Abstract
Many women with a history of childhood sexual abuse (CSA) experience difficulties becoming sexually aroused. This study measured cortisol and physiological sexual arousal during exposure to sexual stimuli in women with and without a history of CSA. CSA survivors showed a smaller decrease in cortisol during sexual arousal than the non-sexually abused, control group potentially due to an increase in cortisol in some of the participants in the CSA group. Physiological sexual arousal was weaker in CSA survivors compared to women with no history of sexual abuse and posttraumatic stress disorder symptoms showed characteristics consistent with mediation for the relationship between a history of CSA and inhibited sexual arousal responses.
Women with a history of childhood sexual abuse (CSA) often report sexual arousal problems (e.g., Becker et al., 1982a; 1982b; Briere, 1992; Fergusson & Mullen, 1999; Westerlund, 1992). Interventions designed to improve sexual arousal in CSA survivors are based on the hypothesis that survivors may have developed a negative response to sexual stimuli because of the nature of the trauma (Maltz, 2002). While this assumption is theoretically feasible, very little empirical evidence is available to either support or reject this hypothesis. Indirect evidence supporting a possible stress response to sexual stimuli in CSA survivors comes from studies on cognitive networks. A study found that CSA survivors, but not women with no history of abuse, paired sexual words with negative affect (Meston & Heiman, 2003). Also, studies point to lower physiological sexual responses to sexual stimuli in CSA survivors compared to women with no history of abuse, an indication of a potential inhibition of the sexual response in these women (Laan & Everaerd, 1995; Rellini & Meston, 2006). Currently, no direct evidence is available on a potential stress response to sexual stimuli and the effect this could have on the sexual arousal of CSA survivors.
During a stress response, both the sympathetic nervous system and the hypothalamus-pituitary-adrenal (HPA) axis become activated. These systems could affect sexual arousal independently from conscious cognitive processes. Indeed, the sympathetic system and the HPA axis can be activated by a stressor without the involvement of the prefrontal cortex (LeDoux, 2002). Studies have shown that moderate sympathetic nervous system activity facilitates, but high activity inhibits, sexual responses in women (Meston & Gorzalka, 1996). The inhibitory effect of sympathetic activity is posed to result from the adrenergic contractive effect on the pre-capillary sphincters, a group of smooth muscles that regulate the arterial blood inflow in the genitals (Levin & Wylie, 2007). Given that trauma survivors with posttraumatic stress disorder (PTSD) symptoms have a heightened sympathetic activity (Yehuda, 2003), any further increase in this activity may have an inhibitory effect on their sexual responses. In support of this hypothesis, a study found that, differently from a group of women with no history of abuse, moderate sympathetic activation failed to facilitate genital sexual arousal in women with a history of CSA (Rellini & Meston, 2005).
A paucity of studies have addressed the relationship between the HPA axis and physiological sexual responses. Impairments in the HPA axis are among the most commonly reported problems in trauma survivors with PTSD (for a review, see Yehuda, 2003; Southwick et al., 2005). The cortisol profile of patients with PTSD, in general, has been mixed, though recent research in women with PTSD, in particular, suggests that hyper-reactivity to traumatic stimuli is more prevalent in women with PTSD (Elzinga et al. 2003). Additionally, the HPA axis closely interacts with the sympathetic nervous system in a way that could be aversive to sexual arousal. Cortisol has a potentiating effect on the adrenergic contraction of the smooth muscles (Xiao, Huang, Pearce, Longo & Zhang, 2003). Relevant to physiological sexual arousal, cortisol could increase adrenergic contraction of the pre-capillary sphincters in the vagina resulting in reduced blood inflow. Indeed, in sexually healthy women, physiological sexual arousal is associated with a slight decrease in cortisol levels (Exton et al., 1999; 2000).
The present study addressed whether impaired physiological sexual responses in CSA survivors are associated with a stress response to sexual stimuli that increases cortisol thus potentiating the adrenergic constriction of smooth muscles and reducing physiological sexual arousal. As such, we expected an increase in cortisol in response to sexual stimuli in CSA survivors (Hypothesis 1). We also expected an inverse relationship between physiological sexual arousal and cortisol response during sexual stimuli (Hypothesis 2). Finally, we expected a mediation effect of cortisol on the relationship between CSA and physiological sexual arousal (Hypothesis 3). Given the documented relationship between HPA and PTSD, the analyses included PTSD symptoms to assess the separate role of trauma exposure and PTSD symptoms on sexual arousal. Exploratory analyses were also conducted to examine the relationship between cortisol and emotional responses to sexual stimuli.
