Abstract
A squaraine rotaxane scaffold with four alkynes groups is readily converted into a range of dendritic architectures using high yielding copper catalyzed alkyne azide cycloaddition (CuAAC) chemistry. A convergent synthesis approach is more efficient than a divergent pathway. Dendritic squaraine rotaxanes with peripheral amine groups can be further functionalized to produce multivalent deep-red fluorescent derivatives that exhibit high brightness and outstanding chemical stability in biological solution. The surface groups on these functionalized fluorescent dendrimers include guanidinium, mannose, and phosphatidylcholine.
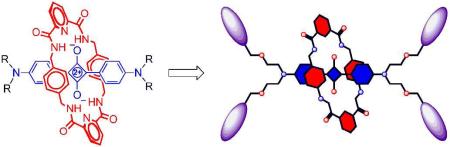
Dendrimers are branched polymers with tree-like structures, and they have been under active investigation for more than twenty five years.1 Interest in fluorescent dendrimers is also ongoing, as they have a wide range of potential applications in biomedical science, energy capture, and nanotechnology.1,2 Most published synthetic approaches directly attach the fluorophores to the dendrimer periphery, which is a straightforward strategy to execute, but a difficult way to control the fluorophore stoichiometry.3 Furthermore, peripheral attachment of dyes can be problematic because the dye molecules are exposed to environmental quenching effects and chemical attack. Bioimaging performance can also be weakened because the appended dyes may alter targeting ability.4 These potential problems are avoided by using a fluorescent core scaffold, and the literature contains scattered reports of this architecture, primarily for light harvesting or oxygen photosensitization.2,5 Here, we describe the synthesis of fluorescent dendrimers that have a squaraine rotaxane as the fluorescent core. We are developing squaraine rotaxanes as high performance, deep-red fluorescent probes for various types of bioimaging applications.6 The rotaxane structure provides outstanding steric protection of the encapsulated squaraine dye, while maintaining the squaraine's favorable photophysical properties of narrow absorption/emission bands, high molar absorptivity, and high fluorescence quantum yield. Previously, we have reported squaraine rotaxane probes that have one or two appended targeting ligands.7 We now describe synthetic methods that attach multiple polyamine dendrons to a squaraine rotaxane core scaffold in high yield and produce dendritic architectures whose peripheries can be further functionalized. Access to these highly fluorescent dendrimers will allow us to develop a range of optical imaging technologies that employ multivalent targeting in cell culture and living animals.
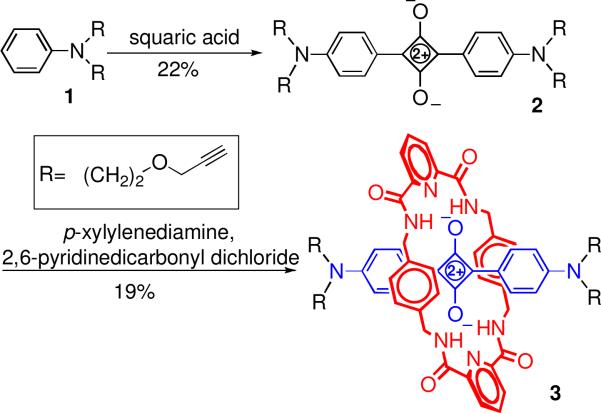
The core squaraine rotaxane scaffold is the novel tetraalkyne 3 which was prepared in three steps using standard dye synthesis and rotaxane templated assembly methods (Scheme 1).6 Squaraine rotaxane 3 exhibits similar photophysical properties (Table 1) as the parent squaraine dye 2, but much better chemical stability because the surrounding tetralactam macrocycle inhibits nucleophilic attack at the electrophilic C4O2 center of the dye. The macrocycle is maintained over the dye by a combination of aromatic stacking interactions and bifurcated hydrogen bonds between the squaraine oxygens and the macrocyclic amide NH residues. At the start of this study it was not obvious if this high level of steric protection would be maintained once polyamine dendrons were attached to the rotaxane scaffold. The following results indicate that this structural change does not introduce any major stability problems.
