Abstract
We have developed a method to analyze the relative contributions of pre- and postsynaptic actions of a particular gene product in neurons in culture and potentially in slices using adenovirus-mediated gene transfer. A recombinant virus directed the expression of both a GFP reporter protein and TrkB.T1, a C-terminal truncated dominant negative TrkB neurotrophin receptor. When expressed in the presynaptic cell at synapses between embryonic hippocampal neurons in culture, the dominant negative TrkB.T1 inhibited two forms of synaptic potentiation induced by the neurotrophin brain-derived neurotrophic factor (BDNF): (i) greater evoked synaptic transmission and (ii) higher frequency of spontaneous miniature synaptic currents. These inhibition effects are not seen if the transgene is expressed only in the postsynaptic cell. We conclude that BDNF-TrkB signal transduction in the presynaptic terminal leads to both types of potentiation and is therefore the primary cause of synaptic enhancement by BDNF in these neurons.
The neurotrophins, including nerve growth factor, brain-derived neurotrophic factor (BDNF), and neurotrophin-3, have recently been shown to enhance transmission at central and peripheral synapses within minutes of application (1–6). Neurotrophins exert their effects on synaptic strength by interacting with the Trk family of receptor Tyr kinases (1, 3, 5, 7, 8). In experiments reporting the effect of BDNF on enhancing synaptic transmission in cultured cells, the data have been interpreted to favor a primarily presynaptic (refs. 1 and 2; Y.-X.L., Y. Zhang, H.A.L., E.M.S., and N.D., unpublished data) or a primarily postsynaptic (4) site for the BDNF/TrkB interaction. The results of Kang and Schuman (in acute hippocampal slices from adult rats) (3, 9) are most plausibly interpreted as indicating both pre- and postsynaptic actions.
It is therefore of considerable interest to develop a method for inhibiting TrkB receptors selectively, either pre- or postsynaptically. We describe such a method and report its use to determine that the primary site of BDNF action for synapses between E18 hippocampal neurons in culture is with presynaptic TrkB receptors.
MATERIALS AND METHODS
Cell Culture.
Pregnant Wistar rats were euthanized by inhalation of CO2, and embryos were immediately removed by cesarean section. Hippocampi were rapidly removed under stereomicroscopic observation under sterile conditions, cut into 1-mm pieces, and digested with 0.25% trypsin/0.25 mg/ml DNase (Sigma) in Hanks’ balanced salt solution without calcium or magnesium (GIBCO) at 36°C for 15 min. The pieces were then gently rinsed in Hanks’ balanced salt solution, washed twice in plating medium [Neurobasal with B27 supplement (GIBCO), with 500 μM Glutamax (GIBCO), and 25 μM glutamate], and gently triturated in 1 ml of plating medium with five passes through the 0.78-mm opening of a blue tip of a P-1000 Pipetman. Suspended cells were decanted and the remaining pieces were triturated once more. Cell suspensions were gravity filtered through a 70-μm nylon mesh (Falcon) to remove large debris and centrifuged for 2 min at 150 × g. Pellets were suspended by gentle trituration as described above. Approximately 35,000 cells were plated in an area 15 mm in diameter at the middle of a 35-mm plastic culture dish (Corning) that had been coated with poly-d-lysine and laminin. Cultures were maintained at 36°C in a 5% CO2 incubator. After the plated neurons had settled, a hippocampal glial cell layer, plated on a Thermonox slide and prepared essentially as described by Goslin and Banker (10), was placed over the culture and maintained there until the culture was used for viral infection. About 2 days later, one-half of the medium over the neurons was replaced with culture medium (Neurobasal with B27 supplement and 500 μM Glutamax), to which 4 μM cytosine arabinoside had been added. One-half of the medium was replaced weekly thereafter. Viral infection and recording was done after about 2 wk in culture.
Electrophysiological Recording.
