Abstract
Poor nutritional supply to the intervertebral disc is believed to be an important factor leading to disc degeneration. However, little is known regarding anisotropic and inhomogeneous transport in human annulus fibrosus (AF) and its relation to tissue morphology. We hypothesized that solute diffusivity in human AF is anisotropic and inhomogeneous and that transport behaviors are associated with tissue composition and structure. To test these hypotheses, we measured the direction-dependent diffusivity of a fluorescent molecule (fluorescein, 332Da) in three regions of AF using a fluorescence recovery after photobleaching (FRAP) technique, and associated transport results to the regional variation in water content and collagen architecture in the tissue. It was found that diffusivity in AF was anisotropic, with higher values in the axial direction than in the radial direction for all regions investigated. The values of the diffusion coefficient ranged from 0.38±0.25×10−6 cm2/s (radial diffusivity in outer AF) to 2.68±0.84×10−6 cm2/s (axial diffusivity in inner AF). In both directions, diffusivity decreased moving from inner to outer AF. Tissue structure was investigated using both Scanning Electron Microscopy (SEM) and Environmental Scanning Electron Microscopy (ESEM) imaging. A unique arrangement of microtubes was found in human AF. Furthermore, we also found that the density of these microtubes varied moving from inner to outer AF. A similar trend of regional variation was found for water content, with the highest value also measured in inner AF. Therefore, we concluded that a relationship exists among the anisotropic and inhomogeneous diffusion in human AF and the structure and composition of the tissue.
Keywords: Intervertebral disc, Diffusion, Anisotropy, Microtube, Fluorescence recovery after photobleaching (FRAP), Scanning electron microscopy (SEM), Environmental scanning electron microscopy (ESEM)
INTRODUCTION
Low back pain represents a significant concern in the United States, with 15-45% of the population affected annually, and over 70% of individuals experiencing symptoms at some point in their lifetime.1 Although the specific cause of low back pain remains unclear, symptoms have been strongly associated with degeneration of the intervertebral discs (IVD) in the spine.2-5 It is generally believed that inadequate nutritional supply to IVD is one of the main mechanisms for disc degeneration.2,3,6-11 The IVD is the largest avascular structure in the human body. Therefore, disc cells receive their nutritional supply from the vascular network surrounding the IVD. Previous studies have suggested two possible pathways for nutrient delivery to the disc: axially via cartilage end-plates, and radially through the periannular route.8,9,12-17 It is commonly believed that the major mechanism for transport of small nutrients within the disc is diffusion.18-20
Several studies have reported the diffusive transport properties of solutes in both animal and human IVDs.7,12-16,19-38 Some of the experimental results for diffusion coefficients, determined by various methods, are reported in Table 1. In particular, experimental observations have indicated that solute diffusion in IVD is anisotropic (i.e., direction-dependent) and inhomogeneous.30,32,33,35,38,39 For instance, our previous research demonstrated the anisotropic diffusion of small solutes (e.g., glucose, fluorescein, and ions) in bovine AF,30,35,38 while magnetic resonance imaging (MRI) techniques have been used to show the anisotropic behavior of water diffusion in porcine, ovine and human IVD tissues.32-34 The unique transport behavior in IVD tissue may be attributed to the complex structure of the disc, which is comprised of the central nucleus pulposus surrounded by the annulus fibrosus (AF) on its periphery.40 The AF is made up of 15 to 25 concentric lamellae41,42 each containing bundles of collagen fibers. Fiber bundles run parallel to each other within each lamella, but opposite to those in adjacent lamella, at angles varying from ±62 to ±45 degrees with respect to the axial direction of the spine.43 Furthermore, recent investigations on animal disc morphology showed that there exist ‘holes’ extending along the collagen fiber direction within the lamella. This unique arrangement of collagen fibers was first observed in murine coccygeal discs, and named ‘microtubules’.44 A similar microstructure was also observed in bovine coccygeal discs and called ‘microtubes’.35,38 While the specific microstructure and the physiological function of microtubes are unknown, it has been suggested that they may play a role in solute transport through the disc.35,38 While several studies reported the effect of tissue morphology on anisotropic diffusion in articular cartilage,45-47 ligament,48 and meniscus,49 to our knowledge, little is known regarding the relationship between the structure and the transport properties of IVD. Knowledge of transport properties of small molecules (e.g., oxygen and glucose) and the relation to tissue morphology is important for better understanding nutritional supply to IVD cells. In this study, the diffusivity of fluorescein was investigated. Fluorescein was chosen as a solute due to its relatively small size (332 Da), making it comparable to important nutrients, such as glucose.
