Abstract
Expression of gastrin and cholecystokinin 2 (CCK2) receptor splice variants (CCK2R and CCK2i4svR) are upregulated in human colonic adenomas where they are thought to contribute to tumor growth and progression. To determine the effects of ectopic CCK2 receptor variant expression on colonic epithelial cell growth in vitro and in vivo, we employed the non-tumorigenic colonic epithelial cell line, NCM356. Receptor expression was induced using a retroviral expression vector containing cDNAs for either CCK2i4svR or CCK2R. RT-PCR and intracellular Ca2+ ([Ca2+]i) imaging of RIE/CCK2R cells treated with conditioned media (CM) from NCM356 revealed that NCM356 cells express gastrin mRNA and secrete endogenous, biologically active peptide. NCM356 cells expressing either CCK2R or CCK2i4svR (71 and 81 fmol/mg, respectively) grew faster in vitro, and exhibited an increase in basal levels of phosphorylated ERK (pERK), compared to vector. CCK2 receptor selective antagonist, YM022, partially inhibited the growth of both receptor-expressing NCM356 cells, but not the control cells. Inhibitors of mitogen activated protein kinase pathway (MEK/ERK) or protein kinase C (PKC) isozymes partially inhibited the elevated levels of basal pERK and in vitro growth of receptor-expressing cells. Vector-NCM356 cells did not form tumors in nude mice, whereas, either CCK2 receptor-expressing cells formed large tumors. Autocrine activation CCK2 receptor variants are sufficient to increase in vitro growth and tumorigenicity of non-transformed NCM356 colon epithelial cells through a pathway involving PKC and the MEK/ERK axis. These findings support the hypothesis that expression of gastrin and its receptors in human colonic adenomas contributes to tumor growth and progression.
Keywords: Gastrin, CCK2 receptors, colorectal tumorigenesis, NCM356 cells
INTRODUCTION
The adenoma- to carcinoma-multistage sequence of colorectal cancer development is characterized by specific histopathologic criteria as well as defined genetic mutations, which result in the activation of oncogenes (e.g., K-ras) and inactivation of tumor suppressors (e.g., adenomatous polyposis coli [APC] and p53).1 It is now well recognized that the key initiating events that underlie most cases of colorectal tumorigenesis, whether familial or sporadic, are the mutations within the Wnt/APC/β-catenin signaling pathway.2 However, increasing evidence also indicate that epigenetic changes in DNA and/or chromatin structure, causing aberrant mRNA splicing and/or inappropriate expression of normal genes, can interact with genetic mutations to contribute to the development of the malignant phenotype.3 Aberrant expression of the gastrin/cholecystokinin 2 (CCK2) receptor, gastrin and its biosynthetic precursors, in a majority of pre-malignant adenomatous polyps strongly implicate a relevant role for this signaling axis in the adenoma-carcinoma sequence.4-6
Carboxyl-terminus-amidated gastrin (i.e., mature gastrin) is produced from the cleavage and post-translational processing of a preprohormone protein. Although both gastrin precursors and mature gastrin are prevalent during the early stages of malignant transformation, as well as in established colon cancers, their exact roles are in each context are controversial (summarized in a recent review7). Several lines of evidence suggest a potentially important function for the peptide hormone particularly in the early stages of colorectal cancer development. First, patients with hypergastrinemia, associated either with Zollinger-Ellison syndrome or chronic autoimmune gastritis, exhibited increased rates of colonic mucosal cell proliferation,8, 9 whereas hypergastrinemia due to pernicious anemia, Helicobacter pylori infection or other causes has been associated with an increased risk of developing colonic polyps and/or cancers.10-12 Second, a mechanistic relationship has been established between mutations in the Wnt/APC/β-catenin pathway, aberrant gastrin gene expression, and gastrin-mediated signal transduction using both in vivo and in vitro rodent models of early colorectal carcinogenesis. Specifically, Koh et al.13 have shown that mice derived from a cross between the APCmin−/+ mouse, a model of familial adenomatous polyposis, and a gastrin gene knockout mouse developed fewer intestinal polyps. Additionally, Watson et al.14 showed in the APCmin−/+ mouse model, that proton pump inhibitor-induced hypergastrinemia increased mucosal proliferation, polyp development, and decreased survival. Treatment with anti-gastrin antibodies inhibited the effects of hypergastrinemia on mucosal proliferation and animal survival. Finally, in vitro experiments demonstrated that induction of the wild-type APC decreased gastrin mRNA expression, while transfection of constitutively active β-catenin increased gastrin promoter activity.13 These data suggest that aberrant gastrin expression is mechanistically linked to initiating genetic mutations within the Wnt/APC/β-catenin pathway and contributes to the development of the malignant phenotype.
Although it is well known that in normal tissues and cells the biologic actions of mature gastrin are mediated by CCK2 receptors, members of the rhodopsin β subclass of G protein-coupled receptors,15 whether CCK2 receptor is the only mediator the tumorigenic activities of gastrin in colorectal cancer remains an unresolved issue. Central to the controversy is the contention that since the biosynthetic precursors of gastrin (i.e., progastrin and glycine-extended gastrin [Gly-G]) bind the CCK2 receptor with significantly lower affinities than that for amidated gastrin, there must also exist unique high-affinity receptor(s) for these precursors. The molecular identity and characteristics of these other receptors, however, currently are not completely elucidated.16, 17 Nonetheless, several studies suggest a potential role for CCK2 receptor variants in the early stages of colorectal carcinogenesis. As the result of alternative splicing at intron 4, at least two functionally distinct CCK2 receptor variants (CCK2R and CCK2i4svR) have been identified in a subset of clinical specimens from human pre-malignant adenomatous polyps when compared to normal colonic mucosa.4, 6 Furthermore, genetic ablation of the CCK2 receptor reduced azoxymethane-induced tumor size and burden compared to wild-type controls and mice overexpressing progastrin,18 suggesting a relevant function for the CCK2 receptor, even in the context of gastrin precursors. Finally, intestinal polyps from hypergastrinemic APCmin−/+ mice exhibited a 6-fold increase in CCK2 receptor mRNA expression compared to normgastrinemic control animals,14 supporting an endocrine mechanism of CCK2 receptor activation. However, human colon adenomas from patient samples demonstrate contemporaneous overexpression of both gastrin peptides and their cognate receptors, implying that a more local or cell-autonomous mechanism of tumor progression may be present. The lack of a non-tumorigenic human colonic epithelial cell model to study potential autocrine/paracrine activation of the CCK2 receptor signaling pathways has limited our understanding of how aberrant expression of gastrin and CCK2 receptor variants lead to the development and the promotion of colorectal carcinogenesis.
To address whether acquisition of the CCK2 receptor is sufficient to convert a non-tumorigenic, gastrin-producing colorectal cell into a tumorigenic cell, we employed the NCM356 epithelial cell line, derived from the normal colon mucosa wide margin resection of a patient with rectal adenocarcinoma.19 The NCM356 cells express colon epithelial antigens, including cytokeratin, villin and mucin. They do not grow in soft agar, are non-tumorigenic in nude mice, and yet, are immortal in cell culture and as we show herein, inappropriately expresses and secretes functional gastrin peptides. We report that ectopic expression of CCK2 receptor splice variants is sufficient to promote enhanced cell adhesion, cell growth, and tumorigenesis, in part, through the activation of protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) pathways.
