Abstract
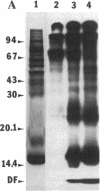
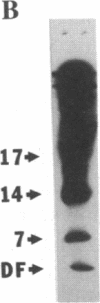
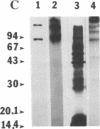
Antidiuretic hormone (ADH) increases the water permeability of the toad urinary bladder. The increase occurs in the apical plasma membrane of granular cells that line the urinary surface of the bladder and is produced by the insertion of water permeability units that have been identified by freeze-fracture electron microscopy as intramembrane particle aggregates. Under water-impermeable conditions, particle aggregates reside in intracellular vesicles called "aggrephores." In response to ADH, the aggrephores fuse with the apical plasma membrane and render it water permeable. When ADH is removed, intramembrane particle aggregates and aggrephores are retrieved from the apical membrane, and it returns to a water-impermeable state. To identify proteins involved in the water permeability response, we used lactoperoxidase/glucose oxidase to 125I-label external apical membrane proteins to compare control and ADH-treated bladders. Several polypeptides were consistently labeled in ADH-treated bladders and not in paired controls. After demonstrating that lactoperoxidase behaves as a fluid-phase marker and is sequestered in aggrephore-like vesicles when ADH is withdrawn, we used the technique of Mellman et al. [Mellman, I.S., Steinman, R. M., Unkeless, J. C. & Cohn, Z. A. (1980) J. Cell Biol. 86, 712-722] to label proteins endocytosed when water permeability declines after ADH is withdrawn to test whether the membrane proteins labeled in ADH-treated bladders behaved like particle aggregates. The internalized membranes contained polypeptides of the same molecular weights (55,000, 17,000-14,000, and 7,000) as those labeled on the apical surface of ADH-treated but not control bladders. These polypeptides are evidently involved in the ADH-stimulated water permeability response and may be components of particle aggregates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguet J., Chevalier J., Hugon J. S. Alterations in membrane-associated particle distribution during antidiuretic challenge in frog urinary bladder epithelium. Biophys J. 1976 Jun;16(6):627–639. doi: 10.1016/S0006-3495(76)85717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown D., Grosso A., DeSousa R. C. Correlation between water flow and intramembrane particle aggregates in toad epidermis. Am J Physiol. 1983 Nov;245(5 Pt 1):C334–C342. doi: 10.1152/ajpcell.1983.245.5.C334. [DOI] [PubMed] [Google Scholar]