Prior to recording, a dish was removed from the incubator and the culture medium was replaced with recording saline: NaCl, 110 mM; KCl, 5.4 mM; CaCl2, 1.8 mM; MgCl2, 0.8 mM; Hepes, 10 mM; d-glucose, 10 mM; and picrotoxin, 0.1 mM, titrated to pH 7.4 with NaOH, and osmolarity was adjusted to 230 mOsmol with sucrose. Dual or single whole-cell recordings were made at room temperature with pipettes pulled in four stages from 1.5-mm o.d. glass capillary tubes (WPI, Sarasota, FL) with a P-80/PC micropipette puller (Sutter Instruments, Novato, CA). Patch pipettes were filled with a solution containing: potassium gluconate, 100 mM; CaCl2, 0.1 mM; EGTA, 1.1 mM; MgCl2, 5 mM; Hepes, 10 mM; ATP, 3 mM; phosphocreatine, 3.0 mM; and GTP, 0.3 mM, pH 7.2 (215 mOsmol). Ionic currents or voltages were measured with patch-clamp amplifiers (Axopatch 200 and Axopatch-1D; Axon Instruments, Foster City, CA), filtered at 2 kHz, digitized at 10 kHz, and recorded on a computer (pCLAMP 6; Axon Instruments) and monitored on both a storage oscilloscope and a chart recorder. Synaptic activities were simultaneously recorded digitally at 9 kHz on pulse–code-modulated magnetic tape (Instrutech VR-100; Instrutech, Great Neck, NY) for off-line analysis. The series resistance was monitored by measuring the instantaneous current in response to a 5-mV voltage step command. Results were discarded if the series resistance changed by more than 10% during the course of an experiment.
In the presence of 100 nM tetrodotoxin, miniature excitatory postsynaptic currents (mEPSCs) were recorded. The mEPSCs were detected and measured by FETCHEX in the pCLAMP6 series (Axon Instruments). Threshold detection levels were between 3 and 10 pA (with lower series resistance, the size of both the noise and signal was larger, thus requiring a higher detection threshold (≈10 pA). The selected threshold level remained constant for the duration of the experiment. All events larger than this level were included if they had rise times <3 ms and had a waveform similar to that of the evoked EPSC. Events that began during the decay phase of a previous event were not included in the amplitude measurements but were included in the frequency measurements. The frequency of mEPSCs was measured over 30-s intervals. For dual whole-cell recording, both presynaptic and postsynaptic cells were voltage clamped at a holding potential of −70 mV. When the presynaptic neuron was stimulated with an 8-ms positive voltage step (to −20 mV or −10 mV), a large (several nA) transient inward current was activated in the presynaptic cell and an EPSC in the postsynaptic neuron. Depolarizing currents were injected every 15 s, except in experiments on reciprocally connected neurons. For that case, the time interval was 12 s, and pre- and postsynaptic neurons were switched every minute. We studied only monosynaptic EPSCs as judged by two criteria: (i) short and constant latency (<5 ms) and (ii) a waveform with a single peak (11). Values reported in the text and figure legends are mean ± SEM. Statistical comparisons were performed using paired and unpaired t tests to analyze differences within an experiment and between experimental groups, respectively. P values reported in brackets in the text represent significance levels for paired t tests performed on pre- versus post-BDNF values. P values >0.05 were considered not significant (NS) and are indicated.
Our general methods for preparing recombinant Ad viruses, including the specific pAC plasmid used as an adenovirus transfer plasmid and the viruses with which they are recombined, have been described (12). The TrkB cDNA was a gift from George Yancopoulos (Regeneron Pharmaceuticals, Inc., Tarrytown, NY). It contained sequences from nucleotides 253 to 3631 of the GenBank Sequence (accession no. M55291), with the initiating ATG at 664 and the termination codon at 3127. The TrkB.T1 cDNA was a gift from Tony Hunter (Salk Institute, San Diego, CA) and has been described (13). Each cDNA was cloned in the pAC transfer vector. The trkB cDNA was then recombined with AdPacI and the TrkB.T1 cDNA with Ad PacI-GFP as described (12). To assess the dominant negative activity of Ad-TrkB.T1-GFP, Chinese hamster ovary cells were coinfected with 1 × 107 plaque-forming units (pfus)/ml of Ad-TrkB and 1 × 108 pfus/ml Ad-ΔE1-GFP or Ad-TrkB.T1-GFP for 2 hr. Forty-eight hours later cells were treated with 100 ng/ml BDNF for 15 min. Following BDNF treatment, immunoprecipitation with a TrkB antibody (gift from Andy Welcher, Amgen, Thousand Oaks, CA) was performed followed by Western blot analysis with an anti-phosphotyrosine antibody.