Scheme 1.
Syntheis of Squaraine Rotaxane 3.
Table 1.
Photophysical Properties of Squaraine Rotaxanes.
| compound | λabs(nm) | λem(nm) | log ε | Φfa |
|---|---|---|---|---|
| 2b | 628 | 650 | 5.52 | 0.68 |
| 3b | 639 | 661 | 5.52 | 0.89 |
| 3c | 644 | 668 | 5.55 | 0.43 |
| SR-G1d | 652 | 678 | 5.25 | 0.18 |
| SR-G2d | 653 | 679 | 5.22 | 0.19 |
| SR-G3d | 652 | 681 | 5.29 | 0.19 |
| SR-G1-Gnd | 653 | 672 | 5.26 | 0.24 |
| SR-G2-Gnd | 652 | 673 | 5.23 | 0.25 |
Error ±5%
Chloroform
THF/H2O (1:1)
H2O.
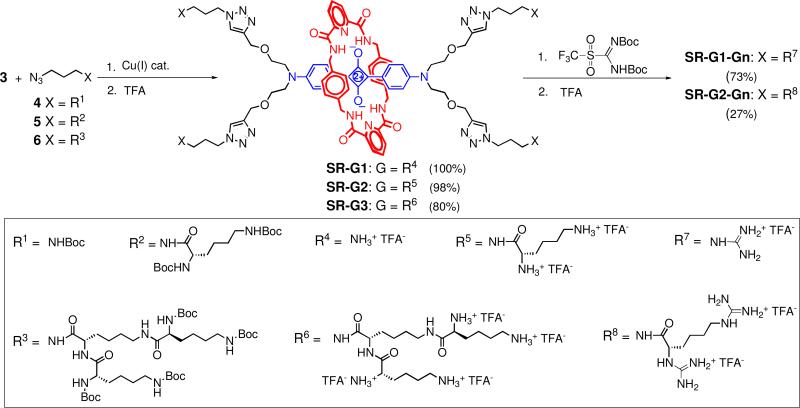
Both convergent and divergent synthesis methods were explored to convert the core scaffold 3 into dendritic architectures.8 The convergent method involved copper catalyzed azide alkyne cycloaddition (CuAAC) of tetralkyne scaffold 3 with Boc-protected azido lysine dendrons 4, 5, or 6, and subsequent removal of the Boc groups with TFA (Scheme 2).9,10 The CuAAC reactions were conveniently monitored by TLC and by the loss of alkyne signal at ~2.5 ppm in the 1H NMR spectrum. The reactions of scaffold 3 with azido dendrons 4, 5, and 6 under standard catalytic conditions (CuSO4/sodium ascorbate; CHCl3:H2O 1:1; room temperature) gave product yields of 99%, 81%, and 0%, respectively. Changing the catalyst to CuBr/tris(2-(dioctadecylamino)ethyl)amine8m for the reactions of 3 with 5 and 6 improved the yields to 98% and 80%, respectively. In all three cases, the Boc groups were subsequently removed in quantitative yield to give the dendritic squaraine rotaxanes SR-G1, SR-G2, and SR-G3.
Scheme 2.
Convergent Synthesis of Dendritic Polyamine and Polyguanidinium Squaraine Rotaxanes.