MATERIALS AND METHODS
Materials
The parental NCM356 cell line was acquired under an MTA from InCell Corp. (San Antonio, TX). Antibodies for immunoblotting of phospho-ERK (pERK) and β-actin were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Chemical inhibitors PD98059 and GF109203X were purchased from Calbiochem (San Diego, CA). Receptor-mediated signaling was antagonized with YM022 (Sigma; St. Louis, MO) and JB93182, a gift from the James Black Foundation. Gastrin 1-17 (G17) was purchased from Biomol (Plymouth Meeting, PA), and dimethyl sulfoxide (DMSO) was purchased from Sigma (St. Louis, MO).
Retroviral expression constructs and transduced cell lines
CCK2 receptor variant cDNAs were cloned into a bicistronic packaging murine oncoretroviral vectors based on pFB (Stratagene). The vector contains the murine leukemia retrovirus (MLV) packaging sequence and a multiple cloning site (MCS), flanked by the MLV long terminal repeat (LTR) regions. The 5′ LTR functions as a strong promoter upon chromosomal integration of proviral DNA. The pFB plasmid was modified to contain a cassette comprising an ECMV internal ribosome entry site (IRES) followed by a gene encoding β-galactosidase (modified with a nuclear localization signal), which enabled retrovirus titer and transcript expression levels to be determined by staining for β-galactosidase.20 Retroviruses were made by simultaneous transfection of HEK293FT cells (Invitrogen; Carlsbad, CA) with the CCK2 receptor expression plasmid and plasmids encoding MLV gag-pol and vesicular stomatitis virus envelope protein. Cell supernatants were used to transduce NCM356 cells (MOI=10). The level of receptor expression in transduced cell lines was quantified using [125I]labeled-G17 competition binding to isolated cell membranes as previously described.21 NCM356-CCK2R and -CCK2i4svR cells express 71 and 81 fmol receptor/mg of membrane protein, respectively. All experiments were performed using the original transduced cell line; there was no additional subcloning of the cultures.
Cell Culture
The parental and transduced NCM356 cell lines were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2 in culture media obtained from Incell Corp. (San Antonio, TX). Serum free media M3 ™ (Incell Corp.) was mixed with M3:10™ containing 10% fetal bovine serum (FBS) to obtain 1% FBS media for the in vitro cell proliferation experiments.
Reverse-transcription-polymerase chain reaction (PCR)
Total RNA was extracted using Ultraspec reagent (Biotecx; Houston, TX) and treated with 1 unit of RNase-free DNase I at 37°C for 30 min (Promega, Madison, WI). Messenger RNA was converted to cDNA using Retroscript (Ambion). PCR was performed using the primers for gastrin: sense, 5′-CTTAGGTACAGGGGCCAACA-3′ and anti-sense 5′-TCCATCCATCCATAGGCTTC-3′. The PCR conditions were 94°C for 5 min followed by 40 cycles of 94°C for 30 s, 45°C for 30 s, and 72°C for 1 min. A 1-kb DNA ladder (10 μl) (Life Technologies, Inc.) was used to determine the relative size of the PCR products.
Intracellular Ca2+ ([Ca2+]i) measurements
[Ca2+]i imaging of rat intestinal epithelial (RIE) cells expressing recombinant CCK2R (RIE/CCK2R) was used as a bioassay, to detect gastrin in conditioned culture media collected from parental NCM356 cells. Briefly, RIE/CCK2R cells were plated on 25 mm glass coverslips, washed with a physiological medium (KRH) containing NaCl (125 mM), KCl (5 mM), KH2PO4 (1.2 mM), MgSO4 (1.2 mM), CaCl2 (2 mM), glucose (6 mM), HEPES (25 mM; pH 7.4), and loaded with 2 μM Fura-2AM (Molecular Probes, Eugene, OR) at 25°C for 50 min. The cells were stimulated with NCM356 conditioned media (CM) and single cell changes in the concentration of free [Ca2+]i were recorded with a Nikon Diaphot inverted microscope (Garden City, NY) and a CCD camera (Dage-MITI, Inc., Michigan City, IN). Data points were collected every 1-8 s from approximately 35 cells/coverslip and processed using ImageMaster software.
In vitro cell proliferation assay
To study the effects of receptor variant expression on NCM356 cell growth in vitro with and without chemical inhibitors, single cell suspensions of 2 × 104 cells were seeded into 24-well plates in triplicate and cultured in 1.0 ml M3:10 media (10% FBS) mixed with M3 (serum free) to a final concentration of 1% FCS with or without G17 (10 nM). Cells were trypsinized and counted by a Coulter counter (Beckman Coulter, Inc., Fullerton, CA) daily for 9 days. The doubling time was calculated using the GraphPad Prism program, Version 4.0 (GraphPad Software Inc, San Diego, CA). Each experiment was performed at least twice.
In vivo tumor growth assay
The athymic nude mouse xenograft model was used to assess the effects of receptor variant expression on NCM356 cell growth in vivo. Replicates (n=6 mice for each group) of nu/nu Balb/C female mice (age 8-10 weeks, weight approximately 20 g each) were injected subcutaneously with 5×106 cells per cell line in the left dorsum. Tumor diameters were measured transcutaneously using calipers over a 3-week period. Tumor volumes for the generally spherical tumors were calculated using the formula: volume=4/3πr3. At harvest, the wet weights of the tumors were recorded. T-test comparisons of the tumor weights for each CCK2 receptor group was performed using Graphpad Prism with significance determined at p<0.05. This experiment was repeated three times.
Western blot
Cells were plated into 12-well plates at a density of approximately 105 cells/well in media. After 2 days, cells were washed with ice-cold PBS and solubilized in lysis buffer containing 1% Triton X-100, 150 mM NaCl, 20 mM Tris pH 7.5, 1 mM EGTA, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride and 5 μg/ml each of chymostatin, pepstatin A, leupeptin and antipain at 4°C for 15 min. Triton X-100 insoluble cellular material was removed by centrifugation at 14,000 rpm for 15 min and the protein concentrations of the supernatant were determined using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories; Hercules, CA). Protein (20 μg) from each sample was resolved on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane and probed with anti-pERK1,2 antibody. Immunoreactive proteins were visualized using the ECL Western blotting detection system (Amersham Biosciences, Piscataway, NY). The blots were stripped and reprobed for β-actin to insure the equal protein loading in all lanes. Densitometry analyses were performed using Un-Scan-It (Silk Scientific; Orem, UT).
RESULTS
The human colonic epithelial cell line NCM356 secretes gastrin peptide
Gastrin binding to the CCK2 receptor leads to the Gq-mediated activation of phospholipase C-β, which cleave membrane phosphatidylinositol 4,5-bisphosphate to produce the second messengers 1,4,5-inositol triphosphate (IP3) and 1,2-diacylglycerol. IP3, in turn, induces Ca2+ release from the endoplasmic reticulum by activating inositol-1,4,5-trisphosphate receptors (IP3R), resulting in a transient increase in the concentration of free cytosolic Ca2+ [Ca2+]i.