For electrophysiology experiments, E18 hippocampal neurons were infected by either 3 × 107 or 3 × 108 pfus/ml adenovirus (either Ad-ΔE1-GFP or Ad-TrkB.T1-GFP) for 2 hr in the absence of the glial overlayer. After 2 hr, the viral suspension was diluted by 50% with culture medium and infection was allowed to continue for an additional 12–16 hr. Electrophysiological experiments were performed 24–48 hr later.
RESULTS
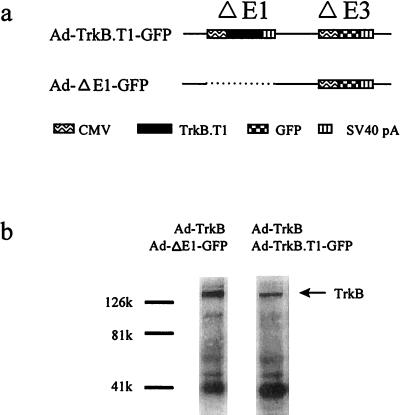
To assess the relative contributions of pre- and postsynaptic neurons to BDNF-induced potentiation in cultured hippocampal neurons, we constructed an adenovirus vector, Ad-TrkB.T1-GFP, expressing cDNAs for both a truncated TrkB receptor (12–14) and a GFP reporter (Fig. 1a). Previous studies show that this truncated TrkB receptor acts as a dominant negative (15). We verified the dominant negative activity of the Ad-TrkB.T1-GFP virus by examining its ability to inhibit BDNF-induced phosphorylation of the TrkB receptor. Chinese hamster ovary cells coinfected with two adenoviruses, one expressing TrkB (Ad-TrkB) and another containing the control virus (Ad-ΔE1-GFP) at a ratio of 1:10, exhibited robust BDNF-induced TrkB phosphorylation (Fig. 1b). In contrast, cells infected with Ad-TrkB and Ad-TrkB.T1-GFP (ratio of 1:10) exhibited significantly reduced TrkB phosphorylation (Fig. 1b), indicating that expression of the truncated Trk receptor can inhibit the ability of full-length TrkB to respond to BDNF. We believe that more complete T1-mediated inhibition of TrkB phosphorylation likely occurs in neurons, owing to the lower amounts of native, endogenous TrkB.
Figure 1.
Ad-TrkB.T1-GFP inhibits BDNF-induced phosphorylation of TrkB. (a) Viral constructs: In Ad-TrkB.T1-GFP, a truncated TrkB receptor expression cassette was inserted into the E1 region of an adenovirus vector containing a GFP expression cassette in the E3 region. In the control Ad-ΔE1-GFP, the GFP gene was inserted into the E3 region of an adenovirus vector deleted in the E1 region. (b) Antiphosphotyrosine blots of anti-TrkB immunoprecipitated fractions from Chinese hamster ovary cells infected with Ad-TrkB and Ad-ΔE1-GFP. The full-length TrkB receptor is detected near 130,000. Treatment with Ad-TrkB.T1-GFP substantially reduced the amount of phosphorylated TrkB receptor detected.
The basic experimental design is illustrated at the top of Figs. 2–5. Dual whole-cell recordings were made from two cells that were close and appeared to be synaptically connected. For dual whole-cell recording, both presynaptic and postsynaptic cells were voltage clamped at a holding potential of −70 mV. When the presynaptic neuron was stimulated with an 8-ms positive voltage step (to −20 mV or −10 mV) every 15 s, an inward current was induced in the presynaptic cell due to Na+ channel activation, and an EPSC was elicited in the postsynaptic neuron (Fig. 2c, Lower and Upper, respectively). Only monosynaptic EPSCs were studied. The criteria for recognizing monosynaptic EPSCs are given in Materials and Methods.
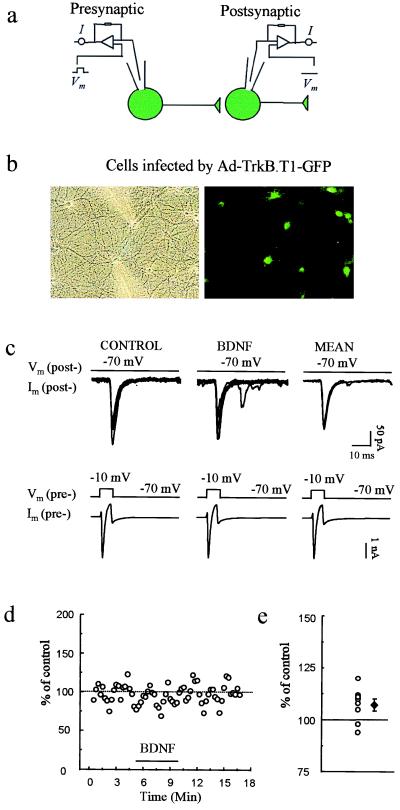
Figure 2.