Method
Participants
Participants were recruited from the community through advertisements in free local newspapers and fliers addressing women with and without a history of unwanted sexual experiences. During a phone screening to assess eligibility, participants were described the study and equipment. Inclusion criteria for all women included: between the ages of 25 and 35, currently sexually active with a male partner and mostly heterosexual orientation. Women were excluded from the study if they reported a traumatic event in the previous three months, current involvement in an abusive relationship, use of medications known to affect cardiovascular function (e.g., beta blockers), or a diagnosis of psychotic disorder in the past 6 months. If participants were receiving medications known to affect sexual function (e.g., antidepressants) they were accepted in the study if they reported taking the same medication and dose for the 3 months prior to the study and denied any sexual problem that started within a few weeks from starting the medication. Participants reported using the following: Seroquil (CSA = 2, no sexual abuse = 2), Wellbutrin (CSA = 2, no sexual abuse = 1), Ambien (CSA = 1), Effexor (CSA = 2), hydrocodone (CSA = 1, no sexual abuse = 1), and marijuana within a week from the call (CSA = 2; no sexual abuse = 2). Of the 187 women who called to inquire about the study, 116 did not qualify because of health problems that affected sexuality (18.0%), medications that affect sexuality (2.4%), lack of sexual activities (13.8%), outside the age range (16.2%), lesbian orientation (7.3%), sexual abuse outside the adopted definition (8.9%), and sexual abuse during adulthood but not during childhood (13.8%). Of the 81 women who qualified, 20 cancelled or failed to show for their appointments (CSA = 7; no sexual abuse = 13), and data for 17 participants was not included because of lack of compliance with the study protocol (10 women reported using street drugs a few days prior to the study, 4 smoked just prior to the study, and 3 reported having sex the day of the study). A total of 11 women in the study (CSA = 6) were regular smokers (frequency 1–3 cigarettes/day for the CSA group and 2–3 cigarettes/day for the no sexual abuse group). The cancellation rates were similar to those of prior studies (e.g., Rellini, unpublished data). Recent studies have reported no differences in personality characteristics between women who participate in studies using vaginal photoplethysmography versus self-report measures (Woo, Brotto, & Yule, in press).
In agreement with Finkelhor et al. (1989), we defined CSA as an unwanted sexual encounter that included touching or penetration of the genitals before the age of 16 with someone at least 5 years older. The CSA group (n = 24) was on average 31 years old (SD = 6) Caucasian (79%) and with at least some college education (83%). The majority of women was either in a committed relationship or married (75%) and most reported a household income less than $50,000 (92%). Ten percent of the women were ethnic African-American and 8% were Hispanic.
Exclusion criteria for the no sexual abuse group included self report of physical abuse or neglect during childhood and any unwanted sexual experience. On average, the no sexual abuse group (n = 20) was 28 years old (SD = 3), mostly Caucasian (65%), in a committed relationship or married (80%) and reported a household income below $50,000 (80%). A total of 2% were Hispanic and 2% were African American, and 3 women reported more than one ethnicity.
As shown in Table 1, no group differences were observed in demographics except for a slightly higher age in the CSA group. Although statistically significant, this difference in age was not considered clinically meaningful for differences relevant to sexual responses and therefore was not used as covariate.
Table 1.
Demographics on Non-Abused Women (NSA) and Women who Experienced CSA
| NSA (n = 20) | CSA (n = 24) | ||||
|---|---|---|---|---|---|
| M | SD | M | SD | t | |
| Age | 27.6 | 2.76 | 31.25 | 5.96 | −2.52* |
| N. of adults in household | 1.9 | 0.85 | 1.87 | 0.74 | <1 |
| N. of children in household | 0.2 | 0.6 | 0.6 | 0.14 | −2.00 |
| N. sexual experiences (not forced) a | |||||
| Non-invasive b | 1.65 | 1.78 | 1.76 | 1.66 | −0.22 |
| Sexual touchc | 2.28 | 1.66 | 1.30 | 1.56 | 1.99 |
| Vaginal Intercourse | 1.45 | 1.82 | 1.75 | 1.62 | <1 |
| Oral Sex | 1.32 | 1.75 | 1.04 | 1.55 | <1 |
| Anal Penetration | 0.15 | 0.49 | 0.13 | 0.450 | <1 |
| N. Sexual Experiences (forced) a | |||||
| Non-invasive b | 2.16 | 1.71 | |||
| Sexual touch c | 2.04 | 1.60 | |||
| Vaginal intercourse | 0.63 | 1.21 | |||
| Oral sex | 1.70 | 1.81 | |||
| Anal penetration | 0.13 | 0.44 | |||
| CAPS current | |||||
| CAPS B | 0.0 | 0.0 | 1.0 | 1.2 | 3.47*** |
| CAPS C | 0.1 | 0.3 | 2.0 | 1.7 | 4.94*** |
| CAPS D | 0.1 | 0.3 | 1.6 | 1.3 | 4.95*** |
| n | % | n | % | pd | |
| Education | |||||
| At least some college | 20 | 100.0 | 20 | 83.3 | .072 |
| Household income | |||||
| Less than $ 50,000 per year | 16 | 80.0 | 22 | 92.0 | .248 |
| Relationship status | |||||
| Single, not dating | 2 | 10.0 | 0 | 0.0 | .201 |
| Single, dating | 2 | 10.0 | 6 | 25.0 | .187 |
| In a committed relationship | 13 | 65.0 | 12 | 50.0 | .244 |
| Married | 3 | 15.0 | 6 | 25.0 | .322 |
| Ethnicity | |||||
| Caucasian | 13 | 65.0 | 19 | 79.2 | .238 |
| Hispanic/Latina | 3 | 15.0 | 2 | 8.3 | .411 |
| Pacific Islander | 2 | 10.0 | 0 | 0.0 | .201 |
| African American or Black | 2 | 10.0 | 2 | 10.0 | .624 |
| Asian American | 2 | 10.0 | 0 | 0.0 | .455 |
| American Indian/Alaska Native | 0 | 0.0 | 1 | 4.2 | .545 |
| Other (Bolivian) | 1 | 5.0 | 0 | 0.0 | .195 |
Note: CSA = child sexual abuse group. NSA = women with no history of child sexual abuse group. CAPS current = Clinician Administered PTSD Scale refers to number of symptoms meeting diagnostic criteria based on frequency and intensity of symptoms in the past month.
