodium (mmol/L) | 137 ± 3 | 137 ± 2 | 136 ± 4 | 137 ± 2 | 138 ± 3 | 138 ± 2 | 137 ± 4 | 137 ± 3 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 2.8 ± 0.5 | 4.4 ± 0.8 | 2.8 ± 0.4 | 4.9 ± 1.0 | 2.9 ± 0.4 | 4.6 ± 0.1 | 3.0 ± 0.7 |
| Urea (mmol/L) | 19.6 ± 4.7 | 5.8 ± 2.1 | 17.8 ± 5.6 | 5.4 ± 2.4 | 21 ± 4.6 | 6.5 ± 2.2 | 23.3 ± 8.7 | 7.0 ± 2.9 |
| Creatinine (μmol/L) | 665 ± 165 | 258 ± 74 | 618 ± 165 | 250 ± 87 | 658 ± 213 | 267 ± 107 | 725 ± 257 | 300 ± 148 |
| Phosphate (mmol/L) | 1.8 ± 0.4 | 0.8 ± 0.1 | 1.7 ± 0.5 | 0.7 ± 0.2 | 1.5 ± 0.5 | 0.8 ± 0.3 | 1.8 ± 0.6 | 0.8 ± 0.3 |
| Bicarbonate (mmol/L) | 23.8 ± 1.9 | 27.1 ± 4.1 | 22.8 ± 2.2 | 27 ± 1.6 | 23.1 ± 2.5 | 27.9 ± 2.4 | 24.2 ± 2.6 | 26.8 ± 1.7 |
| Corrected calcium (mmol/L) | 2.4 ± 0.2 | 2.3 ± 0.1 | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.2 | 2.3 ± 0.1 |
| Albumin (g/L) | 34.4 ± 2.9 | 35.8 ± 3.8 | 30.6 ± 6.2 | 32.3 ± 7.4 | 35.3 ± 4.7 | 36.6 ± 5.5 | 35.2 ± 4.4 | 37.1 ± 4.9 |
| cTnT (μg/L) | 0.07 ± 0.05 | – | 0.12 ± 0.12 | – | 0.09 ± 0.06 | – | 0.08 ± 0.05 | – |
| Kt/Vurea | 1.3 ± 0.2 | – | 1.4 ± 0.2 | – | 1.3 ± 0.3 | – | 1.3 ± 0.4 | – |
There were no significant differences between different or within the same groups at either time point (P > 0.1).
Discussion
This study demonstrates for the first time that LV segments that develop myocardial stunning during HD can progress over time into areas of fixed systolic dysfunction that have distinct systemic hemodynamic consequences. These manifest as reductions in LVEF at rest and a reduced ability to increase LV contractile function to maintain BP during HD with fluid removal. This could potentially create a vicious cycle for further HD-induced cardiac injury.
We found no significant association between the progression of LV regional wall dysfunction and demographic variables. In the HD population, decline in LVEF is usually progressive over time and often accelerated (19), but there is increasing evidence that this is not due to traditional cardiac risk factors or conventional atherosclerotic coronary artery disease (CAD) (2). It is therefore not surprising that the presence or absence of IHD and diabetes mellitus were not different between the two groups.
There were no observed differences between the number or type of antihypertensive medications. Calcium channel antagonists may be cardioprotective in the setting of myocardial stunning (20), although no benefit was observed in the group who did not develop fixed systolic dysfunction. However, the numbers of patients were small and this study was not powered to observe such differences so no conclusion was drawn.
No significant difference in dialysis vintage was observed; however, both groups had been receiving HD for a considerable time (>2 yr). There is currently no evidence as to whether a critical time period on dialysis exists after which the process of irreversible myocyte damage begins and the potential for improvement in LV EF is lost. In reality this is likely to differ considerably from patient to patient and be determined by a complex interrelationship between several underlying factors that affect cardio-renal performance. Some of these pathologic processes associated with HD that predispose to demand cardiac ischemia (coronary and peripheral arterial calcification (21), impaired microcirculation, and increased pulse wave velocity (22)) are now more clearly understood, but there are an equal number of humoral and genetic factors whose roles are very poorly defined (23).
At baseline, around half of all LV regions developed RWMAs on HD. At rest, these regions had a significantly higher regional SF than those unaffected by HD. This may reflect vulnerability to demand ischemia in more kinetic areas of the LV in response to hemodynamic stress in HD patients with reduced coronary flow reserve. At follow-up, these regions had significantly reduced their SF in comparison to unaffected regions at baseline. The magnitude of that reduction varied, but a considerable number (32.2%) showed a reduction of >60% that remained fixed during HD.
As a result of these areas of fixed systolic dysfunction, affected patients showed a decline in resting and demand EF (on HD) after 12 mo that was not seen in the unaffected group. This is in keeping with other published work looking at the effect of chronic myocardial stunning and myocardial hibernation on LV function in patients with IHD. However, in our dialysis patients this reduction in EF seems to have a direct effect on intradialytic hemodynamics.
