Abstract
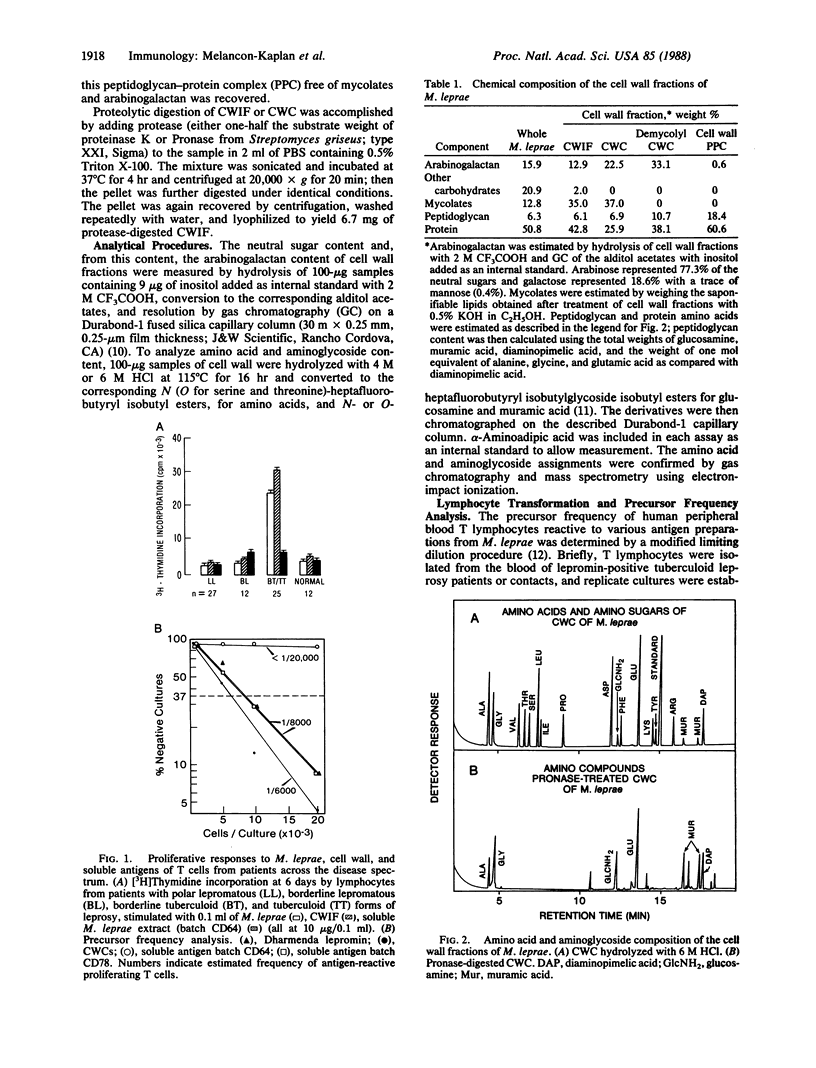
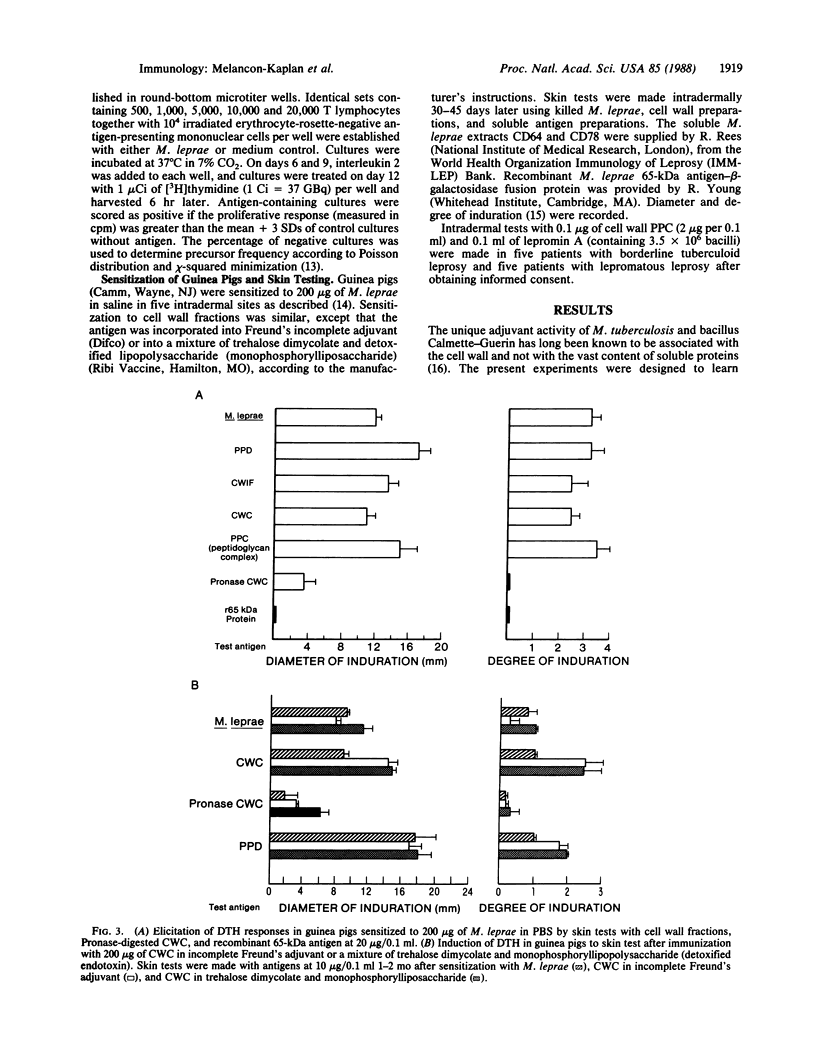
Cell walls of Mycobacterium leprae, prepared by differential solvent extraction, were shown to contain arabinogalactan, mycolates, and peptidoglycan. In addition, amino acid analysis revealed the unexpected presence of large amounts of protein that retained potent immunological reactivity. Purified cell walls stimulated proliferation of T cells from tuberculoid, but not from lepromatous leprosy, patients and elicited delayed-type hypersensitivity skin reactions in guinea pigs and patients sensitized to M. leprae. Analysis of the precursor frequency of antigen-reactive human peripheral T cells revealed that as many cells (approximately equal to 1/6000) proliferate to antigen contained in cell walls as to intact M. leprae. Sequential removal of mycolates and arabinogalactan resulted in a large peptidoglycan-protein complex that retained all the immunological activity. This immunological reactivity and the inherent protein were destroyed by proteolysis. Thus, cell wall protein is a major contributor to cell-mediated immune reactivity to this pathogenic mycobacterium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Petit J. F., Lefrancier P., Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981 Dec 4;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Kingston A. E., Colston M. J. Limiting dilution analysis of the human T cell response to mycobacterial antigens from BCG vaccinated individuals and leprosy patients. Clin Exp Immunol. 1987 Jun;68(3):510–520. [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Johnson W. D., Barreto E., Marsden P. D., Costa J. L., Reed S., Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985 Dec;135(6):4144–4148. [PubMed] [Google Scholar]
- Draper P. Cell walls of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):95–98. [PubMed] [Google Scholar]
- Draper P., Dobson G., Minnikin D. E., Minnikin S. M. The mycolic acids of Mycobacterium leprae harvested from experimentally infected nine-banded armadillos. Ann Microbiol (Paris) 1982 Jul-Aug;133(1):39–47. [PubMed] [Google Scholar]
- Draper P. Wall biosynthesis: a possible site of action for new antimycobacterial drugs. Int J Lepr Other Mycobact Dis. 1984 Dec;52(4):527–532. [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss B., Uhlén M., Nilsson B., Lindberg M., Sjöquist J., Sjödahl J. Region X, the cell-wall-attachment part of staphylococcal protein A. Eur J Biochem. 1984 Jan 16;138(2):413–420. doi: 10.1111/j.1432-1033.1984.tb07931.x. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Kusaka T., Kohsaka K., Fukunishi Y., Akimori H. Isolation and identification of mycolic acids in Mycobacterium leprae and Mycobacterium lepraemurium. Int J Lepr Other Mycobact Dis. 1981 Dec;49(4):406–416. [PubMed] [Google Scholar]