Table 1.
Summary of experimental results for diffusion coefficient, D, in IVD from recent studies. For studies investigating anisotropic diffusivity (†), the ratio of the smallest to the largest value of the diffusion coefficient is reported.
| Solute | Tissue | Method | D (x 10−10 m2/s) | Ratio | Reference |
|---|---|---|---|---|---|
| Na+ | Human lumbar IVD (T = 25°C) |
Radiotracer | 7.4 | - | 21 |
| Bovine coccygeal AF (T = 22°C) |
Electrical conductivity |
2.75 - 4.4 | 0.62† | 30 | |
| Cl− | Human lumbar IVD (T = 25°C) |
Radiotracer | 11.4 | - | 21 |
| Bovine coccygeal AF (T = 22°C) |
Electrical conductivity |
4.25 – 6.75 | 0.63† | 30 | |
| Oxygen | Bovine lumbar AF (T = 22°C) |
Steady-State Diffusion |
14.3 | - | 31 |
| Porcine lumbar IVD (T = 22°C) |
Electrochemical | 25 | - | 27 | |
| Water | Porcine lumbar AF (T = 20°C) |
MRI | 10.6 – 13.6 | 0.75† | 32 |
| Human lumbar AF (T ∼ 0°C) |
MRI | - | 0.84† | 33 | |
| Ovine lumbar AF | MRI | 10.3 - 11.4 | 0.9† | 34 | |
| SO4− | Canine IVD | Radiotracer | 2.78 - 3.89 | - | 19 |
| Glucose | Bovine coccygeal AF (T = 22°C) |
Steady-State Diffusion |
0.917 - 1.38 | 0.66† | 35 |
| Human lumbar AF (T = 37°C) |
Radiotracer | 2.5 | - | 12 | |
| Lactate | Human lumbar AF | Indirect measurement |
4.86 | - | 36 |
| Bovine AF | Fluorescence and Radiotracer |
3.4 | - | 37 | |
| Fluorescein (332Da) | Bovine coccygeal AF (T = 22°C) |
FRAP | 0.814 – 1.26 | 0.64† | 38 |
| Dextran (70kDa) | Bovine AF | Fluorescence and Radiotracer |
0.14 | - | 37 |
Disc degeneration is closely linked to an inadequate supply of nutrients to disc cells2,3,6-11; therefore, the overall goal of our research is to further elucidate the mechanisms and pathways of nutritional transport in IVD in order to better understand the etiology of disc degeneration. However, to our knowledge, the implications of the complex IVD structure (e.g., microtubes) and composition for the inhomogeneous and anisotropic solute diffusivity in the tissue have not been investigated. Therefore, we hypothesized that, for human lumbar AF tissue, (1) diffusion of solutes is anisotropic and inhomogeneous; and (2) transport properties are associated with tissue composition and structure.
In this study, the diffusivity of fluorescein in human AF was determined using a Fluorescence Recovery After Photobleaching (FRAP) technique. The anisotropic behavior of diffusive transport was investigated by measuring the diffusion coefficient in two directions (axial and radial), while its inhomogeneous behavior was considered by determining the diffusivity in three different regions of human AF: inner AF (IAF), middle AF (MAF), and outer AF (OAF). In order to investigate the relationship between tissue morphology and solute diffusivity, we measured the water content as well as the microtube density in the three regions of AF. The density of microtubes in human tissue was determined from images obtained using Scanning Electron Microscopy (SEM); images were also used to qualitatively examine the structure of the tissue and its implications for transport behavior in the disc.
MATERIALS AND METHODS
Specimen Preparation
Human lumbar spines (n=3; 41, 45, and 45 y.o.) were obtained during autopsy within approximately 24 hours of death and stored at −80°C until dissected. On the day of dissection, spines were partially thawed, and three L3-L4 discs (one from each spine) were isolated. According to Thompson's morphologic grading scheme,50 one disc (41 y.o.) was classified as grade I, another disc (45 y.o.) as grade II, and the third disc (45 y.o.) as grade III. Specimens were obtained from AF blocks harvested from the inner AF (IAF), middle AF (MAF), and outer AF (OAF) of both anterior and posterior parts of three human IVDs, see Figure 1. In our preliminary studies, no statistical difference was found between the values of the diffusion coefficients in anterior and posterior regions of the disc; therefore, specimens from both anterior and posterior regions were combined in the same groups. For measurement of diffusivity, 7 IAF specimens, 8 MAF specimens, and 6 OAF specimens were harvested from each disc. Thus, the total number of specimens for IAF, MAF, and OAF regions was: 21, 24, and 18, respectively. For each region, a total of 36 FRAP tests were performed (at least one test for each specimen). For water content measurement, from each disc, 2-3 specimens were harvested from each of the AF regions. There were a total of 8 specimens for each region. For measurement of microtube area fraction, 4 specimens from each of the 3 regions were harvested from one disc (Grade III). On each specimen, 2-3 SEM images were taken. The total number of measurements was 10 for each of AF regions.