Infection by the control Ad-ΔE1-GFP reporter virus allows BDNF-induced potentiation of evoked synaptic transmission. (a) Scheme of recording arrangement indicating that whole-cell recordings were obtained from two synaptically connected neurons, both expressing GFP. (b) Phase-contrast and fluorescence images of GFP-expressing cells during recordings. (c) Representative voltage-clamp currents in presynaptic (Lower) and postsynaptic (Upper) cells; the postsynaptic traces show EPSCs. Recordings are shown before (Control) and during exposure to BDNF (3 min after the start of BDNF perfusion). Traces labeled MEAN are averaged from eight consecutive EPSCs before or after BDNF application. The EPSC amplitude before and during BDNF application was 103.5 ± 19.1 and 147.2 ± 28.6 pA, respectively (mean ± SEM). (d) Plot of EPSC amplitude in an exemplar experiment before, during, and after BDNF application. (e) Percentage of potentiation (at 3 min after start of perfusion with BDNF) for each of the nine individual pairs studied (○). ♦, mean and SEM.
Figure 5.
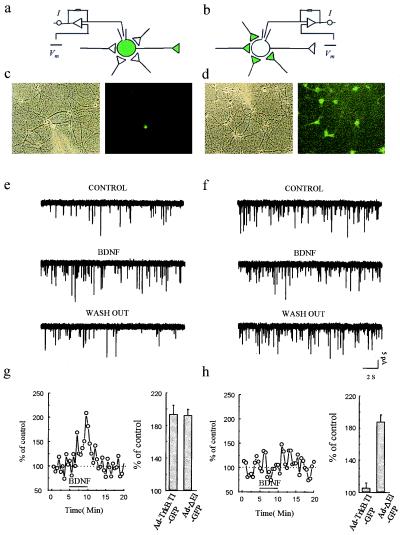
Expression of Ad-TrkB.T1-GFP in presynaptic but not postsynaptic neurons inhibits BDNF-induced increases in mEPSC frequency. (a and b) Schemes of recording arrangement indicating that whole-cell recordings were obtained from single neurons that were either (a) infected with Ad-TrkB.T1-GFP and innervated by many uninfected neurons or (b) uninfected but innervated by many infected neurons. (c and d) bright-field and fluorescence images of GFP-expressing cells during recording schematized in a and b, respectively. (e) Representative sweeps containing mEPSCs before, during, and after BDNF application in an exemplar experiment schematized in a. (g) Plot of mEPSC frequency before, during, and after BDNF application in an experiment like that shown in e. The mean percentage of control is expressed relative to values obtained from uninfected cultures at 3 min after the start of BDNF perfusion. (f) Representative sweeps containing mEPSCs before, during, and after BDNF application in an exemplar experiment schematized in b. (h) Plot of mEPSC frequency before, during, and after BDNF application in an experiments like that in f. Mean percentage of control is expressed relative to values obtained from uninfected cultures at 3 min after the start of BDNF perfusion.
At the time the present work was initiated, we had already observed (Y.-X.L., Y. Zhang, H.A.L., E.M.S., and N.D., unpublished data) that application of BDNF (100 ng/ml) to synaptically connected pairs of cultured neurons results in a rapid increase in evoked synaptic transmission. Typical results are illustrated in Fig. 2 c–e. These observations were after infection of both cells with the control virus Ad-ΔE1-GFP, but identical results were observed for uninfected cells (data not shown; Y.-X.L., Y. Zhang, H.A.L., E.M.S., and N.D., unpublished data). Specifically, six of nine synaptically coupled pairs of neurons exhibited BDNF-induced potentiation which did not differ in magnitude or duration from our previous study of uninfected neurons [mean percentage of baseline: 144.1 ± 11.5%, P ≤ 0.01 (n = 9)] (Y.-X.L. et al., unpublished data).