Sexual experiences indicate experiences before the age of 16. Scores: 1 = one time; 2 = 2 to 4 times, 3 = 5 to 7 times, and 4 = more than 8 times.
Non-invasive sexual experiences include being kissed or hugged in a sexual way, having someone show their genitals, or showing one’s own genitals.
Sexual touch includes being fondled and touching of the genitals.
Fisher exact test p values.
p < .05.
Measures and Materials
To induce sexual arousal, participants viewed a 14-min video: 1 minute of the word “Relax,” 3 minutes of a non-sexual video (a travel documentary), and 10 minutes of a sexual video. The non-sexual video provided a within-person baseline control since vaginal photoplethysmography lacks of an absolute zero (for a review, please see Prause & Janssen, 2005). The scenes shown in the sexual videos were extracted from films produced and directed by women, and have been demonstrated to be an effective means for eliciting both physiological and subjective sexual arousal during laboratory studies in women with (e.g., Rellini & Meston, 2006) and without (e.g., Laan, Everaerd, & van Bellen, 1994) a history of CSA. The non-sexual video was 3 min in compliance with prior studies (Janssen, Carpenter, Graham, 2003; Laan, Everaerd, Bellen, & Hanewald, 1994). The sexual video was twice as long as sexual videos used in prior studies because currently no data exists on the average time it takes women to reach their maximum subjective sexual arousal.
Saliva samples were collected using Sarstedt salivettes with untreated cotton swabs. Samples were centrifuged for 10 minutes at 3,500 rpm, frozen until processed, and then analyzed with Salimetrics Cortisol EIA kit. Intra-assay variability ranged from 5–11% and interassay variability was 7.3%. Cross-reactivities for endogenous steroids were all below 1% (as reported by Salimetrics). Saliva samples were collected prior to (Cort-Baseline) and during (Cort-Video) sexual video. Percentage increase in cortisol from baseline to sexual video was calculated: Cort-Response = (Cort-Video − Cort-Baseline)/Cort-Baseline.
Perceived sexual arousal (Film Scale) and physiological sexual arousal (photoplethysmography) were measured. The Film Scale, a 17-item self-report questionnaire (Heiman and Rowland’s scale, 1983), measures perception of sexual arousal and is administered prior to and after the sexual video. This scale includes questions on physiological sexual arousal, i.e., “tingling sensations in the genitals,” “sense of warmth in the genitals;” and mental sexual arousal, i.e., “feeling ‘turned on’,” “a sense of mental sexual arousal.” Items are rated on a 7-point Likert scale from “not at all” to “intensely.” Vaginal photoplethysmography (Sintchak & Geer, 1975) was used to measure vaginal pulse amplitude, an indication of blood flow in the vaginal walls, which is considered a sensitive and specific measure of physiological sexual arousal. The device looks like a transparent plastic tampon containing a light source and a light detector. The light source illuminates a circumscribed area of the capillary bed of the vagina and the light detector measures the amount of backscattered light. During physiological sexual arousal, the vaginal walls become engorged making the capillary bed darker and thus reducing the backscattered light. The signal is sampled 80 times/sec and the amplitude of each pulse wave is recorded in millivolts (mV) throughout the video. The signal is a sinusoid curve and the amplitude is the primary measure for physiological sexual arousal vaginal pulse amplitude. Artifacts caused by movements are deleted following visual inspection of the data (e.g., Rellini & Meston, 2006; Laan et al., 1995). Average vaginal pulse amplitude is calculated during 30 sec of the non-sexual video (vaginal pulse amplitude non-sexual) and 30 sec of the maximum sexual arousal during the sexual video (vaginal pulse amplitude sexual). The vaginal pulse amplitude score is: (vaginal pulse amplitude sexual − vaginal pulse amplitude non-sexual)/vaginal pulse amplitude non-sexual.
We used the Positive and Negative Affect Schedule (PANAS) for exploratory analyses on affective responses to sexual stimuli (Watson, Clark, & Tellegen, 1988). The PANAS consists of 20-item comprising 10 positive and 10 negative affect items with high internal reliability (αs = .84–.90).
The Female Sexual Function Index comprises 19-items divided into the domains: desire, arousal, lubrication, orgasm, satisfaction, and pain (Rosen et al., 2000). Since the main focus of the study was sexual arousal, only scores for arousal (3 items) were utilized. The Female Sexual Function Index reliably discriminates between women with and without female sexual arousal disorder (Rosen et al., 2000). The maximum score is 6, with higher numbers indicating better sexual functioning. On average, women with female sexual arousal disorder score 3.09 (SD = 1.5) and women with no sexual problems score 5.08 (SD = 1.1) on the Female Sexual Function Index-arousal domain (Wiegel et al., 2005).
