Abstract
Background and objectives: The prevalence and significance of remission and relapse in children, adolescents, and young adults with lupus nephritis in the United States are poorly understood. Patterns and predictors of disease progression in a southeastern U.S. pediatric cohort with severe lupus nephritis are presented.
Design, settings, participants, & measurements: Individuals age 21 or less with kidney biopsy-proven lupus nephritis followed in the Glomerular Disease Collaborative Network were included. Cox regression models were used to evaluate predictors of relapse and end stage kidney disease (ESKD).
Results: Seventy-three subjects with a mean age of 15.6 ± 3.4 yr were included. Five-year kidney survival was 77%. Complete and partial remission rates within 1 yr of induction therapy were 25 and 64%, respectively. Relapse and ESKD rates were similar between complete and partial responders. Relapse occurred in 35% of responders (complete or partial) in 45 ± 32 mo. Disease relapse was a predictor of ESKD (HR = 10.12, P < 0.0001). Treatment resistance was documented in African Americans more often than non-African Americans (eight versus 0; P = 0.03). ESKD HR associated with treatment resistance was 6.25, P < 0.002.
Conclusions: Remission whether complete or partial is associated with improved kidney survival in children with lupus nephritis. Nephritis relapse is a strong predictor of progression to ESKD. Treatment resistance portends a high risk of ESKD and disproportionately affects African American children with lupus nephritis.
Systemic lupus erythematosis (SLE) is a chronic autoimmune disorder affecting approximately 1.4 million U.S. residents of whom 15% have onset before age 16 yr (1). Lupus nephritis affects 20 to 80% of juvenile onset lupus, and 10 to 50% of these individuals progress to end stage kidney disease (ESKD) (2–6). The prevalence and significance of disease relapse and impact of available treatments in pediatric patients with lupus nephritis are poorly understood. Most published studies in pediatrics are small, single-center reports, and outcome data are often difficult to generalize to populations that are demographically diverse (2,7–10). This retrospective study entails a predominantly African American cohort of pediatric patients with lupus nephritis and a high prevalence of proliferative disease. We compare outcomes in patients with partial versus complete treatment response and evaluate patterns and predictors of relapse. We also examine the impact relapse patterns have on progression to ESKD.
Materials and Methods
Study Population
Eligible patients were identified through the Glomerular Disease Collaborative Network (GDCN) and the University of North Carolina at Chapel Hill (UNC) nephropathology database. The GDCN is a network of 800 nephrologists, rheumatologists, and nephropathologists from over 300 private practice nephrology clinics and academic sites located primarily throughout the southeastern United States. The UNC Nephropathology Laboratory evaluates more than 1500 biopsies each year. Individuals with various forms of glomerular disease are invited to provide consent to participate in GDCN disease-specific registries by their participating physician, as described previously (11,12). We identified 330 patients less than 21 yr of age at the time of initial lupus nephritis diagnosis on kidney biopsy between 1985 and 2007.
The medical records of 109 consented eligible subjects were reviewed. Patients with proliferative lupus nephritis lesions (classes III to V) by either 1995 World Health Organization classification or 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification were included (13,14). Twenty-four patients were excluded due to follow-up time less than 6 mo and another 12 patients were excluded for class II (mesangioproliferative) disease, yielding a study cohort of 73 patients. Comparison of the study cohort to the total of 330 pediatric patients with renal biopsy-proven lupus nephritis by the UNC nephropathology laboratory was made to assess whether the study sample is representative of the pediatric lupus nephritis patients registered in the GDCN. In accordance with the Declaration of Helsinki, this study was approved by the UNC Committee for the Protection of Human Subjects.