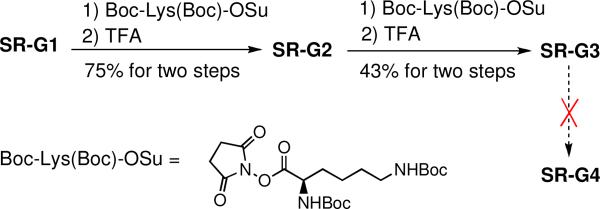
The divergent synthesis employs an interative series of reactions to build up the dendrimer in an expanding stepwise fashion. In this case, the chemistry involves a two-step cycle of amine coupling with Boc-protected lysine followed by removal of the Boc groups. The starting scaffold is SR-G1 with four peripheral amine groups. As shown in Scheme 3, the first reaction cycle produced the dendrimer SR-G2 in 75% yield, and the second cycle produced SR-G3 with sixteen peripheral amines in 42% yield. Repeated attempts to synthesize the next generation SR-G4 by this divergent methodology were unable to produce a reasonable yield of pure product. A significant drawback with this divergent synthesis pathway was undesired squaraine decomposition during the amide bond formation process. The coupling reactions were conducted in DMF, a polar organic solvent that disrupts the hydrogen bonds and stacking interactions within the squaraine rotaxane scaffold and transiently exposes the electrophilic C4O2 center of the squaraine dye to nucleophilic attack by the attached amines. Overall, the convergent method is a more efficient strategy for synthesis of dendritic squaraine rotaxanes with attached polyamine dendrons. Dendrimers SR-G1, SR-G2, and SR-G3 with 4, 8, and 16 peripheral ammoniums, respectively, are highly water soluble compounds that exhibit the typical excellent fluorescent properties of squaraine rotaxanes (Table 1). The quantum yields of ~0.19 in water are quite good considering that unprotected squaraine dyes are severely quenched by aqueous solvent (Table 1 illustrates the quenching effects of water). In addition, the absorption spectra show no band broadening indicating that these dendrimers do not aggregate in aqueous solution. Typically, dendrimers with fluorescent cores exhibit photophysical properties that vary as the dendrimer generation increases due to enhanced isolation of the core from the solvent. However, the photophysical properties for squaraine rotaxane polyamine dendrimers hardly change with dendrimer generation because in each case the squaraine dye is encapsulated inside a tetralactam macrocycle. This insensitivity to dendrimer generation is likely to be a useful feature in many quantitative imaging applications.
Scheme 3.
Divergent Synthesis of Dendritic Polyamine Squaraine Rotaxanes.
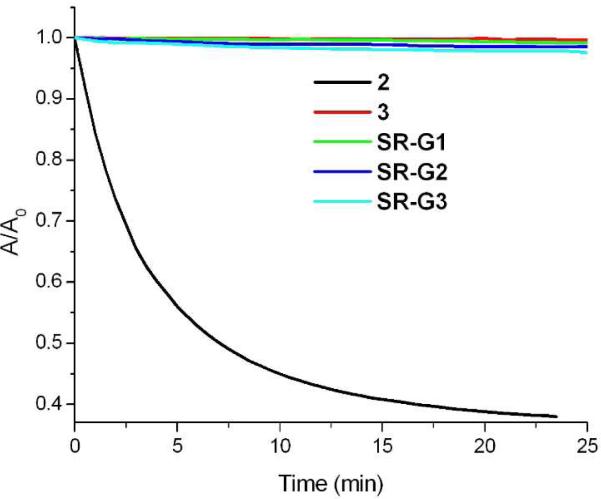
High chemical stability is another desired property for many modern biological imaging applications, and therefore hydrolytic bleaching experiments were conducted to evaluate the stability of these dendritic polyamine squaraine rotaxanes. As shown in Figure 1, the parent squaraine dye 2 was unstable in an aqueous solution containing serum and lost most of its blue color after 30 mins. In contrast, the absorption spectra for 3, SR-G1, SR-G2, and SR-G3 were essentially unchanged over the same time period, and each had lost only a few percent of color after 24 h. The fact that steric protection of the encapsulated squaraine is greater in water than in DMF agrees with previous observations that polar aprotic solvents are more effective at disrupting the combined effects of hydrogen bonding and aromatic stacking interactions that hold the surrounding macrocycle over the the electrophilic center of the squaraine dye.11
Figure 1.
Absorption changes in a THF-water (1:1) mixture also containing 8% serum at 22 °C.