Psychological Assessments
Given that impairments in HPA are associated with PTSD (Yehuda, 2003), a trained interviewer administered the Clinician Administered PTSD Scale (CAPS; Blake et al., 1990). In previous studies, the scores from the CAPS conducted by this interviewer showed high correlations (r = .871) with scores on the Distressing Events Questionnaire (Kubany, Leisen, Kaplan, & Kelly, 2000), a self-reported measure for PTSD. For individuals with multiple traumatic experiences, symptoms in response to the worst event were used. In order to keep the procedures consistent across conditions, and to have a comparable measure for traumatic experiences, we also administered the CAPS to the control group. For the control group, participants were asked to respond based on the most stressful event in their lives: car accidents (3 participants), losing a job (2 participants), death of a loved one (4 participants), moving to a different state (4 participants), difficult work environment (8 participants). Symptoms were measured in the past month. As recommended by Blake et al., the F1/I2 scoring rule was adopted to identify clinically significant symptoms for DSM-IV-TR criteria. The sum of intensity and frequency of all symptoms was used as covariate (CAPS-Total). For this study, inter-item correlations were α = 0.83, 0.72, and 0.66 for the frequency dimension and α = 0.84, 0.71, and 0.71 for the intensity dimension of re-experiencing, avoidance and hyperarousal symptoms, respectively.
Type of sexual experiences were assessed with an adapted version of the Child Sexual Abuse Measure (Finkelhor, 1979), a 14 item questionnaire on sexual activities before the age of 16, from less to more invasive, i.e., watching pornography to anal penetration. For each item, participants indicated frequency (1= one time, 2 = 2 to 4 times, 3 = 5 to 7 times, and 4 = 8 or more), whether they felt forced or coerced, the age of the person/perpetrator, and the age when this event started. Sexual behaviors were grouped into abusive (feeling forced/coerced) and wanted (no force/coercion) and were subdivided into non-invasive (e.g., kissed or hugged), touch (e.g., fondled), oral sex (receiving or giving oral sex), vaginal, and anal penetration. This information was used for descriptive purposes.
Procedure
As approved by the Institutional Review Board of the University of Texas, participants who qualified were scheduled for a visit between 2:00 pm and 6:00 pm between days 5 and 10 of their menstrual cycle to control for fluctuations in cortisol rhythms. Three days prior to the study participants received a reminder call that instructed them to avoid caffeine, chocolate, allergy medications, tobacco products, sexual activities and exercise for 12 hours prior to their visit. Participants were also asked not to brush their teeth, eat, or chew gum for 2 hours prior to their visit. If participants reported addiction to cigarettes or caffeine they were asked to restrain to 1 cup of coffee in the early morning and not to smoke for at least 2 hrs prior to the study. In agreement with the McArthur Research Network’s guidelines for salivary cortisol measurement, compliance with these instructions was assessed with an interview administered prior to saliva collection (Stewart, 2000). Women who reported lack of compliance were excluded from data analyses but completed the study and received full compensation.
First, participants completed a demographics questionnaire, the pre-video Film Scale and PANAS. Participants were then left alone in a private room, and, after inserting the photoplethysmograph, they sat in a recliner chair for 10 min (habituation). After 30 min from the beginning of the study participants provided the first saliva samples (Cort-Baseline). Saliva samples were timed to correspond to 30 min after the point of interest, which is an adequate amount of time for cortisol to peak in saliva (Kirschbaum & Hellhammer, 1989). Participants were then exposed to the video and completed the during-video Film Scale and PANAS, plus other questionnaires not pertinent to this study. Thirty minutes following the middle of the sexual video, participants provided the second saliva sample (Cort-Video). Finally, the first author administered the CAPS interview along with interviews not used for this study (sexual function and dissociation). Participants were compensated $30.00 and debriefed.
Data Analysis
To test Hypothesis 1 a repeated measure ANCOVA examined the effect of Group (CSA vs. no sexual abuse) and Time (baseline vs. video) on cortisol response (dependent variable). CAPS-Total was included as covariate based on prior literature indicating cortisol impairments in trauma survivors with PTSD symptoms (Yehuda, 2003).
Hypothesis 2 on the relationship between cortisol and changes in vaginal pulse amplitude (dependent variable) during the sexual video (repeated measure), was tested with a repeated measure ANCOVA. The model tested the main effect of Cort-Response (independent variable) and Video (non-sexual vs. sexual) on vaginal pulse amplitude. We also tested the main effect of Cort-Response on overall vaginal pulse amplitude (independent from video). Given that cortisol prior to exposure to the sexual video may have been affected by states of anxiety related to the study procedure, we included Cort-Baseline as covariate.
To test the potential mediation effect of cortisol on the relationship between CSA and vaginal pulse amplitude (Hypothesis 3), we computed a repeated measure ANCOVA similar to the one described for Hypothesis 2. Two mixed within/between effects were tested for vaginal pulse amplitude: (1) Cort-Response × Video, and (2) Group (CSA vs. no sexual abuse) × Video. Also the main effect for Cort-Response was tested for overall vaginal pulse amplitude. A three-way interaction was included in the model for Group X Cort-Response × Video. Cort-Baseline was used as covariate.