Definitions
The diagnosis of lupus nephritis was based on the kidney histopathology and meeting at least three American College of Rheumatology (ACR) criteria (13). Complete remission was defined according to the ACR 2006 clinical trial criteria, which define remission as (1) a normal estimated GFR (eGFR) ≥90mls/min/1.73m2 or >25% increase from baseline, (2) urine protein-to-creatinine ratio <0.2 or dipstick of 0 to trace, (3) less than five red blood cells (rbcs)/high power field, and (4) no cellular casts in the urine (15). Estimated GFR was calculated using the Schwartz formula (16). Partial remission was defined as meeting the ACR 2006 clinical trial criteria for remission with the exception of a urine protein-to-creatinine ratio between 0.2 and 2. If patients met at least two parameters but were missing information on the other criteria, they were labeled as partial responders. Patients were defined as nonresponders if they failed to meet any of the criteria for remission. Inclusion into one of these response categories was evaluated as the best remission response or cumulative response over the first year after initial kidney biopsy and induction therapy. Disease relapse was characterized as a >25% decline in GFR, a 50% or more increase in proteinuria, or active urine sediment characterized by >5rbc/hpf and/or cellular casts. ESKD was defined by dialysis dependence for greater than 3 mo or kidney transplantation.
Statistical Analyses
Baseline characteristics of the study cohort were summarized using descriptive statistics expressed as mean ± SD or number (%). Kruskal-Wallis test was used to compare continuous measures by treatment response groups. Fisher's exact test was used for comparisons of categorical data. Kaplan-Meier estimates were used to assess and plot time to relapse and time to ESKD separately following biopsy diagnosis. Patients were censored on their last day of follow-up if they did not reach an end point. Cox regression models were used to evaluate the effect of potential predictors of relapse and ESKD as separate outcomes. Results are reported as hazard ratios (HRs) with 95% confidence intervals (CI). All data analyses were completed using Stata statistical software, version 10 (StataCorp, College Station, TX).
Sensitivity Analysis
To evaluate the impact of misclassification, a partial sensitivity analysis was conducted where study participants were reclassified using a less stringent set of remission criteria. Contreras et al. defined overall remission as improvement in serum creatinine >25% of baseline or stable serum creatinine within 25% of baseline and a urine protein-to-creatinine ratio less than three with a baseline of greater than three or a greater than 50% decrease if baseline was less than three (17). Findings from urine sediment were not included in this definition. Because proteinuria is included in each definition, an analysis excluding patients missing proteinuria information was completed.
Results
A total of 73 study participants ages 6 to 21 yr were evaluated in this analysis. The demographic characteristics of the sample were similar to the overall renal biopsy population from which it arose and are summarized in Table 1. Table 2 displays the more detailed baseline characteristics of the study cohort. The mean age was 15.6 ± 3.4 yr and 12 (16%) men were included, yielding a female-to-male ratio of 5:1. Mean follow-up time was 55.4 ± 51.6 mo. African Americans represented nearly two-thirds of the 73 subjects included in this study. Among the African Americans, 70% had class IV disease compared with 17% with class III and 13% with class V disease.
Table 1.
Comparison of Glomerular Disease Collaborative Network pediatric lupus population and study cohort
| Full Databasen = 330 | Study Cohortn = 73 | |
|---|---|---|
| Age | 15.5 ± 5.5 | 15.6 ± 3.4 |
| Male gender | 69 (21%) | 12 (16%) |
| African American | 172 (52%) | 47 (64%) |
| Caucasian | 69 (21%) | 22 (30%) |
| Hispanic | 9 (3%) | 1 (2%) |
| Other | 8 (2%) | 3 (4%) |
| Unknown | 72 (22%) | 0 (0%) |
Table 2.