To provide decisive results on pre- versus postsynaptic actions, we conducted experiments in which only some of the neurons in the culture were infected. A dish was infected with Ad-TrkB.T1-GFP at a titer of either 3 × 107 or 3 × 108 pfus/ml, leading, respectively, to either a small or a large fraction of infected neurons (i.e., expressing T1 and GFP). The effect of adding BDNF on the evoked synaptic current or on the minifrequency, both recorded in the postsynaptic cell, was then observed.
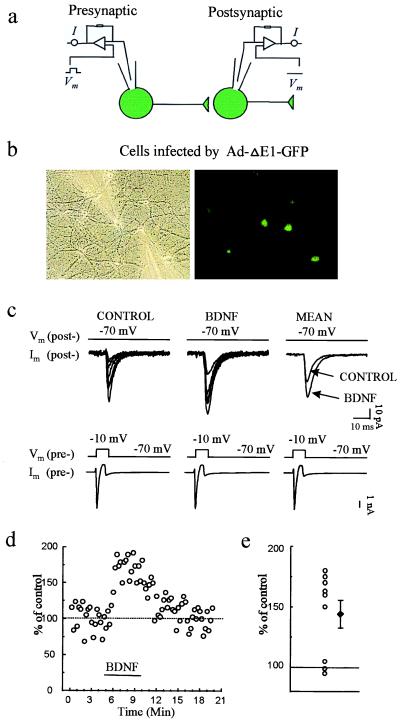
Experiments like those shown in Fig. 3 were conducted on cultures infected with Ad-TrkB.T1-GFP at the higher titer, so that both members of a synaptically connected pair were frequently infected. In contrast to the results with infection by the control virus (Fig. 2), BDNF failed to enhance synaptic transmission significantly in any of the dually infected neuron pairs recorded (Fig. 3) [mean percentage of baseline: 107.3 ± 2.9%, NS (n = 8)]. The magnitude of potentiation observed in T1-infected neurons was significantly less than in Ad-ΔE1-GFP-infected neurons (P < 0.01). This finding indicates that TrkB is required for BDNF to initiate synaptic potentiation between cultured neurons, as has been observed in studies in hippocampal slices (7, 8), but does not distinguish whether presynaptic or postsynaptic TrkB receptors or both are required for this plasticity.
Figure 3.
Ad-TrkB.T1-GFP infection of both pre- and postsynaptic neurons prevents BDNF-induced potentiation of evoked synaptic transmission. (a) Scheme of recording arrangement indicating that whole-cell recordings were obtained from two synaptically connected neurons, both infected with Ad-TrkB.T1-GFP. (b) Phase-contrast and fluorescence images of GFP-expressing cells during recordings. (c) Representative individual and averaged pre- and postsynaptic traces, as in Fig. 2, showing EPSCs elicited before (CONTROL) and during BDNF application. The mean EPSC amplitude before and after BDNF application was 94.3 ± 21.9 and 98.1 ± 21.3 pA, respectively. (d) Plot of EPSC amplitude in an exemplar experiment before, during, and after BDNF application. (e) Percentage of potentiation (at 3 min after the start of perfusion with BDNF) for each of the eight pairs studied (○). ♦, mean and SEM.
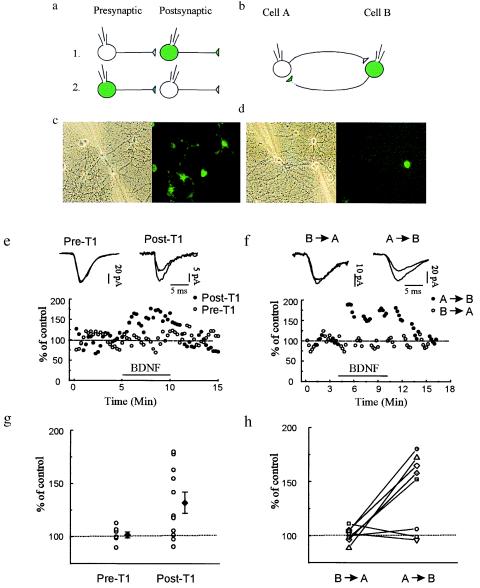
To determine whether BDNF signal transduction occurs pre- or postsynaptically, we recorded from synaptically coupled neurons in which either the pre- or postsynaptic cell was infected with Ad-TrkB.T1-GFP and the synaptic partner was uninfected. In such pairs in which the postsynaptic neuron was infected with Ad-TrkB.T1-GFP, BDNF application produced an increase in evoked synaptic responses comparable to that observed in controls (Fig. 4 e and g) [mean percentage of baseline: 132.1 ± 9.9%, P ≤ 0.05 (n = 11)]. Seven of these 11 pairs exhibited a potentiation of at least 15% above baseline levels. In seven other pairs, only the presynaptic neuron was infected with Ad-TrkB.T1-GFP; none of these pairs exhibited significant potentiation (Fig. 4 e and g) [mean percentage of baseline: 101.9 ± 2.8%, NS (n = 7)] (P ≤ 0.05). These results show that TrkB signaling in the presynaptic neuron is required for BDNF-induced potentiation.