Results
Group Differences
Chi-square tests showed that ethnicity, household income, and relationship status were not statistically different between the two groups (Table 1). A series of t-tests computed on frequency of sexual experiences before age 16 (adapted Child Sexual Abuse Measure) showed no group differences in frequency of wanted sexual experiences (Table 1). Both groups reported that, before age 16, they engaged in consensual non-invasive sexual activities, sexual touch, vaginal intercourse and oral sex less than a handful of times. Within the CSA group, unwanted sexual experiences before age 16 were more frequent than the wanted sexual experiences. Unwanted non-invasive sexual behaviors and sexual touch were reported with frequency 2.16 and 2.04 (1 = one time, 2 = 2 to 4 times), while frequency for oral sex was 1.70 and for vaginal intercourse was 0.63. The types of sexual abuse most commonly reported were genital fondling (33.3%) and intercourse (33.3%). It is noteworthy that 64% of the CSA group reported adult sexual re-victimization. For 66.7%, CSA was chronic (occurred more than 8 times). For 6 participants, at least 1 of the perpetrators was a father or father figure, for 8 was a family member, and for 10 was an acquaintance.
Not surprisingly, the CSA group reported more PTSD symptoms compared to the no sexual abuse group (Table 1). Only 7 of the CSA participants reported PTSD symptoms within 1 SD from the mean symptoms reported by PTSD patients in treatment studies (e.g., Cloitre, Koenen, Cohen, & Han, 2002; M = 69, SD = 16). CAPS-Total was comparable to that of CSA survivors participating in sex studies (i.e., Rellini & Meston, 2006). Scores in Table 1 indicate frequency of symptoms that met the F1/I2 criteria and CAPS-Total. The CSA group ranged between 0 and 4 symptoms out of 5 possible re-experiencing symptoms, 0 to 5 out of 7 potential avoidant symptoms, and 0 to 5 out of the 5 hyperarousability symptoms.
Pearsons’ r correlation coefficients are reported in Table 2 to illustrate the relationship between the PTSD symptoms and responses to the sexual videos. A moderate, although not statistically significant correlation (r = −.21) was observed for CAPS-Total and PANAS-NA. It is unclear whether a larger sample size would have identified a statistical significance. It is noteworthy that CAPS-Total was significantly associated with vaginal pulse amplitude. This relationship is explored in more depth in the a priori analyses that follow.
Table 2.
Zero-Order Pearson’s Correlation Coefficients for all Participants (CSA and NSA Groups)
| CAPS-Total | VPA | PSA | MSA | PANAS-PA | |
|---|---|---|---|---|---|
| VPA | −.31* | ||||
| PSA | −.01 | .22 | |||
| MSA | < .01 | .26 | .79** | ||
| PANAS-PA | .07 | .03 | .55** | .47** | |
| PANAS-NA | −.21 | .10 | .26 | .19 | .24 |
Note. VPA = vaginal pulse amplitude; PSA = physiological sexual arousal; MSA = mental sexual arousal; PANAS-PA = positive affect, PANAS-NA = negative affect.
p < .05.
p < .001.
Group Differences in Cortisol Responses
One participant had a high cortisol level and scored 2 SD above the sample mean in CAPS-total. Analyses were conducted with and without this participant to ensure findings were not accounted for by the outlier. Since no differences were observed in the results the following results include the outlier.
The repeated measure ANCOVA for cortisol showed that, overall, cortisol decreased during the sexual video, F(1, 41) = 16.95, p < .001, η2 = .264. Group did not have an effect on cortisol during the sexual video, F < 1, η2 = .002; however, CAPS-Total did, F(1,41) = 6.15, p < .05, η2 = .096.
To explain the observed relationship between CAPS-Total and video, we ran a linear regression of Cort-Response (criterion) and CAPS-Total (predictor), which indicated that an increase in .05 points in CAPS-Total, a score comparable to an increase in half of a symptom, predicted an increase in 1% of cortisol during the sexual video.
Cortisol and Physiological Sexual Arousal
A repeated measure ANCOVA showed that vaginal pulse amplitude increased during the sexual video, F(1,41) = 13.43, p < .001, η2 = .222. Neither Cort-Baseline, F < 1, η2 = .008, or Cort-Response, F < 1, η2 = .012, explained change in vaginal pulse amplitude during the sexual video. Overall, higher Cort-Response predicted greater overall vaginal pulse amplitude independently from video, F(1,41) = 5.18, p < .05, η 2= .100. The association between overall Cort-Baseline and vaginal pulse amplitude was not significant and cortisol did not explain group differences in vaginal pulse amplitude during the sexual video. All main results were replicated when the smokers were excluded from the analyses.
Cort-Response did not explain changes in vaginal pulse amplitude, F < 1, η 2= .012, nor did the interaction Group × Cort-Response, F(1,37) = 1.33, ns, η2 = .022.