Characteristics of pediatric lupus nephritis cohort
| All n = 73 | Complete Responders n = 18 | Partial Responders n = 47 | Non-Responders n = 8 | P valueb | |
|---|---|---|---|---|---|
| Agea | 15.6 ± 3.4 | 15.3 ± 3.3 | 15.5 ± 3.4 | 16.4 ± 4.1 | 0.70 |
| BMI Z-score | 0.72 ± 1.42 | 0.42 ± 1.72 | 0.73 ± 1.32 | 1.3 ± 1.25 | 0.39 |
| Race | 0.05 | ||||
| Caucasian | 22 (30%) | 3 (16%) | 19 (40%) | ||
| African American | 47 (64%) | 13 (72%) | 26 (55%) | 8 (100%) | |
| Hispanic | 1 (2%) | 1 (6%) | - | ||
| American Indian | 3 (4%) | 1 (6%) | 2 (5%) | ||
| Baseline serum creatinine (mg/dl) | 1.73 ± 1.64 | 1.26 ± 1.2 | 1.4 ± 0.91 | 4.6 ± 2.9 | 0.001 |
| Baseline GFR (ml/min/1.73m2) | 81.3 ± 43.9 | 91.5 ± 38.9 | 86.8 ± 42.2 | 34.3 ± 35.6 | 0.005 |
| Hypertension | 47 (69%) | 13 (76%) | 27 (63%) | 7 (87%) | 0.34 |
| Baseline proteinuria (mg/day) | 3740 ± 3946 | 2802 ± 2999 | 4166 ± 4438 | 3777 ± 3211 | 0.44 |
| Histology class | 0.22 | ||||
| III | 12 (16%) | 6 (33%) | 6 (13%) | - | |
| IV | 54 (74%) | 11 (61%) | 36 (77%) | 7 (88%) | |
| V | 7 (10%) | 1 (6%) | 5 (10%) | 1 (12%) | |
| Relapsec | 23 (35%) | 6 (33%) | 17 (36%) | N/A | 0.54 |
| ESKD | 17 (24%) | 3 (17%) | 8 (18%) | 6 (75%) | 0.004 |
| Time to relapse (months) | 44.6 ± 32.2 | 42 ± 38.6 | 45.5 ± 30.8 | N/A | 0.66 |
| Time to ESKD (months) | 57.8 ± 47.3 | 93.3 ± 58.8 | 67.8 ± 41.4 | 26.7 ± 36.1 | 0.12 |
| Cytoxan | 59 (80%) | 16 (89%) | 37(78%) | 6 (75%) | 0.66 |
| Mycophenolate mofetil | 10 (14%) | 3 (17%) | 5 (11%) | 2 (25%) | 0.40 |
| Hydroxychloroquine | 32 (44%) | 12 (67%) | 19 (40%) | 1 (13%) | 0.04 |
| ACE Inhibitors | 43 (59%) | 14 (78%) | 24 (51%) | 5 (63%) | 0.16 |
Descriptive data represented as mean ± standard error or n (%).
P-values calculated using Fisher's exact test for categorical data and Kruskal-Wallis for continuous variables due to independent variable with more than two levels.
One patient in overall group who developed a partial response beyond first year and eventually relapsed. This patient was excluded from analyses on relapse data. Denominator is based on the total number of responders.
Treatment Response
Overall, 65 (89%) patients had a response to induction therapy, while eight (11%) patients were treatment resistant (Table 2). Among responders, partial response, n = 47 (72%), was more common than complete response, n = 18 (28%), with a ratio of 2.6:1. Among the partial responders, 42 (90%) met criteria by eGFR, 35 (75%) met criteria for improvement in proteinuria, and 20 (43%) had normal urine sediments. In this cohort, 12 (16%) were missing data for proteinuria, and 27 (37%) for urine sediment findings. The eight study participants who were treatment resistant were all African American. Treatment resistance was marked by significant reduction of eGFR at presentation, supporting the likelihood of delayed diagnoses and therefore greater predominance of chronicity at the time of initial therapy. On review of histologic chronicity markers, it was evident that several of the treatment-resistant patients presented with advanced sclerotic disease. Four of the treatment-resistant patients presented with moderate to severe interstitial fibrosis and tubular atrophy, and/or >50% glomeruli with global sclerosis. An additional four treatment-resistant individuals presented with findings suggestive of potentially reversible disease. These individuals had mild to no fibrosis or tubular atrophy, and one of the four showed no cellular crescent formation. One patient had evidence of thrombotic microangiopathy.