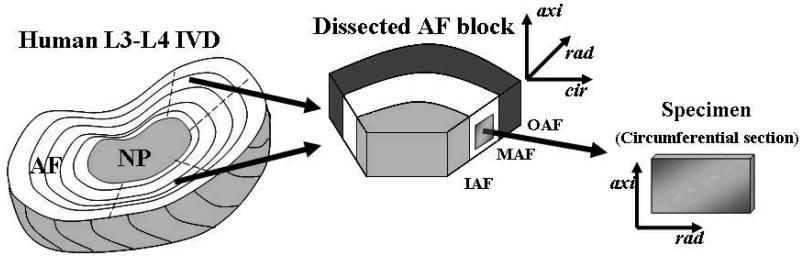
Figure 1.
Schematic of specimen preparation showing the three AF regions analyzed (OAF: outer annulus fibrosus; MAF: middle annulus fibrosus; IAF: inner annulus fibrosus) and sample orientations (axi: axial, rad: radial, cir: circumferential).
Measurement of Diffusivity
In this study the diffusivity of fluorescein molecule in human AF was determined by a Fluorescence Recovery After Photobleaching (FRAP) technique.51 Circumferential sections of AF specimens (area of ∼30 mm2 and thickness of 500 μm) were prepared using a microtome (Figure 1). Prior to testing, the specimens were equilibrated in a phosphate buffered saline (PBS) solution (Sigma®, St. Louis, MO, USA) with 0.1 mol/m3 fluorescein (332 Da, λex 490 nm; λem 514 nm, Fluka-Sigma-Aldrich®, St. Louis, MO, USA) while confined between two sinterized stainless steel plates (20 μm average pore size, Mott Corp., Farmington, CT) and an impermeable spacer, in order to prevent tissue swelling.38 Experiments were conducted at room temperature (22°C). The specimens were photobleached by an argon laser (488 nm wavelength) of a Confocal Scanning Laser Microscope (LSM 510 Zeiss, Jena, Germany) using a Plan-Neofluar 20X/0.50 WD 2.0 objective (Zeiss, Jena, Germany). A Multi-Layer Bleaching (MLB) protocol was used to minimize the error due to the out-of-plane diffusivity contribution.38,49
For each test, a time series of 200 video-images of 128×128 pixels (460.7×460.7 μm2) were collected, including five images prior to bleaching. In order to minimize the contribution of the fluorescence emission of the background, pre-bleach images were averaged and then subtracted from the post-bleach image series. Images were analyzed by custom-made software performing Fast Fourier Transform combined with Karhunen-Loéve Transform (KLT)52,53 to simultaneously determine the fluorescein diffusivity along axial (Daxi) and radial (Drad) directions of the disc.49
Measurement of Water Content
The water content of samples in IAF, MAF, and OAF was expressed in terms of water volume fraction (ϕw). Specimens from each of the three regions of AF were weighed in air (Wwet) and in PBS bathing solution (Ws) using the density determination kit together with a Sartorius analytical balance (Model LA120S, Goettingen, Germany). In order to minimize tissue swelling, measurements of the weight of specimens were performed in less than 15 seconds.30,54-56 Specimens were lyophilized and their dry weight (Wdry) was measured. Thus, ϕw, defined as the ratio of water volume to wet tissue volume, was calculated by30,54-56:
where ρs and ρw are the densities of PBS solution and water, respectively.
Disc Structure Imaging and Measurement of Microtube Area Fraction
For image analysis of AF structure, specimens were harvested from IAF, MAF, and OAF of one disc (grade III). Specifically, two axial sections, and two radial sections were harvested from each of the AF regions. Images of tissue sections were collected by an environmental scanning electron microscope (XL30 ESEM-FEG, FEI Company, Hillsborg, OR, USA) operating in Scanning Electron Microscopy (SEM) mode. For SEM imaging, the specimens were fixed with a solution of 2% gluteraldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in PBS, dehydrated in ethanol and dried by immersion in hexamethyldisilizane (Electron Microscopy Sciences, Hatfield, PA, USA).57 After sputter coating with Pd (Sputter Coater 108auto, Cressington, Watford, UK), SEM images of specimens were captured. To further investigate the tissue structure, two additional axial specimens from OAF region were imaged in Environmental Scanning Electron Microscopy (ESEM) mode. One specimen was observed immediately after dissection. The other specimen was cryogenically frozen after dissection, then observed by ESEM.