Figure 4.
Infection of presynaptic, but not postsynaptic, neurons with Ad-TrkB.T1-GFP prevents BDNF induced potentiation of evoked synaptic transmission. Whole-cell recordings were obtained from 26 pairs of synaptically connected neurons comprising one infected neuron (shown as green) and one noninfected neuron (shown as clear). (a) Scheme of recording arrangement for 18 of the pairs. In seven pairs, the infected neuron was presynaptic; in the other 11 pairs, the infected cell was postsynaptic. (b) Scheme of the recording arrangement for eight other pairs that were reciprocally connected; depolarizing pulses were applied alternatively in each cell, eliciting EPSCs that were measured in the other cell. (c and d) Phase-contrast and fluorescence images of exemplar fields during the recordings schematized in a and b, respectively. (e) Either the pre- (Pre-T1, ○) or postsynaptic (Post-T1, •) neuron was infected with Ad-TrkB.T1-GFP. When the infected neuron was postsynaptic BDNF potentiated the evoked response. Interleaved recordings in which the infected neuron was now presynaptic, revealed that BDNF failed to potentiate evoked transmission. The mean EPSC amplitude before and after BDNF application was 111.8 ± 17.6 and 152.2 ± 26.0 pA, respectively, for pairs with Post-T1. The mean EPSC amplitude before and after BDNF application was 99.9 ± 24.0 and 100.7 ± 24.4 pA, respectively, for pairs with Pre-T1 neurons. (g) Percentage of potentiation (at 3 min after the start of BDNF perfusion) for each pair (○). ♦, mean and SEM. (f) Plot of EPSC amplitude for an exemplar experiment before, during, and after BDNF application for the reciprocally connected pairs schematized in b. During BDNF application, transmission was potentiated when the infected neuron was postsynaptic (•, A → B), but not when the infected neuron was presynaptic, even though the tests were performed during interleaved 1-min intervals. The mean EPSC amplitude before and after BDNF application was 111.8 ± 17.6 and 152.2 ± 26.0 pA, respectively, for stimuli applied to the nonexpressing neuron and recorded postsynaptically in the T1-expressing neuron (A → B). The mean EPSC amplitude before and after BDNF application was 99.9 ± 24.0 and 100.7 ± 24.4 pA, respectively, for stimuli applied to the T-expressing neuron and recorded in the nonexpressing postsynaptic neuron (B → A). (h) Mean percentage of potentiation (at 3 min after start of BDNF perfusion) for each individual experiment with the reciprocally connected pairs.
This conclusion is reinforced by an additional set of experiments on neuron pairs that (i) fortuitously displayed reciprocal synaptic connectivity and (ii) comprised one uninfected and one Ad-TrkB.T1-GFP-infected neuron. With this recording arrangement, we monitored the strength of the synaptic connection in both directions and hence determined the effects of pre- versus postsynaptic T1 expression in the pair of neurons. During 1-min intervals, we (i) alternately stimulated the neuron expressing T1 (“cell B”) and recorded the response in the uninfected cell (“cell A”) or (ii) stimulated cell B and recorded the postsynaptic response in cell A (see Fig. 4). These measurements were carried out before, during, and after washout of applied BDNF. Similar to the previous set of experiments, when the T1-expressing neuron was presynaptic BDNF failed to produce synaptic enhancement (Fig. 4 f and h) [mean percentage of baseline: 100.8 ± 2.4%, NS (n = 8)]. When the same T1-expressing cell was postsynaptic, however, it usually (five of eight experiments) exhibited potentiated EPSCs following application of BDNF (Fig. 4 f and h) [mean percentage of baseline: 141.1 ± 12.3%, P ≤ 0.05 (n = 8)]. The magnitude of potentiation observed when the infected neuron was postsynaptic was significantly greater than when it was presynaptic (P ≤ 0.05). These experiments add strength to the conclusion that signaling occurs through presynaptic rather than postsynaptic TrkB receptors.