Relapse
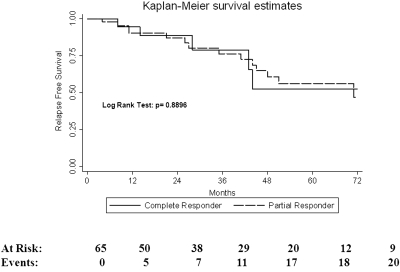
Of the 65 patients who responded to initial therapy, 23 (35%) experienced a renal relapse, with over one-third experiencing relapse within 5 yr of diagnosis. Mean time to relapse overall was 44.6 ± 32 mo. We were unable to identify statistically significant predictors of relapse (Table 3). However, the risk of disease relapse in African American patients was 32% lower than in Caucasians, with a HR of 0.68 (P = 0.37). Overall, ten (53%) of 19 Caucasian responders experienced disease relapse, compared with 12 (33%) of 36 of African American responders. There was no difference in the development of disease relapse between complete and partial responders (Figure 1). Overall, 36% of those who achieved partial remission relapsed compared with 33% of those who achieved complete remission. Only five of the 23 patients who relapsed were on maintenance therapy with a purine analog (mycophenolate mofetil or azathioprine) at the time of relapse. Nine of the relapsers were on no maintenance therapy, and three patients who were supposed to be on maintenance therapy had well-documented histories of nonadherence to prescribed medical therapy. One subject relapsed with the development of pregnancy, and two patients relapsed upon the spacing of cyclophosphamide from a monthly to quarterly dosing regimen.
Table 3.
Univariate predictors of relapse (n = 65)
| Hazard Ratio | 95% CI | P value | |
|---|---|---|---|
| Partial remission | 1.36 | (0.58, 3.19) | 0.48 |
| Age < 16 | 1.52 | (0.66, 3.54) | 0.32 |
| African American | 0.68 | (0.30, 1.56) | 0.37 |
| BMI Z-score | 1.15 | (0.83, 1.59) | 0.39 |
| Baseline eGFRa | 1.00 | (0.89, 1.19) | 0.97 |
| Proteinuriab | 0.97 | (0.86, 1.09) | 0.61 |
| Hypertension | 0.70 | (0.28, 1.79) | 0.46 |
| Class IV nephritis | 0.81 | (0.33, 2.00) | 0.64 |
| IV Cyclophosphamide | 0.78 | (0.31, 2.00) | 0.61 |
Change in eGFR by 10 ml/min/1.73 m2.
Change in proteinuria by 1 g/d.
Figure 1.
Relapse estimates by treatment response.
ESKD
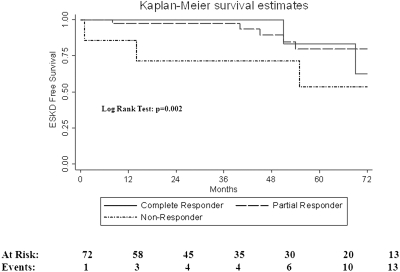
Kidney survival for this cohort was 77% at 5 yr. Five-year survival estimates were 83% for complete and 80% for partial responders. Five-year survival in nonresponders was 54% (Figure 2). Treatment resistance was associated with high risk of progression to ESKD in univariate (HR 6.25; P < 0.002) and multivariate analysis controlling for African American race and baseline eGFR (HR 5.46; P < 0.018) (Table 4). A tenfold risk of progression to ESKD disease was associated with disease relapse; (HR 10.12; P < 0.001) (Table 4). Multivariate analysis controlling for African American race and baseline estimated GFR resulted in similar risk for ESKD associated with relapse (HR 11.76; P < 0.001).
Figure 2.
Kidney survival by treatment responses.
Table 4.
Predictors of End Stage Kidney Diseasec
| Univariate Hazard Ratio | 95% CI | P value | Multivariate Hazard Ratiod | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Relapse (n = 65) | 10.12 | (3.58, 28.98) | <0.0001 | 11.76 | (1.97, 19.76) | <0.002 |
| Complete Remission | 0.77 | (0.16, 3.58) | 0.73 | |||
| Treatment Resistance | 6.25 | (1.97, 19.76) | <0.002 | 5.46 | (1.34, 22.2) | <0.018 |
| Age <16 | 1.13 | (0.41, 3.14) | 0.81 | |||
| African American | 1.17 | (0.40, 3.46) | 0.30 | |||
| BMI Z-score | 1.08 | (0.76, 1.56) | 0.64 | |||
| Baseline GFRa | 0.88 | (0.76, 1.03) | 0.10 | |||
| Proteinuriab | 1.04 | (0.90, 1.19) | 0.62 | |||
| Hypertension | 0.99 | (0.33, 3.01) | 0.99 | |||
| Class IV nephritis | 1.36 | (0.38, 4.86) | 0.63 | |||
| Cyclophosphamide | 1.64 | (0.37, 7.35) | 0.51 |
Change in eGFR by 10 ml/min/1.732.