Another attractive feature with these squaraine rotaxane polyamine dendrimers is ready ability to synthetically alter the dendrimer surface functionality. Shown in Scheme 2 is the efficient conversion of the peripheral amines of SR-G1 and SR-G2 into guanidinium groups by reaction with commercially available 1,3-di-Boc-2-(trifluoromethylsulfonyl) guanidine followed by removal of the protecting Boc groups with TFA. The resulting dendritic polyguanidinium squaraine rotaxanes, SR-G1-Gn and SR-G2-Gn are highly water-soluble and have the same excellent stability and photophysical properties as their amine precursors (Table 1). Future studies will determine if they have different cell penetration capabilities.12
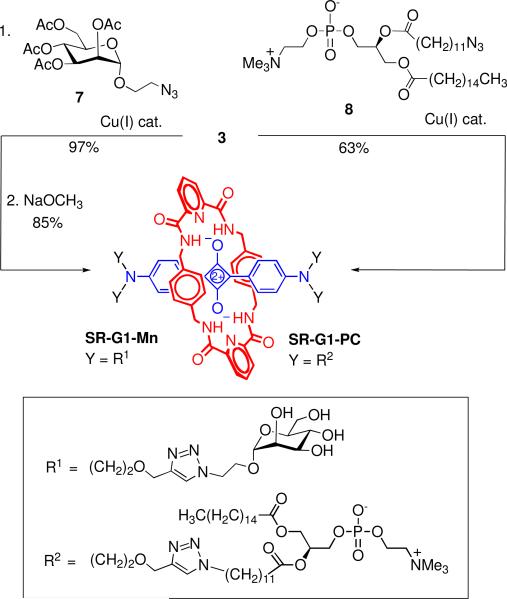
Squaraine rotaxane 3 is a versatile building block that can be conjugated with various biological targeting units using straightfoward CuAAC chemistry. Shown in Scheme 4 is the production of a tetramannose derivative that should have affinity for certain strains of bacteria.13 Reaction of 3 with 2-azidoethyl-2,3,4,6-tetra-O-acetyl-α-D-manno-pyranoside, 7,14 gave the desired conjugated product in 97% yield, which was then deprotected with NaOCH3 to give SR-G1-Mn in 85% yield. It is notable that the squaraine rotaxane core survived exposure with the highly basic and nucleophilic NaOCH3. Also shown in Scheme 4 is the synthesis of a tetravalent phosphatidylcholine derivative, SR-G1-PC, which was obtained in 63% isolated yield by conjugation of 3 with the known azido phosphatidylcholine derivative 8.15 Multivalent phosphatidylcholine derivatives have recently been reported to target proteins that are implicated in inflammation and heart disease;16 thus, fluorescent versions of these compounds, such as SR-G1-PC, may be useful as imaging probes.
Scheme 4.
Synthesis of Dendritic Squaraine Rotaxanes with Appended Mannose or Phosphatidylcholine Units.
In summary, the squaraine rotaxane scaffold 3 is readily converted into a range of dendritic architectures using high yielding CuAAC chemistry. The polyamine versions can be further functionalized to produce multivalent deep-red fluorescent probes that exhibit high brightness and outstanding chemical stability. Fluorescence microscopy and imaging studies that utilize these fluorescent dendrimers as molecular probes will be reported in due course.
Supplementary Material
Acknowledgment
We are grateful for funding support from the NIH, the University of Notre Dame, and the Walther Cancer Insitute.