- Courtoy P. J., Quintart J., Baudhuin P. Shift of equilibrium density induced by 3,3'-diaminobenzidine cytochemistry: a new procedure for the analysis and purification of peroxidase-containing organelles. J Cell Biol. 1984 Mar;98(3):870–876. doi: 10.1083/jcb.98.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz G., Masur S. K., Holtzman E. Quantitative analysis of exocytosis and endocytosis in the hydroosmotic response of toad bladder. J Membr Biol. 1980;52(3):221–235. doi: 10.1007/BF01869191. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Bensinger R., Orloff J. Effect of adrenergic agents on toad bladder response to ADH, 3',5'-AMP, and theophylline. Am J Physiol. 1968 Nov;215(5):1024–1031. doi: 10.1152/ajplegacy.1968.215.5.1024. [DOI] [PubMed] [Google Scholar]
- Harmanci M. C., Stern P., Kachadorian W. A., Valtin H., DiScala V. A. Vasopressin and collecting duct intramembranous particle clusters: a dose-response relationship. Am J Physiol. 1980 Dec;239(6):F560–F564. doi: 10.1152/ajprenal.1980.239.6.F560. [DOI] [PubMed] [Google Scholar]
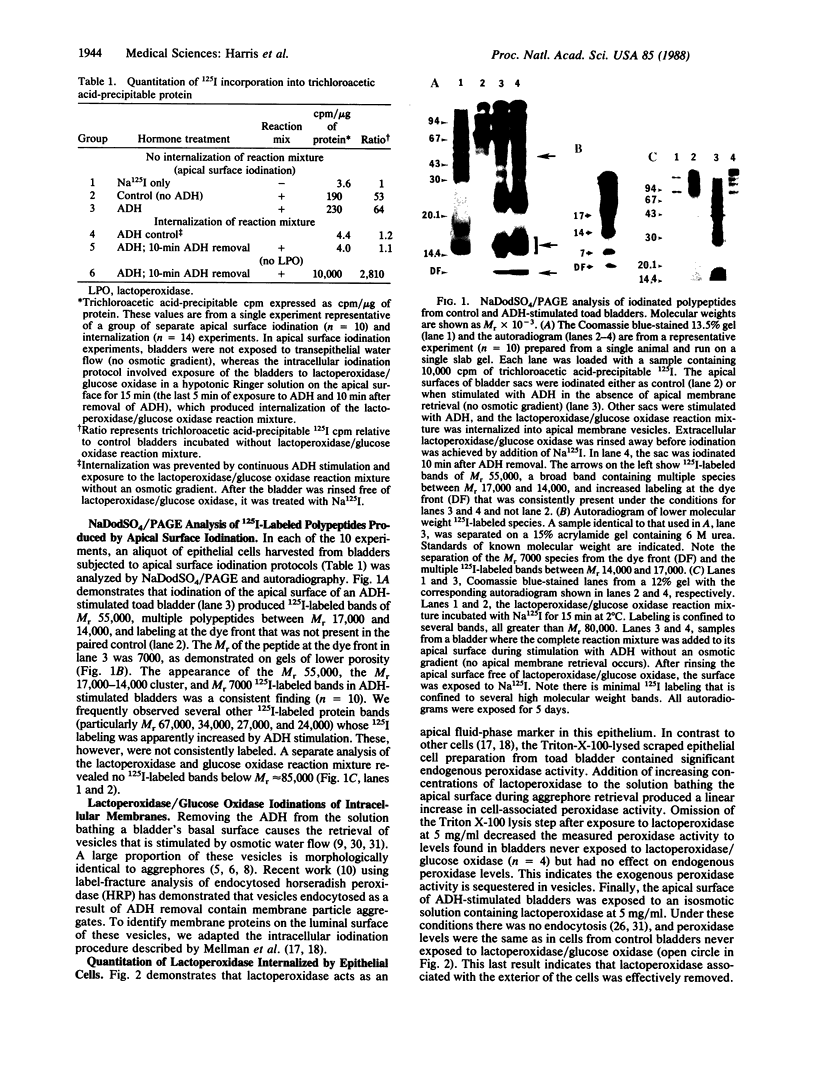
- Harris H. W., Jr, Murphy H. R., Willingham M. C., Handler J. S. Isolation and characterization of specialized regions of toad urinary bladder apical plasma membrane involved in the water permeability response to antidiuretic hormone. J Membr Biol. 1987;96(2):175–186. doi: 10.1007/BF01869243. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Wade J. B., Handler J. S. Fluorescent markers to study membrane retrieval in antidiuretic hormone-treated toad urinary bladder. Am J Physiol. 1986 Aug;251(2 Pt 1):C274–C284. doi: 10.1152/ajpcell.1986.251.2.C274. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Wade J. B., Handler J. S. Transepithelial water flow regulates apical membrane retrieval in antidiuretic hormone-stimulated toad urinary bladder. J Clin Invest. 1986 Sep;78(3):703–712. doi: 10.1172/JCI112630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert F., Montesano R., Grosso A., de Sousa R. C., Orci L. Particle aggregates in plasma and intracellular membranes of toad bladder (granular cell). Experientia. 1977 Oct 15;33(10):1364–1367. doi: 10.1007/BF01920184. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine S. D., Jacoby M., Finkelstein A. The water permeability of toad urinary bladder. II. The value of Pf/Pd(w) for the antidiuretic hormone-induced water permeation pathway. J Gen Physiol. 1984 Apr;83(4):543–561. doi: 10.1085/jgp.83.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur S. K., Cooper S., Rubin M. S. Effect of an osmotic gradient on antidiuretic hormone-induced endocytosis and hydroosmosis in the toad urinary bladder. Am J Physiol. 1984 Aug;247(2 Pt 2):F370–F379. doi: 10.1152/ajprenal.1984.247.2.F370. [DOI] [PubMed] [Google Scholar]
- Mellman I. S., Steinman R. M., Unkeless J. C., Cohn Z. A. Selective iodination and polypeptide composition of pinocytic vesicles. J Cell Biol. 1980 Sep;86(3):712–722. doi: 10.1083/jcb.86.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Galloway C. J. Selective labeling and quantitative analysis of internalized plasma membrane. Methods Enzymol. 1983;98:545–555. doi: 10.1016/0076-6879(83)98181-8. [DOI] [PubMed] [Google Scholar]