Adding CAPS-Total as covariate reduced the relationship between vaginal pulse amplitude and Group (CSA vs. no sexual abuse) which no longer approached significance, F < 1, η2 = .016, suggesting a potential mediation effect of CAPS-Total on the relationship between CSA and vaginal pulse amplitude. CAPS-Total was not significantly associated with vaginal pulse amplitude, F < 1, η2 = .011. Changes in vaginal pulse amplitude in response to the sexual video were significantly associated with Group when CAPS-Total was included in the model, F(1,36) = 13.24, p < .001, η2 = .247.
Exploratory Analyses for Cortisol and Emotional Responses to Sexual Stimuli
Four repeated measure ANCOVAs tested cortisol (dependent variable) based on video (non-sexual vs. sexual) as explained by (1) reported physiological sexual arousal, (2) reported mental sexual arousal, (3) positive affect (PANAS-PA), and (4) negative affect (PANAS-NA). The four analyses included a main effect for group, one of the 4 subjective responses, and the interaction Group × Subjective Response. Given the potential effect of PTSD symptoms, the models also included CAPS-Total as covariate.
Greater CAPS-Total scores explained greater increase in cortisol during the sexual video, F(1,39) = 5.09, p < .05, η2 = .082. An interaction effect was observed for CSA and reported Physiological Sexual Arousal Group × Physiological Sexual Arousal, F (1, 39) = 4.87, p < .05, η2 = .105. Scrutiny of the data showed that this interaction meant that greater physiological sexual arousal explained lower overall cortisol for the no sexual abuse group and greater cortisol for the CSA group. Cortisol changes during the video were not associated with physiological sexual arousal, F(1,39) = 2.16, p = .152, η2 = .035, and also no significant Group × Physiological Sexual Arousal × Video was observed, F < 1, η2 = .002.
A similar pattern was observed for the model testing cortisol changes associated with mental sexual arousal. No significant results were observed for mental sexual arousal, F(1,39) = 2.05, ns, η2 = .033, nor for the interaction between CSA and mental sexual arousal, F < 1, η2 = .011. CAPS-Total explained a significant difference in cortisol changes during the sexual video, F(1,39) = 5.23, p < .05, η2 = .083, with greater PTSD symptoms explaining greater cortisol responses. Different from the previous model, no significant interaction was observed for CSA × Mental Sexual Arousal on overall cortisol, F(1,39) = 1.35, ns, η2 = .032.
The analyses for PANAS-PA and PANAS-NA (independent variables) explaining changes in cortisol (dependent variable) confirmed that CAPS-total significantly explained changes in cortisol levels to sexual video, with greater PTSD predicting greater cortisol responses, with η2 = .121 and .121 for PANAS-PA and PANAS-NA, respectively. The main effects of PANAS-PA and PANAS-NA on cortisol changes were not significant nor were the interaction effects for Group × PANAS-PA and Group × PANAS-NA.
Discussion
This study investigated whether impairments in physiological sexual responses (vaginal pulse amplitude) in CSA survivors are associated with increases in cortisol during exposure to sexual stimuli. Our findings provide evidence for a complex relationship between cortisol and sexual stimuli that is mediated by PTSD symptoms in CSA survivors. PTSD symptoms explained 9.6% of all variance in cortisol changes from the non-sexual to the sexual video, with approximately 0.5 PTSD symptoms predicting a 1% increase in cortisol level from the non-sexual to the sexual video. Interestingly, exploratory analyses did not show a significant association between negative affect and cortisol changes that occurred in response to the sexual video suggesting that if the cortisol increase is a byproduct of negative affect (i.e., anxiety or fear) it is likely to be regulated by affective processes independent from consciousness. In support of this notion, not only previous studies have found that a stress response does not require the involvement of the frontal cortex (LeDoux, 2002), but arousal misattribution theories suggest that individuals experiencing arousal caused by a fear response may indeed misinterpret their arousal and attribute it to the sexual stimuli (Dutton & Aron, 1974). In support to this hypothesis, exploratory analyses showed that, for the CSA survivors, greater cortisol responses were associated with greater perceived states of physiological sexual arousal, while for the no sexual abuse survivors the opposite was true - greater cortisol response was associated with lower perceived physiological sexual responses. Given the preliminary nature of these findings and the lack of a priori hypotheses, future studies are needed to clarify whether the cortisol response observed in CSA survivors with PTSD symptoms is the product of a stress response and whether this response is interpreted as sexual arousal or anxiety.
Noteworthy limitations to our findings are potential biases integral to all laboratory-based and hospital-based studies of sexuality. We cannot deny that the observed changes in cortisol may have resulted from stress caused by embarrassment created by the study protocol. However, baseline levels of cortisol were assessed after each participant was oriented to the laboratory, and it is unlikely that the procedures would have differentially impacted the two groups. Group differences in cortisol responses may have been the result of differences in medications, tobacco (and tobacco withdrawal), or drug and alcohol abuse. Although we attempted to control for these differences, and the two groups were closely matched in types of medications, we only checked self-reports of drugs and medication and we did not statistically control for either the type or dosage of medications. Genetic factors associated with ethnicity could also have had an impact on cortisol reactivity and given that the CSA group had a higher number of African-Americans and Hispanics future studies should attempt to control for these potential confounds.