To assess whether the NCM356 cells secrete gastrin peptides, we developed an [Ca2+]i imaging bioassay, taking advantage of the fact that CCK2 receptors are coupled to IP3R. We generated the rat intestinal epithelial (RIE) cell line which expresses recombinant human CCK2R (RIE/CCK2R). We treated RIE/CCK2R loaded with the Ca2+ indicator dye Fura-2, with known concentrations of agonists to establish the sensitivity, efficacy and specificity of the bioassay. We determined that the RIE/CCK2R cells did not induce a calcium response to 10 nM of Gly-G, but did respond robustly to 1 nM of purified G17 (Fig. 1A). Increasing the Gly-G dose to 100 nM, or a 100-fold excess in comparison to 1 nM G17, did cause an increase in [Ca2+]i, but to a lesser extent as compared to that of 1 nM G17 (Fig. 1B). As expected, pretreatment with the selective CCK2 receptor antagonist L 365,260 (100 nM) abrogated the calcium response to both 100 nM Gly-G and 1 nM G17 in the RIE/CCK2R cells; however, L 365,260 did not affect the increase in [Ca2+]i due to activation of the bradykinin (BK) receptor after application of 100 nM BK (Fig 1C). As previously reported by Hellmich et al.,4 Gly-G binds to the CCK2 receptors with significantly lower affinity than the amidated form of gastrin, G17, and this binding can be blocked with specific inhibitors to CCK2 receptors.
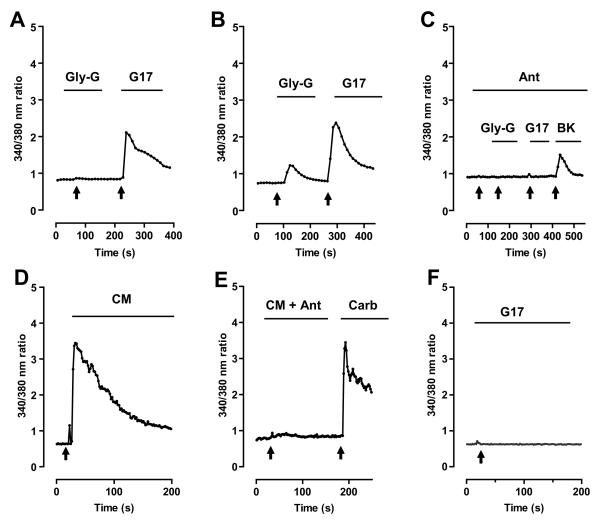
Figure 1. Secretion of functional gastrin-like peptide by non-tumorigenic human colonic epithelial cell line NCM356.
Intracellular Ca2+ ([Ca2+]i) imaging of rat intestinal epithelial (RIE) cells expressing recombinant human CCK2 receptor (RIE/CCK2R) loaded with the Ca2+ indicator dye Fura-2 was used as a bioassay to test the sensitivity, efficacy, and specificity of gastrin peptides. Black arrows indicate the time of addition of each treatment. Black bars indicate duration of exposure to each treatment. (A) Treatment of the RIE/CCK2R cells with 10 nM glycine-extended gastrin (Gly-G) failed to induce an increase in [Ca2+]i, whereas 1 nM of gastrin 1-17 (G17) to RIE/CCK2R cells does. (B) A 100-fold increase in concentration of Gly-G (100 nM) results in calcium response less robust than that of 1 nM of G17. (C) Pretreatment with CCK2 receptor antagonist 100 nM of L365,260 abolishes the Ca2+ response by 100 nM of Gly-G and 1 nM of G17 but not 100 nM of bradykinin (BK). (D) Conditioned media (CM) from NCM365 cultures was applied to the RIE/CCK2R cells, inducing a transient increase in [Ca2+]i in the cells of similar amplitude and duration as treating cells with 1 nM G17 (A,B). (E) Pretreatment with selective CCK2R receptor antagonist 1 μM of JB93182 (JB) abrogates CM-induced Ca2+ response. Cells were treated with 10 μM carbachol (Carb) to insure that the cells were loaded with Fura-2. (F) Although NCM356 cells secrete gastrin, addition of exogenous G17 (100 nM) does not induce an increase in [Ca2+]i indicating the absence of functional CCK2 receptor.
We then applied CM from cultures of parental NCM356 to the RIE/CCK2R cells in our bioassay and showed that the CM produced a transient increase in [Ca2+]i (Fig. 1D). To demonstrate that the NCM356 CM-induced Ca2+ response was mediated by the CCK2R expressed on RIE cells, the CM was supplemented with 1 μM JB93182, a CCK2 receptor-specific antagonist. The antagonist blocked the transient increase in [Ca2+]i induced by CM (Fig. 1E). To verify that the cells were loaded with Fura-2 and capable of responding, we applied the muscarinic receptor agonist carbachol, which induced an increase in [Ca2+]i (Fig. 1E). Treating RIE/CCK2R cells with 1 nM purified G17 induced an increase in [Ca2+]i of similar amplitude and duration (Fig. 1A and B) as cells treated with NCM356 CM (Fig. 1D). Finally, addition of exogenous G17 (100 nM) to parental NCM356 did not induce an increase in [Ca2+]i, indicating the absence of expression of CCK2 receptors by these cells (Fig. 1D). Together, these data demonstrate that, like many pre-malignant polyps, NCM356 secrete forms of gastrin that activate CCK2 receptors, such as Gly-G and G17. Although other precursor forms of gastrin may also be present, the receptors mediating their actions are either not known or well defined, and therefore, were not assessed with our bioassay. Finally, since the NCM356 cells do not express functional CCK2 receptors, this colorectal cell line represents a good model system to address the effects of receptor acquisition, autocrine signaling on cell proliferation, and malignant potential.
Expression of human recombinant CCK2 receptor splice variants in NCM356 cells increases cellular adhesion and proliferation in vitro
Concomitant with the upregulation of gastrin expression, which has been reported to have a prevalence of 78% in polyps, is expression of CCK2 receptor variants in up to 81% of colon adenomas,5 suggesting the existence of a potential autocrine signaling axis. To model the observations from adenomatous polyps, we transduced parental NCM356 cells with retrovirus (pFB-IRES) expression constructs containing the cDNAs for either CCK2R or CCK2i4svR. Cells also were generated containing the pFB-IRES vector alone to control for non-specific effects of transduction. Reverse transcriptase/polymerase chain reaction with primer sequences to human gastrin verified mRNA expression by all three transduced cell lines (Fig. 2A) and the RIE/CCK2R cell bioassay confirmed the presence of secreted gastrin in CM (data not shown).
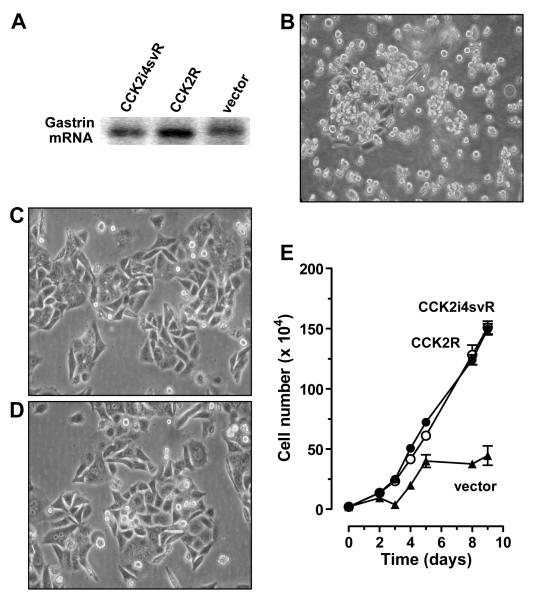
Figure 2. Phenotypic Effects of CCK2 receptor variants expression on NCM356 cell growth in vitro.