- Muller J., Kachadorian W. A. Aggregate-carrying membranes during ADH stimulation and washout in toad bladder. Am J Physiol. 1984 Jul;247(1 Pt 1):C90–C98. doi: 10.1152/ajpcell.1984.247.1.C90. [DOI] [PubMed] [Google Scholar]
- Muller J., Kachadorian W. A., DiScala V. A. Evidence that ADH-stimulated intramembrane particle aggregates are transferred from cytoplasmic to luminal membranes in toad bladder epithelial cells. J Cell Biol. 1980 Apr;85(1):83–95. doi: 10.1083/jcb.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Kachadorian W. A. Regulation of luminal membrane water permeability by water flow in toad urinary bladder. Biol Cell. 1985;55(3):219–224. doi: 10.1111/j.1768-322x.1985.tb00429.x. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Lorenzen M. Antidiuretic hormone-dependent membrane capacitance and water permeability in the toad urinary bladder. Am J Physiol. 1983 Feb;244(2):F195–F204. doi: 10.1152/ajprenal.1983.244.2.F195. [DOI] [PubMed] [Google Scholar]
- Rodriguez H. J., Edelman I. S. Radio-iodination of plasma membranes of toad bladder epithelium. J Membr Biol. 1979 Apr 9;45(3-4):185–214. doi: 10.1007/BF01869285. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Tilles S., Condeelis J., Carboni J., Meiteles L., Franki N., Bolon R., Robertson C., Hays R. M. Electron-microscopic study of the apical region of the toad bladder epithelial cell. Am J Physiol. 1984 Sep;247(3 Pt 1):C268–C281. doi: 10.1152/ajpcell.1984.247.3.C268. [DOI] [PubMed] [Google Scholar]
- Scott W. N., Slatin S. L. Alterations in lactoperoxidase-catalyzed radio-iodination of membrane proteins associated with vasopressin-induced changes in tissue permeability to water. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1038–1044. doi: 10.1016/0006-291x(79)91984-3. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Baron D. A., Sato A., Schulte B. A. Variability of cell surface glycoconjugates--relation to differences in cell function. J Histochem Cytochem. 1981 Sep;29(9):994–1002. doi: 10.1177/29.9.6169762. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum J. M., Edelman I. S. Iodination (125I) of the apical plasma membrane of toad bladder epithelium: electron-microscopic autoradiography and physiological effects. J Membr Biol. 1973 Dec 6;14(1):17–32. doi: 10.1007/BF01868066. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Wade J. B. Membrane structural specialization of the toad urinary bladder revealed by the freeze-fracture technique. III. Location, structure and vasopressin dependence of intramembrane particle arrays. J Membr Biol. 1978;40(Spec No):281–296. doi: 10.1007/BF02026011. [DOI] [PubMed] [Google Scholar]
- Wade J. B. Membrane structural studies of the action of vasopressin. Fed Proc. 1985 Aug;44(11):2687–2692. [PubMed] [Google Scholar]
- Wade J. B., Stetson D. L., Lewis S. A. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]