- Mackenzie S. L., Tenaschuk D. Rapid formation of amino acid isobutyl esters for gas chromatography. J Chromatogr. 1975 Sep 3;111(2):413–415. doi: 10.1016/s0021-9673(00)99292-6. [DOI] [PubMed] [Google Scholar]
- Maugh T. H., 2nd Leprosy vaccine trials to begin soon. Science. 1982 Feb 26;215(4536):1083–1086. doi: 10.1126/science.7063841. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- McNeil M., Wallner S. J., Hunter S. W., Brennan P. J. Demonstration that the galactosyl and arabinosyl residues in the cell-wall arabinogalactan of Mycobacterium leprae and Mycobacterium tuberculosis are furanoid. Carbohydr Res. 1987 Sep 1;166(2):299–308. doi: 10.1016/0008-6215(87)80065-4. [DOI] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R. Induction of cell-mediated immunity to Mycobacterium leprae in guinea pigs. Infect Immun. 1979 Mar;23(3):787–794. doi: 10.1128/iai.23.3.787-794.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki A., Yukawa S., Tsuchiya K., Yamasaki T. Studies on cell walls of Mycobacteria. I. Chemical and biological properties of the cell walls and the mucopeptide of BCG. J Biochem. 1966 Apr;59(4):388–396. doi: 10.1093/oxfordjournals.jbchem.a128314. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Melancon-Kaplan J., Young S. M., Pirmez C., Kino H., Convit J., Rea T. H., Bloom B. R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brehmer W., Goode G., Larson C. L., List R. H., Milner K. C., Wicht W. C. Factors influencing protection against experimental tuberculosis in mice by heat-stable cell wall vaccines. J Bacteriol. 1966 Oct;92(4):869–879. doi: 10.1128/jb.92.4.869-879.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E., Granger D. L., Milner K. C., Yamamoto K., Strain S. M., Parker R., Smith R. W., Brehmer W., Azuma I. Induction of resistance to tuberculosis in mice with defined components of mycobacteria and with some unrelated materials. Immunology. 1982 Jun;46(2):297–305. [PMC free article] [PubMed] [Google Scholar]
- Sansarricq H. Leprosy in the world today. Lepr Rev. 1981 Dec;52 (Suppl 1):15–31. doi: 10.5935/0305-7518.19810053. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Wietzerbin J., Das B. C., Petit J. F., Lederer E., Leyh-Bouille M., Ghuysen J. M. Occurrence of D-alanyl-(D)-meso-diaminopimelic acid and meso-diaminopimelyl-meso-diaminopimelic acid interpeptide linkages in the peptidoglycan of Mycobacteria. Biochemistry. 1974 Aug 13;13(17):3471–3476. doi: 10.1021/bi00714a008. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]
- van Oers M. H., Pinkster J., Zeijlemaker W. P. Quantification of antigen-reactive cells among human T lymphocytes. Eur J Immunol. 1978 Jul;8(7):477–484. doi: 10.1002/eji.1830080706. [DOI] [PubMed] [Google Scholar]