Change in proteinuria by 1 g/d.
n = 73 unless otherwise specified.
Estimate adjusted for African American ethnicity and baseline eGFR.
Sensitivity Analysis
Applying the more liberal overall remission criteria defined by Contreras et al., we found no difference in those identified as nonresponders in our cohort. On the other hand, more than twice as many study participants originally classified as partial responders met the Contreras criteria for overall remission: 37 (51%) compared with 18 (25%) using the ACR 2006 criteria. These differences, however, did not translate into significant changes in outcome. Relapse occurred in nine (25%) of 37 of complete responders classified via the Contreras criteria versus six (33%) of 18 complete responders, using ACR 2006 criteria. Progression to ESKD was similar as well with five (14%) of complete responders using the Contreras criteria compared with three (17%) of complete responders using the ACR 2006 criteria developing ESKD. Exclusion of the 12 partial responders missing information for proteinuria yielded similar patterns of relapse and ESKD events. Relapse occurred in 13 (37%) of 35 partial responders compared with six (33%) of 18 complete responders. ESKD occurred in five (14%) of partial responders compared with three (17%) complete responders.
Discussion
This study is the largest cohort evaluating treatment response and relapse patterns among children, adolescents, and young adults with proliferative lupus nephritis in the United States. We observed treatment resistance in 11% of patients in this cohort and disease relapse in 35% of those with a cumulative response to induction therapy within 1 yr of treatment.
Treatment resistance has been observed in 20 to 32% of adult patients with lupus nephritis (18,19). Prior estimates of treatment resistance in children are more difficult to find as most of these studies report only long-term survival estimates. Treatment resistance was observed in 13% of a cohort of (n = 77) Korean children with lupus nephritis and in 29% of a group of predominately (n = 44) African American children with lupus nephritis (3,9). Treatment resistance was observed only among African Americans in our cohort.
Treatment resistance was highly predictive of ESKD (HR 6.25; P < 0.002). Patients with complete versus partial response did not differ with respect to risk of either disease relapse or progression to ESKD. Similar findings are reported in a recent publication by Chen et al. where they evaluated 86 adult patients with proliferative lupus nephritis previously involved in a randomized clinical trial (19). In this study, even a partial remission defined clinically by changes in serum creatinine and proteinuria predicted better kidney survival than no response to initial therapy.
Treatment resistance in our cohort may be related specifically to chronicity in some patients, but four of the eight treatment-resistant patients had only mild evidence of chronicity by histology. Previous studies have reported similar patterns of treatment resistance among ethnic minorities independent of chronic changes on biopsy (12,20). Treatment resistance among individuals of African descent has been described in areas with universal access to health care, supporting the presence of risk factors independent of socioeconomic disparities (2). Another study evaluated the impact of concomitant lupus involvement in the central nervous system and found similar patterns of treatment resistance among African Americans (21).
The development of disease relapse in 35% of our cohort is similar to observed relapse patterns reported in 32% of 25 children with lupus nephritis in a separate southeastern US cohort (22). Lee et al. observed disease relapse in 45% of 77 Korean children with lupus nephritis (3). We found an absolute difference of only 3% in relapse occurrence between complete (33%) and partial (36%) responders. In pediatrics, relapse patterns in complete compared with partial responders have not been reported previously.
Differences in relapse rates between complete and partial responders in adult cohorts have been reported. Using response criteria comparable to those chosen for our current study, lower rates of relapse occurrence for complete versus partial remission have been reported in Italian and Korean cohorts (23–25). A follow-up study on 145 patients previously involved in the early NIH trials for induction therapy revealed that partial responders were more likely to develop a renal flare, 12 (63%) of 19, compared with complete responders, 29 (40%) of 73 (26). It is interesting to note that all the relapsing patients had been off maintenance therapy for at least 6 mo. In comparison, only 25% of the relapsers in our cohort were on maintenance therapy at the time of relapse, which underscores the question of how long to continue maintenance therapy in patients who have achieved remission.