Contrary to our prediction, we did not find evidence for an inverse relationship between cortisol response to sexual stimuli and vaginal pulse amplitude. Indeed, we found preliminary evidence that an increase in cortisol during the sexual video was associated with greater overall vaginal pulse amplitude levels independent of video type. These findings are difficult to interpret given that individual differences in vaginal pulse amplitude during non-sexual videos are not currently understood. Researchers have not been able to identify factors that explain differences in vaginal pulse amplitude during the non-sexual video (Prause & Janssen, 2004). The fact that we were able to find a significant relationship between overall vaginal pulse amplitude and cortisol changes during the sexual stimuli points to physiological reactivity of the HPA axis as a noteworthy variable for future studies on genital blood flow. It should also be noted that the direction of the observed relationship was opposite with respect to evidence of cortisol potentiating adrenergic constriction of smooth muscles. Basic research on vasoconstriction in the vaginal tissue exposed to cortisol may be able to clarify these results.
It is plausible that the lack of a significant relationship between cortisol and vaginal pulse amplitude may be due to the sample size. However, the effect size observed in our sample indicated that cortisol responses to the sexual video accounted for only 1.2% of variance in vaginal pulse amplitude during the sexual video. Although a larger sample size may render this effect size statistically significant, it is arguable whether it would be clinically meaningful. Moreover, the meaning of a group difference is questionable given that a large effect was observed for both vaginal pulse amplitude and cortisol. The increase in vaginal pulse amplitude for all participants corresponded to 42.2% of vaginal pulse amplitude during the non-sexual video. The cortisol increase for those participants that showed an increase (05 μg/dl or 32.6% ) was comparable to cortisol responses to trauma-scripts in individuals with PTSD reported by prior studies (M change ~ .02 ηg or 18% cortisol increase; Elzinga et al., 2003).
In agreement with prior research (Laan & Everaerd, 1995; Rellini & Meston, 2006), we found a trend for a lower vaginal pulse amplitude response in the CSA group compared to the no sexual abuse group. However, when controlling for PTSD symptoms, the group difference in vaginal pulse amplitude response disappeared and effect sizes were reduced from η2 = .094 to η2 = .016. This finding is consistent with our finding that PTSD symptoms and not trauma exposure predicted cortisol levels, and with prior studies reporting that not all trauma-exposed individuals develop psychiatric symptoms (Browne & Finkelhor, 1986; Laam & Grossman, 1997). Taken together, these results indicate that trauma exposure is not sufficient for the development of a dysfunctional response.
In conclusion, this study found that a history of CSA does not equally affect all women. Some CSA survivors showed an increase in cortisol during exposure to sexual stimuli, which was associated with PTSD symptoms. Data failed to support our hypothesis that an increase in cortisol would cause an inhibition in physiological sexual arousal. Indeed, a significant positive relationship was observed between cortisol responses and overall vaginal pulse amplitude levels. Further investigation is needed to identify the physiological and psychological mechanisms of sexual arousal impairments in patients with PTSD, and to explore whether these results are specific to women with high education and high functioning or generalizable to all CSA survivors including men.
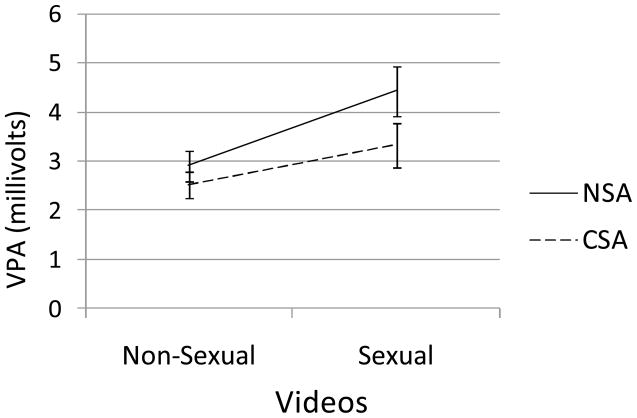
Fig 1.
Group differences between women with a history of child sexual abuse (CSA) vs. women with no history of childhood sexual abuse (NSA) in vaginal pulse amplitude changes from the non-sexual to the sexual video.
Acknowledgments
This publication was made possible by Grant Number F31 MH68165 and Grant Number 1 RO1 HD051676-01 A1. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIMH or NICHD.