(A) All three transduced cell lines express gastrin mRNA by reverse transcriptase/polymerase chain reaction assay. (B) Photomicrograph of NCM356 cells stably transduced with the pFB-IRES retroviral expression vectors. Vector cells exhibited a small round, non-adherent (NA) phenotype indistinguishable from the parental line. In contrast, NCM356 cells transduced either with pFB-IRES containing recombinant human CCK2R (C) or CCK2i4svR (D) exhibit a spread, adherent (A) phenotype with few floating cells. (E) Change in cell number in cell culture over time. NCM356-CCK2R (open circle) or -CCK2i4svR (filled circle) grew faster compared to -vector control cells (filled triangle) in 10% FCS media conditions.
In culture, the parental NCM356 cells grow predominantly (>98%) as a suspension of loosely aggregated round cells with a small (<2%) population of adherent (A) cells.19 The NCM356 vector-transduced cells were phenotypically indistinguishable from parental NCM356 cells (Fig. 2B). However, in contrast to the vector transduced cells, both CCK2R- and CCK2i4svR-expressing NCM356 cell lines exhibited predominantly (>99%) an A and spread phenotype in culture with <1% non-adherent (NA) cells (Fig. 2C and D, respectively).
To assess the effects of CCK2 receptor variant expression on NCM356 cell proliferation, cells were plated in multi-well plates and counted using a Coulter cell counter. The doubling time for vector-transduced NCM356 cells was 3.9 days (95% confidence intervals of 2.5, 6.2). In contrast, both CCK2R- and CCK2i4svR-expressing cells grew faster, with a shorter doubling times of 2.5 days (2.2, 2.8); and 2.7 (2.4, 3.1), respectively (Fig. 2D). Comparison of the best fit curve of exponential growth between vector- versus either CCK2R- or CCK2i4svR-expressing cell was statistically significant (p=0.0001). However, there were no differences in doubling times between each the two CCK2 receptor variants (p=0.48; comparison of best-fit values).
YM022, a CCK2 receptor-specific antagonist/inverse agonist, partially inhibits the proliferation of CCK2R and CCK2i4svR cells in vitro
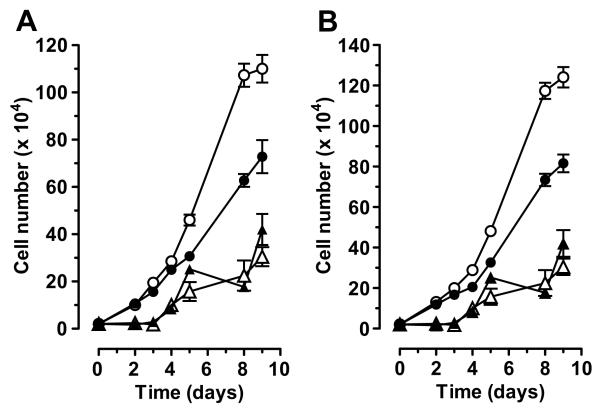
To determine whether the enhanced proliferation of receptor-expressing NCM356 cells was due to autocrine activation of the CCK2 receptor variants by endogenous gastrin, the cells were treated at a 48-h interval beginning two days after plating either with vehicle (DMSO) or the CCK2R antagonist/inverse agonist YM02222, 23 over a 9-day time course (Fig. 3). YM022 partially inhibited the proliferation of both CCK2R- and CCK2i4svR-expressing cells when compared to cells treated with vehicle (Fig. 3A and B, respectively). Vector-transduced cells grew slower than receptor-expressing cells and were not inhibited by YM022 treatment (Fig. 3A and B). The p-values for best-fit linear regression growth curve between DMSO- versus YM022-treated were statistically significant for both CCK2 receptor-expressing cell lines (p<0.001), but not for the vector-expressing cells (p=0.38). These data suggest that autocrine activation of the CCK2 receptor signaling axis is, in part, responsible of the enhanced proliferation rate observed with receptor-transduced NCM356 cells. Additionally, YM022 inhibited the growth response of both CCK2R- and CCK2i4svR-expressing cells to the same extent, suggesting that there was no significant constitutive activity associated with CCK2i4svR in this cell system. If CCK2i4svR had significant constitutive growth promoting effects, YM022 would have suppressed the growth of these cells to a greater extent when compared to the CCK2R-expressing cells.
Figure 3. Effect of YM022 on cell growth in NCM356 cells expressing CCK2 receptor variants.
Change in cell number in cell culture over time with application of either vehicle (DMSO) or YM022 (1 μM) to the growth media. Receptor expressing cells were treated at a 48 h interval beginning on day 2 either with vehicle [DMSO (open circle)] or 1 μM YM022 (filled circle) over a 9-day time course. Vector transduced NCM356 cells were also treated with either vehicle (open triangle) or 1 μM YM022 (filled triangle) over the same time course. Data from the vector transduced cells are plotted on both graphs for comparison. (A) The growth curves for NCM356-CCK2R compared to -vector cells. (B) The growth curves for NCM356-CCK2i4svR cells compared to -vector cells.
CCK2 receptor expression is sufficient to transform NCM356 cells
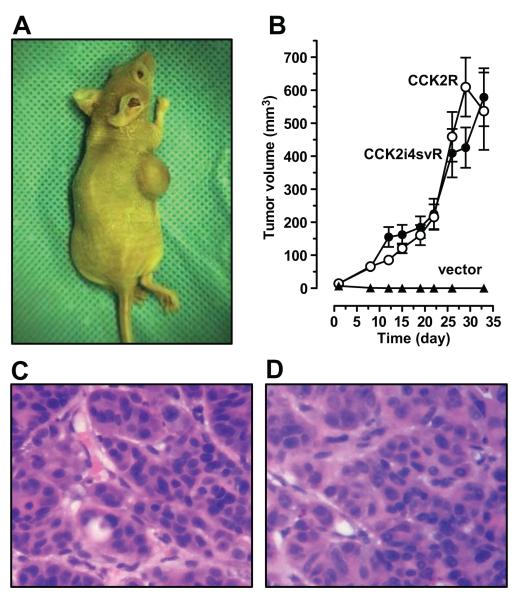
Having established the presence of an in vitro autocrine growth loop, we next investigated whether acquisition of either CCK2 receptor variant affected the NCM356 cell tumorigenic potential. Transduced cells were injected into the flanks of athymic nude mice (5×106 cells per mouse, 6 replicates per condition). This experiment was performed three times and a representative experiment is shown in Fig. 4A. Neither parental NCM356 cells (data not shown) nor the vector-transduced cells formed tumors in athymic nude mice (Fig. 4B). However, both CCK2R- and CCK2i4svR-expressing cells formed large tumor masses at the subcutaneous injection site (Fig. 4B). At sacrifice on day 33, no statistically significant differences (p=0.6, t-test) were noted between the mean weights of CCK2R- (0.68 ± 0.05 g) and CCK2i4svR-expressing tumors (0.63 ± 0.09 g). Hematoxylin and eosin (H&E)-stained tissue sections from both CCK2R- (Fig. 4C) and CCK2i4svR-expressing (Fig. 4D) tumors showed similar histopathology. The tumors appeared to be moderately differentiated adenocarcinoma, with clusters of tumor cells exhibiting marked nuclear atypia surrounded by scant intervening stromal tissue including murine blood vessels. Together, these data demonstrate that acquisition of either CCK2 receptor variant is sufficient to transform the non-tumorigenic NCM356 cells into tumor-forming cells.