Renal relapse was a strong predictor of progression of ESKD (HR 10.12; P < 0.001) in this study. This finding is in agreement with prior lupus nephritis cohorts (27,28). Mosca et al. found that nephritic flares and early proteinuric flares among adults were highly associated with doubling of serum creatinine (27,29). Similar findings have been reported in another adult cohort (28). The relationship between relapsing disease and ESKD has been reported less frequently in pediatric cohorts. In the Korean pediatric lupus nephritis cohort, Lee et al. found nephritic flares to be independently associated with chronic kidney failure (3).
One of the strengths of our study is the length of follow-up. With a mean follow-up time of 55 ± 51 mo, the ability to capture relapses and ESKD is strengthened. Review of the literature on adults with lupus nephritis reveals mean time to first episode of relapses between 32 and 42 mo (27,28,30). We found similar results with a mean time to first relapse episode of 43 mo in this pediatric cohort. Our 5-yr kidney survival estimate for complete responders of 83% is poor compared with a report by Chen et al., who reported a 10-yr survival estimate for complete responders of 95% (19). Of note, African Americans represented only 24% of the Chen cohort, which is quite different from our southeast U.S. cohort with 64% African Americans. An additional strength of our study is how representative our cohort is of the overall biopsied 309 pediatric lupus nephritis patients in the GDCN registry.
Limitations of this study exist and warrant discussion. The retrospective design of the study makes the introduction of biases possible. Uniform review of medical records with application of strict definitions for remission limits these biases. There is also a concern with the strict nature of the definition used for complete and partial remission in this study. Data were required for each of the three criteria to be classified as a complete responder. If a subject met at least two of the criteria for complete remission but did not have information for at least one of the other criteria, the subject was classified as a partial responder. As a result, there were patients classified as partial responders in this cohort that would be classified as complete responders using alternative definitions for response. Inconsistency of classification of a positive response to therapy however did little to change the measurable differences in outcomes of relapse or ESKD when using less strict definitions or excluding patients missing information for proteinuria. Despite being a large cohort for this rare disease, the number of participants in this study limits the power to reveal small differences.
Conclusion
Clinical response to therapy defined as complete or partial remission yielded superior long-term kidney survival than observed in nonresponders. Treatment resistance in African Americans is a major concern and may not be related to advanced chronic disease defined histologically. Relapsing disease in 35% of this cohort with a mean time of 44 mo to first relapse is comparable to published data in adult patients. Disease relapse is a strong predictor for risk of ESKD, yet larger studies to investigate this question are warranted. Studies of both children and adults with lupus nephritis focusing on biologic influences for treatment resistance also warrant exploration.
Disclosures
None.
Acknowledgments
We extend our sincere gratitude to the patients, physicians, and medical staff who participate in the Glomerular Disease Collaborative Network. One published abstract included preliminary results that contributed to this manuscript: J Am Soc Nephrol 19: 782A, 2008.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Perfumo F, Martini A: Lupus nephritis in children. Lupus 14: 83–88, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Hagelberg S, Lee Y, Bargman J, Mah G, Schneider R, Laskin C, Eddy A, Gladman D, Urowitz M, Hebert D, Silverman E: Longterm followup of childhood lupus nephritis. J Rheumatol 29: 2635–2642, 2002 [PubMed] [Google Scholar]
- 3.Lee BS, Cho HY, Kim EJ, Kang HG, Ha IS, Cheong HI, Kim JG, Lee HS, Choi Y: Clinical outcomes of childhood lupus nephritis: A single center's experience. Pediatr Nephrol 22: 222–231, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Baqi N, Moazami S, Singh A, Ahmad H, Balachandra S, Tejani A: Lupus nephritis in children: A longitudinal study of prognostic factors and therapy. J Am Soc Nephrol 7: 924–929, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Yang LY, Chen WP, Lin CY: Lupus nephritis in children–a review of 167 patients. Pediatrics 94: 335–340, 1994 [PubMed] [Google Scholar]