The cohort as a whole represents patients with HD-induced cardiac dysfunction. Both individual study groups had some reduction in absolute SBP during HD. However, the only patients that had a significant deterioration in their SBP during HD after 12 mo were in the group that developed regional fixed systolic reductions of >60%. This is not attributable simply to volume overload. A small subset of patients with below-average ultrafiltration volumes had significant reductions in SBP. In addition, SBP at the end of HD is primarily determined by cardiac output (24) and therefore it is likely that the absolute reduction in SBP seen here is secondary to the reduction in LVEF. Intradialytic hypotension (IDH) is known to be associated with increased mortality. Given the underlying vulnerability of hibernating myocardium to increases in demand (12) coupled with decreased coronary flow reserve in HD patients, it may be that this adaptive process actually leads to further segmental injury by exacerbating intradialytic instability. This may be one of the reasons that prevalence of heart failure is so high and survival so poor in HD patients.
This hypothesis is supported by evidence that a continuation of HD results in a significant increase in cardiovascular events as well as myocyte fibrosis and death compared with uremic patients not on HD (25). A study by Wali et al. (26) looking at the effects of renal transplantation in patients with heart failure demonstrated that a longer duration of dialysis in months before transplantation was the only significant factor associated with a decreased likelihood of achieving a normal LV EF in the posttransplantation period. They attributed this to prolonged exposure to potentially negatively inotropic factors and other toxins that are present in uremic plasma, which may be decreased after transplantation (27). Although these potential uremic toxins may play a part, we assert that the observed changes are related to the removal of hemodialytic stress.
Previous studies in our center have shown that myocardial segments that develop stunning during HD have normal resting blood flow but a reduction in blood flow during treatment. Animal studies have shown that repeated myocardial stunning precedes myocardial hibernation and remodeling (28). Such fixed reductions in systolic function in those regions undergoing chronic myocardial stunning would be consistent with myocardial hibernation but may also potentially represent areas that have already undergone fibrosis and remodeling. Therefore, these chronically stunned segments may well have deteriorated over 12 mo and lie somewhere along the continuum of disease classified as myocardial hibernation and remodeling.
Dialysis sessions that are complicated by episodes of IDH are associated with a significant rise in cardiac troponin I and cTnT levels (29), suggesting underlying subclinical myocardial damage. A significant amount of work has been done looking at the clinical significance of biochemical markers of cardiac injury, including cardiac troponins, but their value as long-term prognostic markers is as yet undetermined. We observed no difference in cTnT levels between the two groups of patients; however, this may be because several of the most potentially unstable patients died before 12 mo and were not included in the analysis. More work is needed to assess the use of biochemical markers as a screening tool for the identification of at-risk patients.
The process of myocardial stunning in HD has been shown to be modifiable in several ways (apart from transplantation) that improve intradialytic hemodynamics. These include biofeedback mechanisms and cooled dialysate (4,5), although there are as yet no long-term data looking at improvements in LV EF as a result of the longer-term applications of these interventions. Other dialysis treatment such as nocturnal HD have been shown to result in an improvement in LV EF (30), which may also be in part due to abrogation of HD-induced myocardial stunning through modifications to ultrafiltration rates and IDH. Given the current shortage of donor organs for kidney transplantation, these other dialysis options may offer treatment strategies that can minimize/avoid HD-induced myocardial stunning, myocardial hibernation and remodeling, improve LV function, and thereby reduce heart failure and mortality.
Study Limitations
Echocardiography was the only method used to quantify functional decline; however, this method is repeatable and quantitative. Also, the use of other imaging techniques such as magnetic resonance imaging to assess functional change while the patient was receiving HD would be technically impossible.
There was no angiography or perfusion imaging available to quantify epicardial CAD in these patients. However, HD induces myocardial ischemia even in the absence of epicardial CAD, and myocardial scintigraphy is an imperfect technique for the evaluation of CAD in patients with ESRD (31). Although the mechanism of HD-driven myocardial stunning and hibernation would still apply in this case, it may be that coronary revascularization is a potential intervention that will improve LV EF and heart failure in this group. That question is outside of the scope of this study.
In conclusion, this study demonstrates for the first time that HD-induced cardiac injury results in a reduction in segmental and global LV function. The cycle of intradialytic instability resulting in repetitive cardiac injury leads to a potential for yet more dialysis-induced hypotension. This fits well with the observation that heart failure is associated with very poor outcome in HD patients. However, hemodynamic instability during HD is a potentially avoidable phenomenon. This raises the possibility that addressing HD-induced repetitive injury might prevent the development of myocardial stunning, hibernation, and fibrosis that lead to LV systolic dysfunction in these patients and potentially prevent the development of heart failure.