Footnotes
Supporting Information Available. Synthetic procedures and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Tekade RK, Kumar PV, Jain NK. Chem. Rev. 2009;109:49–87. doi: 10.1021/cr068212n. [DOI] [PubMed] [Google Scholar]; b Lee CC, MacKay JA, Frechet JM, Szoka FC. Nat. Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]; c Tomalia DA, Frechet JMJ. J. Polym. Science: Part A: Polym. Chem. 2002;40:2719–2728. [Google Scholar]; d Tomalia DA, Naylor AM, Goddard WA., III Angew. Chem., Int. Ed. Engl. 1990;29:138–175. [Google Scholar]
- 2.a Bouit P-A, Westlund R, Feneyrou P, Maury O, Malkoch M, Malmstrom E, Andraud C. New. J. Chem. 2009;33:964–968. [Google Scholar]; b Caminade A-M, Hameau A, Majoral J-P. Chem. Eur. J. 2009;15:9270–9285. doi: 10.1002/chem.200901597. [DOI] [PubMed] [Google Scholar]
- 3.a Hayek A, Ercelen S, Zhang X, Bolze F, Nicoud J-F, Schaub E, Baldeck PL, Mely Y. Bioconjugate Chem. 2007;18:844–851. doi: 10.1021/bc060362h. [DOI] [PubMed] [Google Scholar]; b Talanov VS, Regino CS, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Nano Lett. 2006;6:1459–1463. doi: 10.1021/nl060765q. [DOI] [PubMed] [Google Scholar]
- 4.Puckett CA, Barton JK. J. Am. Chem. Soc. 2009;131:8738–8739. doi: 10.1021/ja9025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a Yilmaz MD, Bozdemir OA, Akkaya EU. Org. Lett. 2006;8:2871–2873. doi: 10.1021/ol061110z. [DOI] [PubMed] [Google Scholar]; b Zeng Y, Li YY, Li M, Yang GQ, Li Y. J. Am. Chem. Soc. 2009;131:9100–9106. doi: 10.1021/ja902998g. [DOI] [PubMed] [Google Scholar]; b Yuan MJ, Yin XD, Zheng HY, Ouyang CB, Zuo ZC, Liu HB, Li YL. Chem. Asian J. 2009;4:707–713. doi: 10.1002/asia.200800391. [DOI] [PubMed] [Google Scholar]
- 6.a Arunkumar E, Forbes CC, Noll BC, Smith BD. J. Am. Chem. Soc. 2005;127:3288–3289. doi: 10.1021/ja042404n. [DOI] [PubMed] [Google Scholar]; b Arunkumar E, Fu N, Smith BD. Chem. Eur. J. 2006;12:4684–4690. doi: 10.1002/chem.200501541. [DOI] [PubMed] [Google Scholar]; c Gassensmith JJ, Baumes JM, Smith BD. Chem. Commun. 2009:6329–6338. doi: 10.1039/b911064j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JR, Fu N, Arunkumar E, Leevy WM, Gammon ST, Piwnica-Worms D, Smith BD. Angew. Chem,. Int. Ed. 2007;46:5528–5531. doi: 10.1002/anie.200701491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Camponovo J, Ruiz J, Cloutet E, Astruc D. Chem. Eur. J. 2009;15:2990–3002. doi: 10.1002/chem.200801999. [DOI] [PubMed] [Google Scholar]; b Lee JW, Kim B-K, Han SC, Kim JH. Bull. Korean Chem. Soc. 2009;30:157–162. [Google Scholar]; c Kimura M, Nakano Y, Adachi N, Tatewaki Y, Shirai H, Kobayashi N. Chem. Eur. J. 2009;15:2617–2624. doi: 10.1002/chem.200801557. [DOI] [PubMed] [Google Scholar]; d Kohman RE, Zimmerman SC. Chem. Commun. 2009:794–796. doi: 10.1039/b818183g. [DOI] [PubMed] [Google Scholar]; e Joralemon MJ, O'Reilly RK, Hawker CJ, Wooley KL. J. Am. Chem. Soc. 2005;127:16892–16899. doi: 10.1021/ja053919x. [DOI] [PubMed] [Google Scholar]; f Camponovo J, Hadad C, Ruiz J, Cloutet E, Gatard S, Muzart J, Bouquillon S, Asturc D. J. Org. Chem. 2009;74:5071–5074. doi: 10.1021/jo900554b. [DOI] [PubMed] [Google Scholar]; g Franc G, Kakkar A. Chem. Commun. 