Figure 4. Phenotypic effects of CCK2 receptor variants expression on NCM356 cell in vivo.
The athymic nude mouse model was used to assess whether acquisition of either CCK2 receptor variant was sufficient to transform non-tumorigenic NCM356 cells into tumor forming cells. (A) Photograph of representative nude mouse with subcutaneous xenograft at harvest on day 33. (B) Change in tumor volume over time. NCM356 cells infected with the control vector (filled triangle) did not form tumors in nude mice; whereas, both -CCK2R (open circle) and -CCK2i4svR cells (filled circle) formed subcutaneous tumors (~600 mm3) over a 33-day time course. The graph represents summary data from 6 mice /group. (C, D) Photomicrograph of formalin-fixed, hematoxylin-eosin stained section of NCM356-CCK2R and -CCK2i4svR tumors, respectively (400X magnification). Tumors, at day 33, showed similar moderately-differentiated adenocarcinomas with marked nuclear atypia are surrounded by scant stroma.
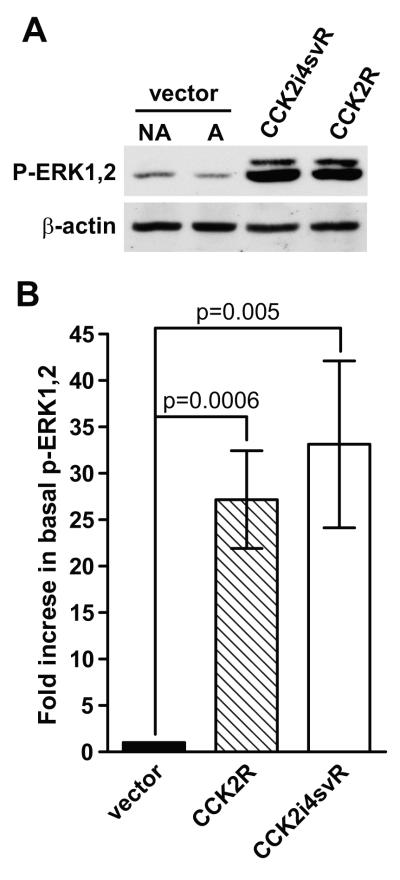
Basal phosphorylation of extracellular signal-regulated kinase (ERK)-1 and ERK2 are elevated in NCM356 cells expressing CCK2 receptor variants
Extracellular signal-regulated kinases are members of the MAPK family that includes c-Jun N-terminal kinase (JNK), and p38MAPK. The ERK subfamily is comprised of multiple isoforms, of which ERK1 and ERK2 are the most extensively characterized. Both are activated by phosphorylation of the threonine and tyrosine residues with the T-E/D-Y consensus motif by the dual-specificity kinases, MAP/ERK kinase (MEK)1 and/or MEK2. Previous studies have shown that CCK2R and CCK2i4svR can couple gastrin stimulation to the activation (phosphorylation) of ERK1 and ERK2.24, 25
To begin to elucidate potential signaling mechanisms for the enhanced proliferation and tumorigenicity of CCK2 receptor variant-expressing NCM356 cells, we compared the basal phosphorylation state of ERK1 and ERK2 in protein extracts from CCK2R-, CCK2i4svR- and vector-transduced NCM356 cells. Since the vector-transduced cells contain both A and NA cells, we separately evaluated the levels of phosphorylated ERK1 and ERK2 (p-ERK1,2) in each subpopulation of cells. Compared to either NA or A vector-transduced cells, both CCK2 receptor variants exhibited a marked increase in basal levels of activated ERK1 and ERK2 (Fig. 5A). No differences were observed between the basal levels of p-ERK1 and p-ERK2 in the NA and A subpopulations of vector-transduced cells (Fig. 5A). Densitometric analyses of immunoblot data from 6 independent experiments, after normalization using β actin as a protein loading control, demonstrated a significant difference in the basal p-ERK1 and p-ERK2 levels comparing vector-transduced cells to either CCK2R- (p=0.0006) or CCK2i4svR-expressing lines (p=0.005) (Fig. 4B). Additionally, we evaluated whether the Src or PI3K/Akt pathways were activated by CCK2 receptor expression. Western blots revealed that neither phospho-Src nor phospho-Akt levels increased in CCK2R- and CCK2i4svR-expressing cells when compared to vector-transduced control cultures (data not shown).
Figure 5. Effect of basal levels of phosphorylated (activated) ERK1 and ERK2 in NCM356 cells expressing CCK2 receptor variants.
(A) Western blot comparing the basal levels of phospho-ERK1 and 2 (p-ERK1,2) levels in the non-adherent (NA) cell population NCM356-vector cells, the adherent (A) -vector cells, -CCK2R pooled cells, and the -CCK2i4svR pooled cells. Blots were reprobed with an antibody to β-actin to insure the equal loading and transfer of proteins in each lane. (B) Graph representing densitometric analyses of immunoblot data from 6 independent experiments comparing the fold change in basal p-ERK1,2 levels between NCM 356-vector, -CCK2R and -CCK2i4svR transduced cell lines. Statistical analysis using the t-test demonstrated significance between the basal p-ERK1,2 levels from vector and NCM356-CCK2R (p=0.0006) and between vector and -CCK2i4svR cells (p=0.005).
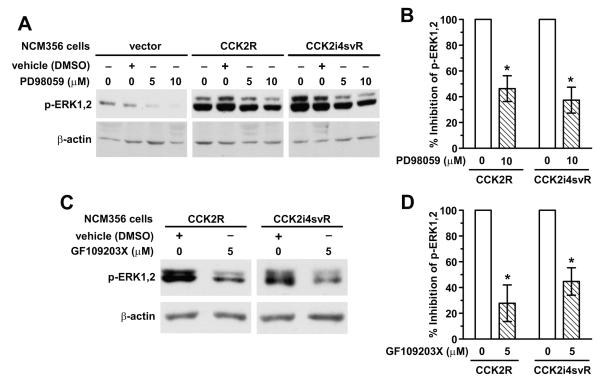
The MEK inhibitor PD98059 and the PKC inhibitor GF109203X partially inhibit basal ERK phosphorylation in CCK2R- and CCK2i4svR-expressing NCM356 cells
Todisco et al.26 reported that gastrin-stimulation of CCK2R expressed on the rat pancreatic acinar cell line AR42J activated ERK by both MEK- and PKC-dependent mechanisms. To further define the mechanisms involved in enhanced basal activation of ERK1 and ERK2 in NCM356 cells, we treated CCK2R- and CCK2i4svR-expressing cells either with the MEK inhibitor PD98059 or the PKC inhibitor, GF109203X. Immunoblot analyses of protein extracts from vector-transduced as well as CCK2R- and CCK2i4svR-expressing cells, showed a partial but dose-dependent inhibition of p-ERK1 and p-ERK2 in cells treated with PD98059 (Fig. 6A). Densitometric analyses from three independent experiments comparing p-ERK1 and p-ERK2 levels between vehicle- and PD98059-treated groups for CCK2R- and CCK2i4svR-expressing cells demonstrated a 54 ± 9.9% (p=0.006) and 63 ± 10% (p=0.003) inhibition of ERK activity, respectively, when treated with 10 μM PD98059 for 30 min (Fig. 6B). The basal p-ERK1 and p-ERK2 levels were also partially decreased when receptor-expressing cells were treated with the PKC inhibitor, GF109203X (5 μM), for 30 min (Fig. 6C). Comparison of p-ERK1 and p-ERK2 levels between vehicle- and GF109203X-treated groups for CCK2R- and CCK2i4svR-expressing cells demonstrated a 72 ± 14% (p=0.036) and 55 ± 10% (p=0.035) inhibition of ERK activity, respectively, when treated with 5 μM GF109203X for 30 min (n=3) (Fig. 6B). Together these data indicate that receptor expression enhances basal ERK activation in NCM356 cells, in part, through a pathway involving GF109203X-sensitive PKC isozymes.