Disclosures
None.
Acknowledgments
This study was funded by a grant from Kidney Research (United Kingdom) and jointly sponsored by the University of Nottingham and Derby Hospitals NHS Foundation Trust
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9: S16–S23, 1998 [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]
- 3.U.S. Renal Data System. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the US Bethesda, MD, National Institutes of Health, Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 4.Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW: Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. Am J Kidney Dis 47: 830–841, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Selby NM, Burton JO, Chesterton LJ, McIntyre CW: Dialysis induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol 1: 1216–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG: Hemodialysis induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silberberg JS, Barre PE, Prichard SS, Sniderman AD: Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 36: 286–290, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Sigrist MK, McIntyre CW: Vascular calcification is associated with impaired microcirculatory function in chronic hemodialysis patients. Nephron Clin Pract 108: c121–c126, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ, Powers ER: Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J 147: 1017–1023, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Bos WJ, Bruin S, van Olden RW, Keur I, Wesseling KH, Westerhof N, Krediet RT, Arisz LA: Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis 35: 819–826, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Homans DC, Laxson DD, Sublett E, Lindstrom P, Bache RJ: Cumulative deterioration of myocardial function after repeated episodes of exercise-induced ischemia. Am J Physiol 256: H1462–H1471, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Vanoverschelde JL, Wijns W, Depre C, Essamri B, Heyndrickx GR, Borgers M, Bol A, Melin JA: Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation 87: 1513–1523, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Narula J, Dawson MS, Singh BK, Amanullah A, Acio ER, Chaudhry FA, Arani RB, Iskandrian AE: Noninvasive characterization of stunned, hibernating, remodeled and nonviable myocardium in ischemic cardiomyopathy. J Am Coll Cardiol 36: 1913–1919, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Braunwald E, Rutherford JD: Reversible ischemic left ventricular dysfunction: evidence for the “hibernating myocardium”. J Am Coll Cardiol 8: 1467–1470, 1986 [DOI] [PubMed] [Google Scholar]
- 16.Schulz R, Rose J, Martin C, Brodde OE, Heusch G: Development of short-term myocardial hibernation. Its limitation by the severity of ischemia and inotropic stimulation. Circulation 88: 684–695, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Fallavollita JA, Canty JM, Jr: Differential 18F-2-deoxyglucose uptake in viable dysfunctional myocardium with normal resting perfusion: Evidence for chronic stunning in pigs. Circulation 99: 2798–2805, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Bosch JG, Savalle LH, van Burken G, Reiber JH: Evaluation of a semiautomatic contour detection approach in sequences of short-axis two-dimensional echocardiographic images. J Am Soc Echocardiogr 8: 810–821, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE: Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol 11: 912–916, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Birnbaum Y, Kloner RA: Therapy for myocardial stunning. Basic Res Cardiol 190: 291–293, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Sigrist M, Bungay P, Taal MW, McIntyre CW: Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant 21: 707–714, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM: Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 63: 1852–1860, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Freedman BI: Genetic factors in common complex renal and cardiovascular diseases. Adv Chronic Kidney Dis 13: 105–109, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Stewart GA, Mark PB, Johnston N, Foster JE, Cowan M, Rodger RS, Dargie HJ, Jardine AG: Determinants of hypertension and left ventricular function in end stage renal failure: A pilot study using cardiovascular magnetic resonance imaging. Clin Physiol Funct Imaging 24: 387–393, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Mall G, Huther W, Schneider J, Lundin P, Ritz E: Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant 5: 39–44, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, Drachenberg C, Papadimitriou J, Brisco MA, Blahut S, Fink JC, Fisher ML, Bartlett ST, Weir MR: Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol 45: 1051–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Vanholder R, Glorieux G, Lameire N: Uremic toxins and cardiovascular disease. Nephrol Dial Transplant 18: 463–466, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Shivalkar B, Flameng W, Szilard M, Pislaru S, Borgers M, Vanhaecke J: Repeated stunning precedes myocardial hibernation in progressive multiple coronary artery obstruction. J Am Coll Cardiol 34: 2126–2136, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Hung SY, Hung YM, Fang HC, Yeh JH, Hung GC, Wu CJ, Chou KJ, Chung HM: Cardiac troponin I and creatine kinase isoenzyme MB in patients with intradialytic hypotension. Blood Purif 22: 338–343, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Chan C, Floras JS, Miller JA, Pierratos A: Improvement in ejection fraction by nocturnal hemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrol Dial Transplant 17: 1518–1521, 2002 [DOI] [PubMed] [Google Scholar]
- 31.De Lima JJ, Sabbaga E, Vieira ML, de Paula FJ, Ianhez LE, Krieger EM, Ramires JA: Coronary angiography is the best predictor of events in renal transplant candidates compared with noninvasive testing. Hypertension 42: 263–268, 2003 [DOI] [PubMed] [Google Scholar]