Measurements of the microtube area fraction in IVD were performed on axial sections of AF. A standard gray-scale threshold method was applied to SEM images. The microtube area fraction was defined as the percentage ratio of the voids (microtube cross-sectional areas) to the total area of the SEM image. The analysis was performed by means of the Particle Analyzer tool provided by ImageJ software (Version 1.39f, by Wayne Rasband, National Institutes of Health, USA).58-62
Statistical Analysis
A two-way analysis of variance (ANOVA) was performed using SPSS software (SPSS, Inc., Chicago, IL) to analyze the results for diffusivity in human AF. The two factors were direction (2 levels: axial and radial) and region (3 levels: IAF, MAF, OAF). Single factor ANOVA tests were performed using SPSS software to determine if regions within AF (i.e., IAF, MAF, and OAF) significantly affected microtube area fraction and water content. Student-Newman-Keuls post hoc test was used in order to determine between which levels of each factor the differences were significant. For all tests, the significance level was set at p<0.05. All data are given in mean ± standard deviation.
RESULTS
The results for diffusivity of fluorescein in human AF tissue are shown in Table 2. The values of the diffusion coefficients in the axial direction were significantly higher than those in the radial direction in all three regions of AF investigated (p<0.05), with Daxi being ∼1.4 to ∼2.7 times of Drad, indicating a significant anisotropic trend for diffusion of fluorescein in human AF tissue. Moreover, disc region significantly affected solute diffusivity (p<0.05), with the highest values of both axial and radial diffusivity found in IAF, and the lowest in OAF.
Table 2.
Results for diffusivity of fluorescien in axial (Daxi) and radial (Drad) directions, water volume fraction (ϕw), and microtube area fraction in the three regions of AF tissue investigated. Microtube area fraction was measured in axial specimens only. The value of n indicates the number of tests performed in each region.
| n | IAF | MAF | OAF | |
|---|---|---|---|---|
| Daxi (x10−6cm2s−1)§ | 36 | 2.68 ± 0.84 | 1.54 ± 0.70 | 1.04 ± 0.45 |
| Drad (x10−6cm2s−1)§ | 36 | 1.19 ± 0.76 | 0.58 ± 0.37 | 0.38 ± 0.25 |
| ϕw | 8 | 0.78 ± 0.16 | 0.75 ± 0.27 | 0.69 ± 0.13 |
| Microtube Area Fraction (%)* | 10 | 33.18 ± 2.37 | 27.45 ± 2.85 | 17.01 ± 3.58 |
Significant difference in region and direction: IAF > MAF > OAF; Daxi > Drad (p < 0.05)
Significant difference in region: IAF > MAF > OAF (p < 0.05)
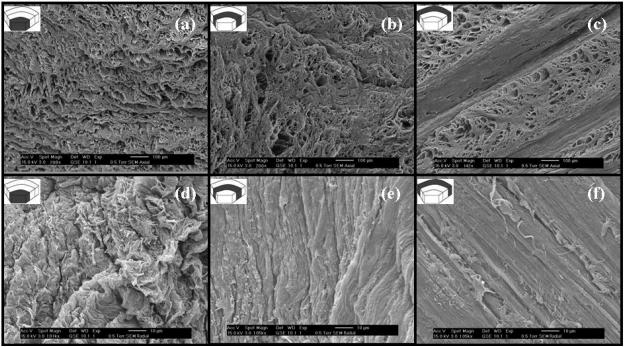
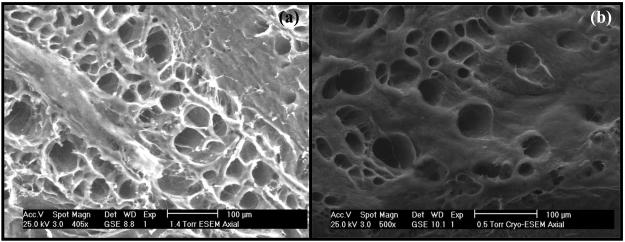
SEM images of radial and axial sections of AF showed differences in the organization of the collagen fibers among IAF, MAF, and OAF. Images showed that, in axial sections, non-contiguous microtubes, with diameters varying from 5 to 50 μm, are clearly visible and extend along the direction of the collagen fiber bundles in AF, see Figure 2(a-c). On the other hand, no microtube structures were evident in images of radial sections of AF, see Figure 2(d-f), likely due to their orientation parallel to the plane of the radial section. Microtubes were also evident in the images captured in ESEM mode. Figure 3a-b shows the images of axial sections of OAF obtained using both ESEM protocols (see ‘Materials and Methods’). The density and the diameter of the microtubes are similar to those observed in SEM images.