Figure 6. Effects of the MEK inhibitor PD98059 and the PKC inhibitor GF109203X on basal p-ERK1,2 levels.
(A) Western blot of basal pERK levels in NCM356-vector, -CCK2R, or -CCK2i4svR expressing cells in the presence of vehicle (DMSO) or MEK inhibitor PD98059 (5 or 10 μM) for 30 min. Blots were reprobed with an antibody to β-actin to insure the equal loading and transfer of proteins in each lane. (B) Graph representing densitometric analyses of immunoblot data from 3 independent experiments comparing the % inhibition of basal p-ERK1,2 levels between treatment with vehicle or 10 μM PD98059 on NCM 356 -CCK2R and -CCK2i4svR transduced cell lines. T-test demonstrates statistical significance comparing the % inhibition of basal p-ERK1,2 levels between vehicle-treated and PD98059-treated NCM356-CCK2R (p=0.006) and—CCK2i4svR cells (p=0.003). (C) Western blot of basal pERK levels in NCM356-vector, -CCK2R, or -CCK2i4svR expressing cells in the presence of vehicle (DMSO) or PKC inhibitor GF109203X (5 μM) for 30 min. An antibody to β-actin was used to insure the equal loading and transfer of proteins in each lane. (D) Graphical representation of immunoblot data from 2 independent experiments comparing the % inhibition of basal p-ERK1,2 levels between treatment with vehicle or 5 μM GF109203X on NCM 356 -CCK2R and -CCK2i4svR transduced cell lines. Statistically significant difference between basal p-ERK1,2 levels of vehicle-treated compared to inhibitor-treated cells were noted in the NCM356-CCK2R (p=0.036) and -CCK2i4svR cells (p=0.035) using t-test analyses.
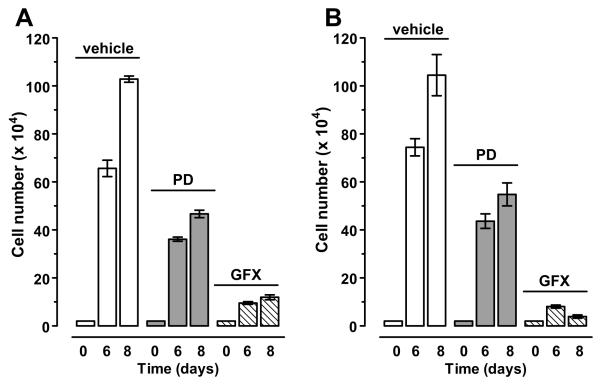
Inhibition of MEK and PKC slow the proliferation of CCK2R- and CCK2i4svR-expressing NCM356 cells
To assess the involvement of the MEK/ERK pathway and GF109203X-sensitive PKC isozymes in receptor-stimulated NCM356 cell proliferation, 2 days after plating (20,000 cells/well), cells were treated at 48-h intervals either with vehicle (DMSO), PD98059 (10 μM) or GF109203X (5 μM) over an 8-day time course. Cell proliferation was determined by counting the total number of attached cells per well on day 6 and 8 using a Coulter Cell Counter. Both PD98059- and GF109203X-treatment inhibited the proliferation of receptor-transduced NCM356 cells. GF109203X was more effective inhibiting 86% and 88% of CCK2R-associated (Fig. 6A) and 90% and 96% of CCK2i4svR-associated (Fig. 6B) cell proliferation at days 6 and 8, respectively. By comparison, PD98059 inhibited only 45% and 54% of CCK2R-associated (Fig. 6A) and 41% and 47% of CCK2i4svR-associated cell proliferation (Fig. 6B) at days 6 and 8.
DISCUSSION
The role of gastrin peptides in colorectal cancer is complex and multifactorial. Experimental models using the hypergastremic APCmin−/+ mice14 have demonstrated that both CCK2 receptors and gastrin are upregulated in intestinal epithelial cells early in the development of colorectal carcinogenesis, indicating a positive role for endocrine gastrin. However, clinical data have shown that most adenomatous polyp specimens coexpress gastrin peptides and CCK2 receptors in the absence of hypergastrinemia,6 suggesting that an autocrine mechanisms may be more important to tumor progression. Here we have provided evidence demonstrating neoplastic transformation of a human colorectal epithelial cell line by autocrine activation of two CCK2 receptor variants. Additionally, we have shown that gastrin precursors such as Gly-G may also contribute to the initiation of colorectal tumorigenesis through calcium imaging of the RIE/CCK2R cell line, a useful bioassay for the activation of the CCK2 receptor. Although a 100-fold concentration of Gly-G (compared to the amidated G17) is required to generate an increase in [Ca2+]i through the CCK2 receptor, these incompletely processed forms of gastrin peptide are abundantly expressed in human colonic polyps. Smith et al.,5 using immunohistochemistry to identify the expression of gastrin peptides, reported that among 55 human polyps that were examined, the prevalence for progastrin, Gly-G, and amidated G17 was 91, 80, and 47%, respectively. Our study does not specifically examine, and does not preclude, the potential contributions of progastrin or other receptors to the precursor forms of gastrin. Most importantly, we demonstrate that expression of either CCK2R or CCK2i4svR in the gastrin-producing, non-malignant, human colonic mucosa NCM356 cells, in distinct contrast to the vector-expressing cells, is sufficient to cause the cells to spread, adhere, and proliferate faster in cell culture, upregulate basal p-ERK 1,2 protein expression, and also, confer a tumorigenic phenotype in athymic nude mice.