2008:5267–5276. doi: 10.1039/b809870k. [DOI] [PubMed] [Google Scholar]; h Elmer SL, Man S, Zimmerman SC. Eur. J. Org. Chem. 2008:3845–3851. doi: 10.1002/ejoc.200800401. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Ornelas C, Aranzaes JR, Cloutet E, Alves S, Astruc D. Angew. Chem,. Int. Ed. 2007;46:872–877. doi: 10.1002/anie.200602858. [DOI] [PubMed] [Google Scholar]; j Lee JW, Kim JH, Kim B-K, Kim JH, Shin WS, Jin S-H. Tetrahedron. 2006;62:9193–9200. [Google Scholar]; j Wu P, Malkoch M, Hunt JN, Vestberg R, Kaltgrad E, Finn MG, Fokin VV, Sharpless KB, Hawker CJ. Chem. Commun. 2005:5775–5777. doi: 10.1039/b512021g. [DOI] [PubMed] [Google Scholar]; k Rijkers DTS, van Esse GW, Merkx R, Brouwer AJ, Jacobs HJF, Pieters RJ, Liskamp RMJ. Chem. Commun. 2005:4581–4583. doi: 10.1039/b507975f. [DOI] [PubMed] [Google Scholar]; l Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Frechet JMJ, Sharpless KB, Fokin VV. Angew. Chem,. Int. Ed. 2004;43:3928–3932. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]; m Candelon N, Lastecoueres D, Diallo AK, Aranzaes JR, Astruc D, Vincent J-M. Chem. Commun. 2008:741–743. doi: 10.1039/b716306a. [DOI] [PubMed] [Google Scholar]
- 9.Gassensmith JJ, Barr L, Baumes JM, Paek A, Nguyen A, Smith BD. Org. Lett. 2008;10:3343–3346. doi: 10.1021/ol801189a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Lee JW, Kim JH, Kim HJ, Han SC, Kim JH, Shin WS, Jin S-H. Bioconjugate Chem. 2007;18:579–584. doi: 10.1021/bc060256f. [DOI] [PubMed] [Google Scholar]; b Lee JW, Kim JH, Kim B-K. Tetrahedron Lett. 2006;47:2683–2686. [Google Scholar]; c Betancourt JE, Rivera JM. Org. Lett. 2008;10:2287–2290. doi: 10.1021/ol800701j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gassensmith J, Arunkumar E, Lorna Barr L, Baumes JM, Noll BC, Smith BD. J. Am. Chem. Soc. 2007;129:15054–15060. doi: 10.1021/ja075567v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a Wender PA, Kreider E, Pelkey ET, Rothbard J, VanDeusen CL. Org. Lett. 2005;7:4815–4818. doi: 10.1021/ol051496y. [DOI] [PubMed] [Google Scholar]; b Bagnacani V, Sansone F, Donofrio G, Baldini L, Casnati A, Ungaro R. Org. Lett. 2008;10:3953–3956. doi: 10.1021/ol801326d. [DOI] [PubMed] [Google Scholar]
- 13.a Imberty A, Chabre YM, Roy R. Chem. Eur. J. 2008;14:7490–7499. doi: 10.1002/chem.200800700. [DOI] [PubMed] [Google Scholar]; b Gouin SG, Wellens A, Bouckaert J, Kovensky J. ChemMedChem. 2009;4:749–755. doi: 10.1002/cmdc.200900034. [DOI] [PubMed] [Google Scholar]
- 14.a Gu L, Luo PG, Wang H, Meziani MJ, Lin Y, Veca LM, Cao L, Lu FS, Wang X, Quinn RA, Wang W, Zhang PY, Lacher S, Sun YP. Biomacromolecules. 2008;9:2408–2418. doi: 10.1021/bm800395e. [DOI] [PubMed] [Google Scholar]; b Chabre YM, Contino-Pepin C, Placide V, Shiao TC, Roy R. J. Org. Chem. 2008;73:5602–5605. doi: 10.1021/jo8008935. [DOI] [PubMed] [Google Scholar]
- 15.O'Neil EJ, DiVittorio KM, Smith BD. Org. Lett. 2007;9:199–203. doi: 10.1021/ol062557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenzweig BA, Ross NT, Tagore DM, Jayawickramarajah J, Saraogi I, Hamilton AD. J. Am. Chem. Soc. 2009;131:5020–5021. doi: 10.1021/ja809219p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.