Figure 2.
SEM images of anterior AF samples. (a-c) Axial sections of IAF, MAF, and OAF. (d-f) Radial sections of IAF, MAF, and OAF.
Figure 3.
ESEM images of axial sections of anterior OAF. (a) Image captured directly following specimen dissection. (b) Image captured after cryogenically freezing the dissected specimen.
The regional variation of microtube area fraction is also shown in Table 2. It was found that disc region significantly affected the microtube distribution (p<0.05), decreasing from IAF to OAF. The trend for decreasing microtube area fraction was similar to the trend for decreasing diffusivity in the axial direction moving from IAF to OAF.
The values for water volume fraction were highest in IAF and lowest in OAF, see Table 2. While no statistically significant difference in water content was found among different groups (p=0.69), a decreasing trend moving from IAF to OAF was found.
DISCUSSION
The results of this study demonstrate that solute diffusive transport in human AF tissue is anisotropic (with Daxi = ∼1.4 to ∼2.7 times of Drad) and inhomogeneous (diffusivities are the highest in IAF and the lowest in OAF). Furthermore, both tissue water content and microtube area fraction were found to be highest in the IAF and smallest in OAF for human AF. The same trend was found for diffusivity in both the axial and radial directions in human AF.
The values of fluorescein diffusion coefficients determined in this study are lower than those previously determined for ions (Na+ and Cl−),21 glucose,12 and lactate36 in human IVD, see Table 1. This was expected, since, among these solutes, fluorescein has the highest molecular weight. Moreover, the results found here, showing the anisotropic diffusive transport in IVD tissue, are in agreement with experimental observations previously reported in the literature: the values for the diffusion coefficients of ions (Na+ and Cl−), glucose, and fluorescein in bovine coccygeal AF were determined to be higher in the axial direction than in the radial.30,35,38 Similar results were also found by MRI measurements of water diffusion in ovine and human AF.33,34 Furthermore, in each AF region investigated, the ratio of the radial to the axial fluorescein diffusion coefficient was found to be approximately 0.4. By comparison, studies on water and glucose anisotropic diffusion in AF indicated that the ratios of the smallest to the largest diffusion coefficient, measured in different directions of the tissue, are 0.75-0.9 for water,32-34 and 0.66 for glucose,35 see Table 1. These findings would suggest that hindrance to diffusion in the radial direction, caused by the collagen fiber organization within the disc, increases with the size of the solute diffusing in IVD.
Our findings suggest that diffusive transport in human AF tissue is dependent upon tissue composition (i.e. water content) and morphology. As is evident from Table 2, there is a decrease in the diffusivity of fluorescein as tissue water content decreases, moving from IAF to OAF. Previous studies have shown that solute diffusivity in cartilaginous tissues is dependent upon tissue porosity;63 this is reflected in the findings of this study. Moreover, we also found that the distribution of microtubes varied moving from IAF to OAF, with the density decreasing from IAF to OAF. This trend, similar to that of decreasing diffusivity along the same direction, highlights the effect of tissue morphology on the transport properties in human AF tissue. As it has been previously suggested by Travascio and Gu (2007)38 and Jackson et al. (2008),35 the presence of microtubes may provide justification for the anisotropic behavior of diffusive transport in AF tissue. That is, in the axial direction of AF, solute diffusion is facilitated due to the orientation of the microtubes running parallel to the bundles of collagen fibers within the lamella. In contrast, in the radial direction of AF, solute diffusive transport is hindered by the collagen fiber network, since the microtubes appear to be non-contiguous. This hypothesis is supported by our findings in this investigation, which show significantly lower values for diffusivity of fluorescein in the radial direction as compared with those in the axial direction of human AF tissue.
Some limitations of our investigation are noted here. The values of the area fraction of microtubes may be affected by both the sampling and the measurement technique. Due to the limited number of discs available for study, all the samples used for the measurement of microtube area fraction were harvested from the same disc (grade III). Moreover, in this study, microtube area fraction was determined by measuring the values of cross-sectional areas of microtubes in axial sections of the samples. As discussed, microtube orientation is not perpendicular to the axial section of AF. Therefore, the values of microtube area fraction measured in this study might differ from those determined by analyzing images of tissue sections orthogonal to microtube orientation.