Another potent effect of BDNF on hippocampal synaptic transmission is the enhancement of the frequency, but not the amplitude, of mEPSCs in cultured neurons (Y.-X.L., Y. Zhang, H.A.L., E.M.S., and N.D., unpublished data). Although increases in mEPSC frequency have traditionally been interpreted as reflecting a presynaptic change (16), recent studies (17–19) have suggested that the uncovering of previously silent postsynaptic receptors might represent a plausible alternative interpretation. We have tested the synaptic locus of BDNF signaling in mEPSC frequency enhancement by expressing T1 in either a postsynaptic or a presynaptic compartment. In the first set of experiments, we examined transmission for cases in which a single neuron expressing T1 (or GFP alone) was surrounded by many uninfected neurons. We obtained a whole-cell recording from the T1-expressing neuron and monitored mEPSC frequency before and after the application of BDNF. We found that BDNF still enhanced mEPSC frequency when the postsynaptic neuron was infected with Ad-TrkB.T1-GFP (Fig. 5 e and g) [mean percentage of baseline: 194.0 ± 10.6%, P ≤ 0.001 (n = 6)]. The enhancement observed in postsynaptic T1-expressing neurons was indistinguishable from that observed when postsynaptic neurons were infected with the reporter gene alone, Ad-ΔE1-GFP (Fig. 5g) [mean percentage of baseline: 192.8 ± 7.2%, P ≤ 0.05 (n = 6)].
In complementary experiments, we recorded from an uninfected postsynaptic neuron innervated by many presynaptic neurons infected with Ad-TrkB.T1-GFP (Fig. 5b). When many of the presynaptic neurons were expressing T1, BDNF failed to enhance frequency [mean percentage of baseline: 105.7 ± 6.1%, NS (n = 6)] (Fig. 5 f and h). In control experiments, postsynaptic neurons innervated by presynaptic neurons expressing Ad-ΔE1-GFP exhibited robust BDNF-induced potentiation of mEPSC frequency [mean percentage of baseline: 188.2 ± 2.2%, P ≤ 0.05 (n = 6)]. The increase in mEPSC frequency observed when presynaptic neurons were infected with the control virus, Ad-ΔE1-GFP, was significantly greater than that observed when presynaptic neurons were infected with Ad-TrkB.T1-GFP (P ≤ 0.05). These data demonstrate that presynaptic but not postsynaptic TrkB is critical for BDNF-induced increases in frequency.
In summary, the expression of a dominant negative TrkB in either pre- or postsynaptic embryonic neurons shows that applied BDNF transiently potentiates both evoked and spontaneous synaptic transmission by interacting with presynaptic Trk B receptors.
DISCUSSION
Together with our previous results (Y.-X.L., Y. Zhang, H.A.L., E.M.S., and N.D., unpublished data), the data presented here indicate that the primary synaptic action of BDNF in E18 hippocampal cultures is to augment presynaptic function. BDNF does not appear to alter the size of miniature synaptic events nor the size of the postsynaptic response to exogenous glutamate (Y.-X.L., Y. Zhang, H.A.L., E.M.S., and N.D., unpublished data). We note that under somewhat different culture conditions, Levine et al. (4) report that postsynaptic injection of the receptor Tyr kinase inhibitor, K252a, prevented the synaptic action of BDNF. This experiment was taken to indicate that the primary locus of action was postsynaptic. The differing results may be due to differences in the culture conditions; alternatively, it is possible that because K252a is membrane permeant, it diffused from the injected postsynaptic cell and affected the TrkB receptors or downstream Tyr kinases in the adjoining presynaptic membranes.