Acquisition of the CCK2 receptor has afforded the non-tumorigenic NCM356 cell line a means to transmit gastrin-mediated, growth-stimulatory signals and to activate the MEK/ERK pathway. Perhaps, as postulated by Hanahan and Weinberg,27 the functional consequence of CCK2 receptor overexpression into the context of the NCM356 cell, is to phenocopy the actions of a mutated K-ras, an early oncogene implicated in the progression of colorectal adenoma to carcinoma in about 40% of cases.1 Thus, CCK2 receptor activation, early in the development of an adenoma, in effect, may substitute for one of the multiple effector pathways downstream of K-ras, such as activation of the Raf/MEK/ERK pathway.28 As a non-malignant polyp transitions to carcinoma, additional genetic mutations are gained, some of which may also sustain the MEK/ERK pathway, decreasing the selective pressure to maintain the gastrin-CCK2 receptor autocrine loop for cellular proliferation and survival. Carcinomas maintain genetic mutations, while epigenetic regulation of other genes, perhaps genes with redundant functions, such as the expression of CCK2 receptors, may become less sustainable, as a carcinoma progresses toward greater chromosomal instability. This theory is consistent with the finding that while the majority (81%) of adenomatous polyps examined express the CCK2 receptor,5 colorectal carcinomas only express the receptor in 30-40% cases examined.29, 30
We and others31-35 have shown that CCK2R and CCK2i4svR regulate intracellular pathways in an agonist-dependent manner involving MAPKs. Both MEK inhibitor PD98095 and PKC inhibitor GF109203X partially decreased the steady-state p-ERK levels to a similar extent in both the NCM356-CCK2R and -CCK2i4svR cells, corresponding to the MEK-mediated partial decrease in the cell growth. Partial growth inhibition was also achieved by the specific antagonist for CCK2 receptor, YM022, verifying that autocrine activation of the CCK2 receptor in the transfected cells has a role in cell growth. The possibility that an endogenous receptor, not inhibited by YM022 and activated by precursor gastrin peptides, also may be responsible for partial cell growth. The in vitro cell growth mediated by the CCK2 receptor variants was almost entirely inhibited in a PKC-dependent manner, suggesting that the MEK/ERK pathway is only partially responsible for cell growth. Furthermore, the enhanced cell growth conferred by ectopic expression of either CCK2R or CCK2i4svR, which can be almost fully attenuated with GF109203X, implicates additional growth mechanisms involving other PKC isozymes that do not utilize the MEK/ERK pathway. Future studies will delineate the specific PKC isozymes responsible for MEK/ERK-dependent and -independent mechanisms of G17-stimulated cell growth in CCK2 receptor variant-expressing NCM356 cells.
In conclusion, our results support the concept that inhibition of CCK2 receptor activation may be an important component in the prevention of adenoma to carcinoma progression in colorectal cancer. Indeed, G17-DT (Gastrimmune), an antibody therapy which neutralizes gastrin, was successful in preclinical murine studies, reducing the incidence of polyps and increasing survival.14 However, in a Phase I/II clinical trial of 50 patients with advanced colorectal cancer, Gastrimmune failed to reduce tumor burden in the later stages of disease,36 suggesting that Gastrimmune may be more relevant for use in prevention of high-risk patients. Additionally, several CCK2R antagonists have been developed and used in human studies for diagnostic imaging of and therapeutic radiotherapy of receptor-expressing tumors (reviewed in Berna, et al.37). In particular, one CCK2 receptor antagonist, JB95008 (Gastrazole) has in two clinical trials for advanced pancreatic cancer, another tumor type that aberrantly overexpresses CCK2 receptors.38 Chau and colleagues38 demonstrated a significant one-year survival benefit (log rank p=0.03) in a small trial of 18 patients. In a follow-up study of 98 patients with inoperable, advanced pancreatic cancer, however, the survival of patients treated with Gastrazole was equivalent to that of 5-fluorouracil, with a median survival of 3.6 and 4.2 months, respectively. These clinical trials were directed at late-stage colorectal and pancreatic patients, and therefore, it is not surprising that a survival benefit was not realized for either treatment. Our study supports the hypothesis that early targeting of the gastrin and the CCK2 receptor autocrine loop, perhaps at the adenomatous polyp stage with CCK2 receptor antagonists or antibodies, may be a successful therapeutic option for colorectal cancer prevention. Currently, chemoprevention with nonsteroidal anti-inflammatory drugs are very efficacious, but are not widely adopted for use due to risk of gastrointestinal bleeding or cardiovascular events.39 In contrast, both the Gastrazole the Gastrimmune trials established acceptable toxicity profiles, which do not include cardiovascular side-effects. Blockade of CCK2 receptors, therefore, may prove to be effective alternative chemopreventative agents.
Figure 7. Effects of the MEK inhibitor PD98059 (PD) and the PKC inhibitor GF109203X (GFX) on the proliferation of NCM356-CCK2R (A) and -CCK2i4svR (B) cells.
Bar graphs comparing the growth of cells in 3 different conditions are shown. 2×105 cells were plated in triplicate on day 0 and starting on day 2, treated every 48 h with inhibitor. The cells were harvested and counted by Coulter Counter on days 6 and 8: vehicle-treated media (open bars), addition of PD98059 (5 μM) (bars filled with dots), and GF109302X (5 μM) (bars filled with slashes). Data represent the average cell numbers on days 6 and 8 ± S.E.M for 3 separate experiments.
ACKNOWLEDGMENTS
We thank the James Black Foundation for the CCK2R antagonist, JB93182. We also thank Nathan To for his technical assistance, as well as Eileen Figueroa and Steve Schuenke for their assistance in the preparation of this manuscript. This work is supported by grants from the National Institutes of Health (R01—DK058119, R01—DK048345, and T32—DK007639).
Abbreviations
- (CCK2)
cholecystokinin 2
- (pERK)
phosphorylated ERK
- (APC)
adenomatous polyposis coli
- (PKC)
protein kinase C
- (MAPK)
mitogen-activated protein kinase
- (G17)
Gastrin 1-17
- (MLV)
murine leukemia retrovirus
- (MCS)
multiple cloning site
- (LTR)
long terminal repeat
- (DMSO)
dimethyl sulfoxide
- (IRES)
internal ribosome entry site
- (PCR)
polymerase chain reaction
- (CM)
conditioned media
- (H&E)
hematoxylin and eosin
- (RIE)
rat intestinal epithelial
- (JNK)
Jun N-terminal kinase
- (Gly-G)
glycine-extended gastrin
- (BK)
bradykinin
- (FBS)
fetal bovine serum
- (A)
adherent
- (NA)
non-adherent
Footnotes
Ectopic expression of CCK2 receptor splice variants in the non-tumorigenic colonic epithelial cell line, NCM356, is sufficient to promote enhanced cell adhesion, cell growth, and tumorigenesis, in part, through autocrine activation of protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) pathways. This data supports the hypothesis that CCK2 receptors and gastrin contribute to colorectal tumor development and growth.