Collagen fibers exhibit autofluorescence when exposed to the laser source used in these experiments (488 nm wavelength, see ‘Materials and Methods’).64 However, the effect of collagen autofluorescence on the measurement of solute diffusivity by FRAP is expected to be negligible. In a FRAP experiment, the solute diffusivity is determined by the measurement of the time variation of the fluorescence intensity.65 The variation of the fluorescence intensity is mainly due to the diffusive transport of the fluorescent probes, since collagen fibers are immobile.
In summary, the present study represents the first quantitative measurement of diffusivity of a small solute in human AF using a FRAP technique. It was found that the diffusivity of fluorescein in human lumbar AF is anisotropic and inhomogeneous. The anisotropic diffusivity may be attributed to the orientation of the microtubes seen in both SEM and ESEM images (i.e., oriented in axial, but not radial direction in AF specimens), suggesting a preferred pathway for axial diffusion. Moreover, the regional variations in water content and microtube area fraction indicate further implications of the structure and the composition of the tissue for solute diffusivity. This is the first study to show a relationship between tissue morphology and transport properties in human IVD tissues. These findings are crucial for a better understanding of transport properties in IVD tissues, as well as for the future development of numerical models on nutritional supply to IVD.
Acknowledgments
The project described was supported by Grant Number AR050609 from the NIH (NIAMS). The authors wish to thank Dr. Patricia L. Blackwelder and Mr. Husain Al Sayegh for their assistance in scanning electron microscopy imaging.
REFERENCES
- 1.NIH Research on low back pain and common spinal disorders. NIH Guide. 1997;26 [Google Scholar]
- 2.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Eyre DR, Benya P, Buckwalter J, et al. Intervertebral disk: Basic science perspectives. In: Frymoyer JW, Gordon SL, editors. New Perspectives on Low Back Pain. American Academy of Orthopaedic Surgeons; Park Ridge, IL: 1989. pp. 147–207. [Google Scholar]
- 4.Kelsey JL, Mundt DF, Golden AL. Epidemiology of low back pain. In: Malcolm JIV, editor. The Lumbar Spine and Back Pain. 4th ed. Churchill Livingstone; New York: 1992. pp. 537–549. [Google Scholar]
- 5.White AA. Biomechanics of lumbar spine and sacroiliac articulation: relevance to idiopathic low back pain. In: White AA, Gordon SL, editors. Symposium on Idiopathic Low Back Pain; St. Louis: CV Mosby Co.; 1981. pp. 296–322. [Google Scholar]
- 6.Bibby SR, Fairbank JC, Urban MR, Urban JP. Cell viability in scoliotic discs in relation to disc deformity and nutrient levels. Spine. 2002;27:2220–8. doi: 10.1097/00007632-200210150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Holm S, Nachemson A. Nutritional changes in the canine intervertebral disc after spinal fusion. Clin Orthop. 1982;169:243–58. [PubMed] [Google Scholar]
- 8.Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–9. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Nachemson A, Lewin T, Maroudas A, Freeman MA. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970;41:589–607. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- 10.Urban JP. The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans. 2001;30:858–64. doi: 10.1042/bst0300858. [DOI] [PubMed] [Google Scholar]
- 11.Urban JP, Smith S, Fairbank JC. Nutrition of the Intervertebral disc. Spine. 2004;29:2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 12.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Urban JPG, Holms S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: An in vivo study of solute transport. Clin Orthop. 1977;129:101–14. [PubMed] [Google Scholar]
- 14.Ogata K, Whiteside LA. 1980 Volvo award winner in basic science. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine. 1981;6:211–6. [PubMed] [Google Scholar]
- 15.Holm S, Maroudas A, Urban JP, et al. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–19. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 16.Roberts S, Urban JP, Evans H, Eisenstein SM. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21:415–20. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Brown MD, Tsaltas TT. Studies on the permeability of the intervertebral disc during skeletal maturation. Spine. 1976;1:240–4. [Google Scholar]
- 18.Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233–48. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- 19.Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: as in vivo study. Biorheology. 1978;15:203–21. doi: 10.3233/bir-1978-153-409. [DOI] [PubMed] [Google Scholar]