References
- Becker JV, Skinner LJ, Abel GG, Howell J, Bruce K. The effects of sexual assault on rape and attempted rape victims. Victimology. 1982;7:106–113. [Google Scholar]
- Becker JV, Skinner LJ, Abel GG, Treacy EC. Incidence and types of sexual dysfunction in rape and incest victims. Journal of Sex & Marital Therapy. 1982;8:65–74. doi: 10.1080/00926238208405813. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, et al. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behavior Therapist. 1990;18:187–188. [Google Scholar]
- Briere J. Child abuse trauma: Theory and treatment of the lasting effects. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- Cloitre M, Koenen KC, Cohen LR, Han H. Skills training in affective and interpersonal regulation followed by exposure: A phase-based treatment for PTSD related to childhood abuse. Journal of Consulting and Clinical Psychology. 2002;70:1067–1074. doi: 10.1037//0022-006x.70.5.1067. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CS, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Exton MS, Bindert A, Kruger T, Scheller F, Hartmann U, Schedlowski M. Cardiovascular and endocrine alterations after masturbation-induced orgasm in women. Psychosomatic Medicine. 1999;61:280–289. doi: 10.1097/00006842-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Exton NG, Truong TC, Exton MS. Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrinology. 2000;25:187–199. doi: 10.1016/s0306-4530(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Mullen PE. Childhood sexual abuse: An evidence based perspective. Thousand Oaks, Ca: Sage Publications; 1999. [Google Scholar]
- Finkelhor D, Hotaling GT, Lewis IA, Smith C. Sexual abuse and its relationship to later sexual satisfaction, marital status, religion, and attitudes. Journal of Interpersonal Violence. 1989;4:379–399. [Google Scholar]
- Heiman JR, Rowland DL. Affective and physiological sexual response patterns: The effects of instructions on sexually functional and dysfunctional men. Journal of Psychosomatic Research. 1983;27:105–116. doi: 10.1016/0022-3999(83)90086-7. [DOI] [PubMed] [Google Scholar]
- Janssen E, Carpenter D, Graham CA. Selecting films for sex research: Gender differences in erotic film preference. Archives of Sexual Behavior. 2003;32:243–251. doi: 10.1023/a:1023413617648. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhamer DH. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Leisen MB, Kaplan AS, Kelly MP. Validation of a brief measure of posttraumatic stress disorder: The Distressing Event Questionnaire (DEQ) Psychological Assessment. 2000;12:197–209. doi: 10.1037//1040-3590.12.2.197. [DOI] [PubMed] [Google Scholar]
- Laan E, Everaerd W, van Bellen G, Hanewald G. Women’s sexual and emotional responses to male- and female-produced erotica. Archives of Sexual Behavior. 1994;23:153–169. doi: 10.1007/BF01542096. [DOI] [PubMed] [Google Scholar]
- Laan E, Everaerd W, Evers A. Assessment of female sexual arousal: Response specificity and construct validity. Psychophysiology. 1995;32:476–485. doi: 10.1111/j.1469-8986.1995.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Laam JN, Grossman FK. Resiliency and adult adaptation in women with and without self-reported histories of childhood sexual abuse. Journal of Traumatic Stress. 1997;10:175–196. doi: 10.1023/a:1024821927574. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Synaptic self- How our brains become who we are. New York: Viking; 2002. [Google Scholar]
- Levin RJ, Wylie K. Vaginal vasomotion- Its appearance, measurement, and usefulness in assessing the mechanisms of vasodilatation. Journal of Sexual Medicine. 2007;5:377–386. doi: 10.1111/j.1743-6109.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- Maltz W. Treating the sexual intimacy concerns of sexual abuse survivors. Sexual and Relational Therapy. 2002;17:321–327. [Google Scholar]
- Meston CM, Gorzalka BB. The effects of immediate, delayed and residual sympathetic activation on physiological and subjective sexual arousal in women. Behavior Research and Therapy. 1996;34:143–148. doi: 10.1016/0005-7967(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Prause N, Janssen E. Blood flow: Vaginal photoplethysmography. In: Goldstein I, Meston C, Davis S, Traish A, editors. Women sexual function and dysfunction: Study, diagnosis and treatment. London: Taylor & Francis; 2005. pp. 98–104. [Google Scholar]
- Rellini AH, Meston CM. Psychophysiological sexual arousal in women with a history of childhood sexual abuse. Journal of Sex & Marital Therapy. 2006;32:1–18. doi: 10.1080/00926230500229145. [DOI] [PubMed] [Google Scholar]
- Rosen R, Brown C, Heiman J, Leiblum S, Meston CM, Shabsigh R, et al. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex &Marital Therapy. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Sintchak G, Geer JH. A vaginal plethysmograph system. Psychophysiology. 1975;12:113–115. doi: 10.1111/j.1469-8986.1975.tb03074.x. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Rasmusson A, Barron J, Arnsten A. Neurobiological and neurocognitive alterations in PTSD: A focus on norepinephrine, serotonin, and the hypothalamic-pituitary-adrenal axis. In: Vasterling JJ, Brewin CR, editors. Neuropsychology of PTSD: Biological, cognitive, and clinical perspectives. New York: Guilford Press; 2005. pp. 27–58. [Google Scholar]
- Stewart J. Salivary cortisol measurement. Summary of findings by the John D. and Catherine T. MacArthur Research Network on Socioeconomic Status and Health. 2000 http://www.macses.ucsf.edu/Research/Allostatic/notebook/salivarycort.html.
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wiegel M, Meston CM, Rosen RC. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cutoff scores. Journal of Sex & Marital Therapy. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- Woo Y, Brotto L, Yule T. Do East-Asian and Euro-Canadian women differ in sexual psychophysiology research participation? Journal of Sex Research. doi: 10.1080/00224490902999294. In press. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Adult neuroendocrine aspects of PTSD. Psychiatry Annals. 2003;33:30–36. [Google Scholar]