REFERENCES
- 1.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 2.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 4.Hellmich MR, Rui XL, Hellmich HL, Fleming RY, Evers BM, Townsend CM., Jr. Human colorectal cancers express a constitutively active cholecystokinin-B/gastrin receptor that stimulates cell growth. J Biol Chem. 2000;275:32122–8. doi: 10.1074/jbc.M005754200. [DOI] [PubMed] [Google Scholar]
- 5.Smith AM, Watson SA. Gastrin and gastrin receptor activation: an early event in the adenoma-carcinoma sequence. Gut. 2000;47:820–4. doi: 10.1136/gut.47.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson SA, Morris TM, McWilliams DF, Harris J, Evans S, Smith A, Clarke PA. Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Br J Cancer. 2002;87:567–73. doi: 10.1038/sj.bjc.6600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta. 2004;1704:1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Renga M, Brandi G, Paganelli GM, Calabrese C, Papa S, Tosti A, Tomassetti P, Miglioli M, Biasco G. Rectal cell proliferation and colon cancer risk in patients with hypergastrinaemia. Gut. 1997;41:330–2. doi: 10.1136/gut.41.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobhani I, Lehy T, Laurent-Puig P, Cadiot G, Ruszniewski P, Mignon M. Chronic endogenous hypergastrinemia in humans: evidence for a mitogenic effect on the colonic mucosa. Gastroenterology. 1993;105:22–30. doi: 10.1016/0016-5085(93)90006-x. [DOI] [PubMed] [Google Scholar]
- 10.Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, Goebell H. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group. Digestion. 1999;60:210–5. doi: 10.1159/000007661. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Chute CG, Larson DE, Epstein R, Lydick EG, Melton LJ., 3rd. Risk for colorectal adenocarcinoma in pernicious anemia. A population-based cohort study. Ann Intern Med. 1989;111:738–42. doi: 10.7326/0003-4819-111-9-738. [DOI] [PubMed] [Google Scholar]
- 12.Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275–80. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- 13.Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J Clin Invest. 2000;106:533–9. doi: 10.1172/JCI9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson SA, Smith AM. Hypergastrinemia promotes adenoma progression in the APC(Min−/+) mouse model of familial adenomatous polyposis. Cancer Res. 2001;61:625–31. [PubMed] [Google Scholar]
- 15.Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev. 1999;51:745–81. [PubMed] [Google Scholar]
- 16.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–39. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 17.Singh P. Role of Annexin-II in GI cancers: interaction with gastrins/progastrins. Cancer Lett. 2007;252:19–35. doi: 10.1016/j.canlet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin G, Dubeykovskiy AN, Pritchard DM, Betz KS, Varro A, Wang TC. Inactivation of cholecystokinin-2 receptor inhibits progastrin-dependent colonic proliferation and colorectal cancer in mice. Gastroenterology. 2008;134:A115. [Google Scholar]
- 19.Stauffer JS, Manzano LA, Balch GC, Merriman RL, Tanzer LR, Moyer MP. Development and characterization of normal colonic epithelial cell lines derived from normal mucosa of patients with colon cancer. Am J Surg. 1995;169:190–5. doi: 10.1016/S0002-9610(99)80135-4. [DOI] [PubMed] [Google Scholar]
- 20.Kolokoltsov AA, Weaver SC, Davey RA. Efficient functional pseudotyping of oncoretroviral and lentiviral vectors by Venezuelan equine encephalitis virus envelope proteins. J Virol. 2005;79:756–63. doi: 10.1128/JVI.79.2.756-763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellmich MR, Battey JF, Northup JK. Selective reconstitution of gastrin-releasing peptide receptor with G alpha q. Proc Natl Acad Sci U S A. 1997;94:751–6. doi: 10.1073/pnas.94.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao C, Goluszko E, Lee YT, Kolokoltsov AA, Davey RA, Uchida T, Townsend CM, Jr., Hellmich MR. Constitutively active CCK2 receptor splice variant increases Src-dependent HIF-1 alpha expression and tumor growth. Oncogene. 2007;26:1013–9. doi: 10.1038/sj.onc.1209862. [DOI] [PubMed] [Google Scholar]
- 23.Chao C, Ives KL, Goluszko E, Kolokoltsov AA, Davey RA, Townsend CM, Jr., Hellmich MR. SRC regulates constitutive internalization and rapid resensitization of a cholecystokinin 2 receptor splice variant. J Biol Chem. 2005;280:33368–73. doi: 10.1074/jbc.M506337200. [DOI] [PubMed] [Google Scholar]
- 24.Cheng ZJ, Harikumar KG, Ding WQ, Holicky EL, Miller LJ. Analysis of the cellular and molecular mechanisms of trophic action of a misspliced form of the type B cholecystokinin receptor present in colon and pancreatic cancer. Cancer Lett. 2005;222:95–105. doi: 10.1016/j.canlet.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Hellmich MR, Hull R, Harper EA, Merchant JL. Gastrin in the New Millenniumed. MedPub Inc.; Belleville: 2004. Structure/function of the CCK2/gastrin receptor; pp. 71–92. [Google Scholar]
- 26.Todisco A, Takeuchi Y, Urumov A, Yamada J, Stepan VM, Yamada T. Molecular mechanisms for the growth factor action of gastrin. Am J Physiol. 1997;273:G891–8. doi: 10.1152/ajpgi.1997.273.4.G891. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 28.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–31. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao C, Tallman ML, Ives KL, Townsend CM, Jr., Hellmich MR. Gastrointestinal hormone receptors in primary human colorectal carcinomas. J Surg Res. 2005;129:313–21. doi: 10.1016/j.jss.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 30.Reubi JC, Waser B, Schmassmann A, Laissue JA. Receptor autoradiographic evaluation of cholecystokinin, neurotensin, somatostatin and vasoactive intestinal peptide receptors in gastro-intestinal adenocarcinoma samples: where are they really located? Int J Cancer. 1999;81:376–86. doi: 10.1002/(sici)1097-0215(19990505)81:3<376::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Dehez S, Daulhac L, Kowalski-Chauvel A, Fourmy D, Pradayrol L, Seva C. Gastrin-induced DNA synthesis requires p38-MAPK activation via PKC/Ca(2+) and Src-dependent mechanisms. FEBS Lett. 2001;496:25–30. doi: 10.1016/s0014-5793(01)02396-1. [DOI] [PubMed] [Google Scholar]
- 32.Guo YS, Cheng JZ, Jin GF, Gutkind JS, Hellmich MR, Townsend CM., Jr. Gastrin stimulates cyclooxygenase-2 expression in intestinal epithelial cells through multiple signaling pathways. Evidence for involvement of ERK5 kinase and transactivation of the epidermal growth factor receptor. J Biol Chem. 2002;277:48755–63. doi: 10.1074/jbc.M209016200. [DOI] [PubMed] [Google Scholar]
- 33.Hocker M. Molecular mechanisms of gastrin-dependent gene regulation. Ann N Y Acad Sci. 2004;1014:97–109. doi: 10.1196/annals.1294.010. [DOI] [PubMed] [Google Scholar]
- 34.Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol Gastrointest Liver Physiol. 2003;284:G75–84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- 35.Stepan VM, Dickinson CJ, del Valle J, Matsushima M, Todisco A. Cell type-specific requirement of the MAPK pathway for the growth factor action of gastrin. Am J Physiol. 1999;276:G1363–72. doi: 10.1152/ajpgi.1999.276.6.G1363. [DOI] [PubMed] [Google Scholar]
- 36.Smith AM, Justin T, Michaeli D, Watson SA. Phase I/II study of G17-DT, an anti-gastrin immunogen, in advanced colorectal cancer. Clin Cancer Res. 2000;6:4719–24. [PubMed] [Google Scholar]
- 37.Berna MJ, Tapia JA, Sancho V, Jensen RT. Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr Opin Pharmacol. 2007;7:583–92. doi: 10.1016/j.coph.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chau I, Cunningham D, Russell C, Norman AR, Kurzawinski T, Harper P, Harrison P, Middleton G, Daniels F, Hickish T, Prendeville J, Ross PJ, et al. Gastrazole (JB95008), a novel CCK2/gastrin receptor antagonist, in the treatment of advanced pancreatic cancer: results from two randomised controlled trials. Br J Cancer. 2006;94:1107–15. doi: 10.1038/sj.bjc.6603058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanghi S, MacLaughlin EJ, Jewell CW, Chaffer S, Naus PJ, Watson LE, Dostal DE. Cyclooxygenase-2 inhibitors: a painful lesson. Cardiovasc Hematol Disord Drug Targets. 2006;6:85–100. doi: 10.2174/187152906777441803. [DOI] [PubMed] [Google Scholar]