- 20.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: Effect of fluid flow on solute transport. Clin Orthop. 1982;170:296–302. [PubMed] [Google Scholar]
- 21.Urban JPG. Fluid and solute transport in the intervertebral disc. London University; London, UK: 1977. PhD Dissertation. [Google Scholar]
- 22.Holm S, Nachemson A. Variations in the nutrition of the canine intervertebral disc induced by motion. Spine. 1983;8:866–74. doi: 10.1097/00007632-198311000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Adams MA, Hutton WC. The effect of posture on diffusion into lumbar intervertebral discs. J Anat. 1986;147:121–34. [PMC free article] [PubMed] [Google Scholar]
- 24.Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine. 1998;23:1–7. doi: 10.1097/00007632-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Holm S, Selstam G. Oxygen tension alterations in the intervertebral disc as a response to changes in the arterial blood. Ups J Med Sci. 1982;87:163–74. doi: 10.3109/03009738209178421. [DOI] [PubMed] [Google Scholar]
- 26.Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci. 1988;93:91–9. doi: 10.1517/03009734000000042. [DOI] [PubMed] [Google Scholar]
- 27.O'Hare D, Winlove CP, Parker KH. Electrochemical method for direct measurement of oxygen concentration and diffusivity in the intervertebral disc: electrochemical characterization and tissue-sensor interactions. J Biomed Eng. 1991;13:304–12. doi: 10.1016/0141-5425(91)90112-k. [DOI] [PubMed] [Google Scholar]
- 28.Stairmand JW, Holm S, Urban JP. Factors influencing oxygen concentration gradients in the intervertebral disc. A theoretical analysis. Spine. 1991;16:444–9. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Urban MR, Fairbank JC, Etherington PJ, et al. Electrochemical measurement of transport into scoliotic intervertebral discs in vivo using nitrous oxide as a tracer. Spine. 2001;26:984–90. doi: 10.1097/00007632-200104150-00028. [DOI] [PubMed] [Google Scholar]
- 30.Jackson AR, Yao H, Brown MD, Gu WY. Anisotropic ion diffusivity in intervertebral disc: an electrical conductivity approach. Spine. 2006;31:2783–9. doi: 10.1097/01.brs.0000245842.02717.1b. [DOI] [PubMed] [Google Scholar]
- 31.Yuan TY, Jackson AR, Huang CY, Gu WY. Strain-Dependent Oxygen Diffusivity in Bovine Annulus Fibrosus. Journal of Biomechanical Engineering. 2009 doi: 10.1115/1.3127254. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu EW, Setton LA. Diffusion tensor microscopy of the intervertebral disc anulus fibrosus. Magn Reson Med. 1999;41:992–9. doi: 10.1002/(sici)1522-2594(199905)41:5<992::aid-mrm19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Chiu EJ, Newitt DC, Segal MR, et al. Magnetic resonance imaging measurement of relaxation and water diffusion in the human lumbar intervertebral disc under compression in vitro. Spine. 2001;26:E437–E444. doi: 10.1097/00007632-200110010-00017. [DOI] [PubMed] [Google Scholar]
- 34.Drew SC, Silva P, Crozier S, Pearcy MJ. A Diffusion and T2 Relaxation MRI Study of the Ovine Lumbar Intervertebral Disc Under Compression In Vitro. Physics in Medicine and Biology. 2004;49:3585–92. doi: 10.1088/0031-9155/49/16/006. [DOI] [PubMed] [Google Scholar]
- 35.Jackson AR, Yuan TY, Huang CY, et al. Effect of compression and anisotropy on the diffusion of glucose in annulus fibrosus. Spine. 2008;33:1–7. doi: 10.1097/BRS.0b013e31815e4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selard E, Shirazi-Adl A, Urban J. Finite Element Study of Nutrient Diffusion in the Human Intervertebral Disc. Spine. 2003;28:1945–53. doi: 10.1097/01.BRS.0000087210.93541.23. [DOI] [PubMed] [Google Scholar]
- 37.Boubriak O, Urban JPG. Measurement of Diffusion Coefficients in the Nucleus and Annulus of the Intervertebral Disc. 84-B ed. 2002. [Google Scholar]
- 38.Travascio F, Gu WY. Anisotropic diffusive transport in annulus fibrosus: experimental determination of the diffusion tensor by FRAP technique. Annals of Biomed Engng. 2007;35:1739–48. doi: 10.1007/s10439-007-9346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohshima H, Tsuji H, Hiarano N, et al. Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine. 1989;14:1234–44. doi: 10.1097/00007632-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Research & Therapy. 2003;5:120–30. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickey DS, Hukins DWL. Relation between the structure of the annulus fibrosus and the function and failure of the intervertebral disc. Spine. 1980;5:106–16. doi: 10.1097/00007632-198003000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc annulus fibrosus. Spine. 