The effects of BDNF on synaptic transmission in slices from adult rats clearly differ from those observed here for cultured E18 neurons. In slices it has been observed that protein synthesis, which is probably postsynaptic, is required for BDNF’s actions (9), while there is likely also a presynaptic change in synaptic efficacy (3). In contrast, protein synthesis is not required for BDNF’s actions on synaptic transmission in cultured hippocampal neurons (Y.-X.L., Y. Zhang, H.A.L., E.M.S., and N.D., unpublished data). This difference in synaptic locus in developing versus mature synapses cannot be explained by the absence of TrkB in developing dendrites, because we (H. R. Li, personal communication) and others (20, 21) have observed TrkB in dendrites of cultured hippocampal neurons. [Consistent with the results reported here, we also observed immunostaining for TrkB in axonal projections (H. R. Li, personal communication)].
In the experiments of Schuman and Kang (3, 9), the observed long-lasting enhancement of synaptic transmission was produced by application of BDNF for about 20 min. When we applied BDNF for 20 min to neurons in culture, synaptic enhancement decayed slightly over the time period and recovered to baseline within 10 min of washout (data not shown). In effect, the results for the 20-min application of BDNF did not substantially differ from those for 5-min applications.
We also wish to note that a full understanding of the BDNF/TrkB interaction is important for the study of long-term potentiation (LTP). Specifically, Kang et al. (8) showed the function-blocking antibodies to TrkB caused a reduction in LTP from about 20 min after tetanus. These effects suggest that activity-dependent BDNF secretion plays a role in LTP. Several other recent studies also demonstrate the importance of the BDNF/TrkB interaction in LTP (22–24). Figurov et al. (22) suggest that in part the BDNF effect on LTP is presynaptic, as did Kang and Schuman (3) for the effect of BDNF on synaptic transmission in adult slices.
Finally, we suggest that the method used here for analyzing the relative pre- and postsynaptic effects of a particular gene product in synaptic function will find many useful applications in studies of synaptic function of neurons in culture and in slices.
Acknowledgments
We thank Dr. Andy Welcher (Amgen) for the generous gift of BDNF and TrkB antibody. We thank Sheri McKinney for tissue culture work. This work was supported by National Institutes of Health Grant MH-49176. E.M.S. is a Pew Biomedical Scholar, Beckman Young Investigator, Merck Scholar, and an Assistant Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- LTP
long-term potentiation
- mEPSC
miniature excitatory postsynaptic current
References
- 1.Lohof A M, Ip N, Poo M-M. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 2.LeBmann V, Gottmann K, Heumann R. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 3.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 4.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8078. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo D C. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 6.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 7.Kang H, Jia L Z, Suh K-Y, Tang L, Schuman E M. Learn Mem. 1996;3:188–196. doi: 10.1101/lm.3.2-3.188. [DOI] [PubMed] [Google Scholar]
- 8.Kang H, Shelton D, Welcher A, Schuman E M. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 9.Kang H, Schuman E M. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 10.Goslin K, Banker G. In: Culturing Nerve Cells. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 1991. pp. 251–282. [Google Scholar]
- 11.Arancio O, Kandel E R, Hawkins R D. Nature (London) 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- 12.Ehrengruber M U, Lanzrein M, Xu Y, Jasek M, Kanter D B, Schuman E M, Lester H A, Davidson N. Methods Enzymol. 1998;293:483–503. doi: 10.1016/s0076-6879(98)93030-0. [DOI] [PubMed] [Google Scholar]
- 13.Middlemas D S, Lindberg R A, Hunter T. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein R, Conway D, Parada L F, Barbacid M. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- 15.Eide F F, Lowenstein D H, Reichardt L F. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fatt P, Katz B. J Physiol (London) 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 17.Kullman D M. Neuron. 1994;12:1111–1120. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 18.Isaac J T, Nicoll R A, Malenka R C. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Liao S, Hessler N A, Malinow R. Nature (London) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 20.Wu K, Xu J L, Suen P C, Levine E, Huang Y Y, Mount H T, Lin S Y, Black I B. Mol Brain Res. 1996;43:286–290. doi: 10.1016/s0169-328x(96)00211-2. [DOI] [PubMed] [Google Scholar]
- 21.Tongiorgi E, Righi M, Cattaneo A. J Neurosci. 1997;17:9492–9505. doi: 10.1523/JNEUROSCI.17-24-09492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figurov A, Pozzomiller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 23.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson S L, Abel T, Deuel T A S, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]