- 6.Garin EH, Donnelly WH, Fennell RS, 3rd, Richard GA: Nephritis in systemic lupus erythematosus in children. J Pediatr 89: 366–371, 1976 [DOI] [PubMed] [Google Scholar]
- 7.Bogdanovic R, Nikolic V, Pasic S, Dimitrijevic J, Lipkovska-Markovic J, Eric-Marinkovic J, Ognjanovic M, Minic A, Stajic N: Lupus nephritis in childhood: A review of 53 patients followed at a single center. Pediatr Nephrol 19: 36–44, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Pattaragarn A, Sumboonnanonda A, Parichatikanond P, Supavekin S, Suntornpoch V, Vongjirad A: Systemic lupus erythematosus in Thai children: Clinicopathologic findings and outcome in 82 patients. J Med Assoc Thai 88 [Suppl 8]: S232–S241, 2005 [PubMed] [Google Scholar]
- 9.Lau KK, Jones DP, Hastings MC, Gaber LW, Ault BH: Short-term outcomes of severe lupus nephritis in a cohort of predominantly African-American children. Pediatr Nephrol 21: 655–662, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Barbano G, Gusmano R, Damasio B, Alpigiani MG, Buoncompagni A, Gattorno M, Perfumo F: Childhood-onset lupus nephritis: A single-center experience of pulse intravenous cyclophosphamide therapy. J Nephrol 15: 123–129, 2002 [PubMed] [Google Scholar]
- 11.Falk RJ, Hogan S, Carey TS, Jennette JC: Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative Network. Ann Intern Med 113: 656–663, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Dooley MA, Hogan S, Jennette C, Falk R: Cyclophosphamide therapy for lupus nephritis: Poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int 51: 1188–1195, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25: 1271–1277, 1982 [DOI] [PubMed] [Google Scholar]
- 14.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 15.The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum 54: 421–432, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Contreras G, Pardo V, Leclercq B, Lenz O, Tozman E, O'Nan P, Roth D: Sequential therapies for proliferative lupus nephritis. N Engl J Med 350: 971–980, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Mok CC: Therapeutic options for resistant lupus nephritis. Semin Arthritis Rheum 36: 71–81, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ: Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol 3: 46–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korbet SM, Schwartz MM, Evans J, Lewis E: J Severe lupus nephritis: Racial differences in presentation and outcome. J Am Soc Nephrol 18: 244–254, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Vyas S, Hidalgo G, Baqi N, Von Gizyki H, Singh A: Outcome in African-American children of neuropsychiatric lupus and lupus nephritis. Pediatr Nephrol 17: 45–49, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Askenazi D, Myones B, Kamdar A, Warren R, Perez M, De Guzman M, Minta A, Hicks MJ, Kale A: Outcomes of children with proliferative lupus nephritis: The role of protocol renal biopsy. Pediatr Nephrol 22: 981–986, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, Lai KN: Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med 343: 1156–1162, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Chan TM, Tse KC, Tang CS, Lai KN, Li FK: Long-term outcome of patients with diffuse proliferative lupus nephritis treated with prednisolone and oral cyclophosphamide followed by azathioprine. Lupus 14: 265–272, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C: The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrol Dial Transplant 22: 2531–2539, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Illei GG, Takada K, Parkin D, Austin HA, Crane M, Yarboro CH, Vaughan EM, Kuroiwa T, Danning CL, Pando J, Steinberg AD, Gourley MF, Klippel JH, Balow JE, Boumpas DT: Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: Long-term followup of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum 46: 995–1002, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Mosca M, Bencivelli W, Neri R, Pasquariello A, Batini V, Puccini R, Tavoni A, Bombardieri S: Renal flares in 91 SLE patients with diffuse proliferative glomerulonephritis. Kidney Int 61: 1502–1509, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Mok CC, Ying KY, Tang S, Leung CY, Lee KW, Ng WL, Wong RW, Lau CS: Predictors and outcome of renal flares after successful cyclophosphamide treatment for diffuse proliferative lupus glomerulonephritis. Arthritis Rheum 50: 2559–2568, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Boumpas DT, Balow JE: Outcome criteria for lupus nephritis trials: A critical overview. Lupus 7: 622–629, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Ioannidis JP, Boki KA, Katsorida ME, Drosos AA, Skopouli FN, Boletis JN, Moutsopoulos HM: Remission, relapse, and re-remission of proliferative lupus nephritis treated with cyclophosphamide. Kidney Int 57: 258–264, 2000 [DOI] [PubMed] [Google Scholar]