1990;15:402–10. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23:75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 44.Iatridis JC, ap Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37:1165–75. doi: 10.1016/j.jbiomech.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knauss R, Schiller J, Fleischer G, et al. Self-Diffusion of Water in Cartilage and Cartilage Components as Studied by Pulsed Field Gradient NMR. Magnetic Resonance in Medicine. 1999;41:285–92. doi: 10.1002/(sici)1522-2594(199902)41:2<285::aid-mrm11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Leddy HA, Guilak F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Annals of Biomedical Engineering. 2003;31:753–60. doi: 10.1114/1.1581879. [DOI] [PubMed] [Google Scholar]
- 47.Xia Y, Farquahar T, Burton-Wurster N, et al. Self-Diffusion Monitors Degraded Cartilage. Arch Biochem Biophys. 1995;10:323–8. doi: 10.1006/abbi.1995.9958. [DOI] [PubMed] [Google Scholar]
- 48.Leddy HA, Haider MA, Guilak F. Diffusional anisotropy in collagenous tissues: fluorescence imaging of continuous point photobleaching. Biophys J. 2006;91:311–6. doi: 10.1529/biophysj.105.075283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Travascio F, Zhao W, Gu WY. Characterization of anisotropic diffusion tensor of solute in tissue by video-FRAP imaging technique. Annals of Biomed Engng. 2009;37:813–23. doi: 10.1007/s10439-009-9655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–5. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Axelrod D, Koppel DE, Schlessinger J, et al. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16:1055–69. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Marin FJ. Automatic recognition of biological shapes using the Hotelling transform. Computers in Biology and Medicine. 2001;31:85–99. doi: 10.1016/s0010-4825(00)00027-5. [DOI] [PubMed] [Google Scholar]
- 53.Li ZN, Drew MS. Fundamentals of Multimedia. Pearson Prentice Hall; Upper Saddle River (NJ): 2004. [Google Scholar]
- 54.Gu WY, Justiz MA. Apparatus for measuring the swelling dependent electrical conductivity of charged hydrated soft tissues. J Biomech Engng. 2002;124:790–3. doi: 10.1115/1.1516571. [DOI] [PubMed] [Google Scholar]
- 55.Gu WY, Justiz MA, Yao H. Electrical conductivity of lumbar annulus fibrosis: Effects of porosity and fixed charge density. Spine. 2002;27:2390–5. doi: 10.1097/00007632-200211010-00014. [DOI] [PubMed] [Google Scholar]
- 56.Yao H, Justiz MA, Flagler D, Gu WY. Effects of swelling pressure and hydraulic permeability on dynamic compressive behavior of lumbar annulus fibrosus. Annals of Biomed Engng. 2002;30:1234–41. doi: 10.1114/1.1523920. [DOI] [PubMed] [Google Scholar]
- 57.Hayat MA. Fixation for electron microscope. Academic Press; New York: 1982. [Google Scholar]
- 58.Rahbek O, Kold S, Zippor B, et al. Particle migration and gap healing around trabecular metal implants. International Orthopaedics. 2005;29:368–74. doi: 10.1007/s00264-005-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkataraman R, Das G, Venkataraman B, et al. Image processing and statistical analysis of microstructures of as plasma sprayed Alumina-13 wt.% Titania coatings. Surface and Coatings Technology. 2006;201:3691–700. [Google Scholar]
- 60.Guillon O, Weiler L, Rodel J. Anisotropic microstructural development during the constrained sintering of dip-coated alumina thin films. J Am Ceram Soc. 2007;90:1394–400. [Google Scholar]
- 61.Rahbek O, Kold S, Zippor B, et al. The influence of surface porosity on gap-healing around intra-articular implants in the presence of migrating particles. Biomaterials. 2005;26:4728–36. doi: 10.1016/j.biomaterials.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 62.Venkataraman R, Das G, Singh SR, et al. Study on the infuence of porosity, pore size, spatial and topological distribution of pores on microhardness of as plasma sprayed ceramic coatings. Materials Science and Engineering. 2007:269–74. [Google Scholar]
- 63.Gu WY, Yao H, Vega AL, Flagler D. Diffusivity of ions in agarose gels and intervertebral disc: Effect of porosity. Annals of Biomedical Engineering. 2004;32:1710–7. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 64.Deyl Z, Macek K, Adam M, Vancikova O. Studies on the chemical nature of elastin fluorescence. Biochim Biophys Acta. 1980;625:248–54. doi: 10.1016/0005-2795(80)90288-3. [DOI] [PubMed] [Google Scholar]
- 65.Tsay TT, Jacobson K. Spatial Fourier analysis of video photobleaching measurements. Principles and optimization. Biophys J. 1991;60:360–8. doi: 10.1016/S0006-3495(91)82061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]