Abstract
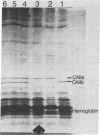
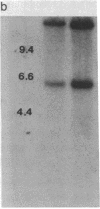
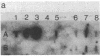
Electrophoretic screening of (C57BL/6J x DBA/2J)F1 progeny of male mice treated with N-ethyl-N-nitrosourea revealed a mouse that lacked the paternal carbonic anhydrase II (CA II). Breeding tests showed that this trait was heritable and due to a null mutation at the Car-2 locus on chromosome 3. Like humans with the same inherited enzyme defect, animals homozygous for the new null allele are runted and have renal tubular acidosis. However, the prominent osteopetrosis found in humans with CA II deficiency could not be detected even in very old homozygous null mice. A molecular analysis of the deficient mice shows that the mutant gene is not deleted and is transcribed. The CA II protein, which is normally expressed in most tissues, could not be detected by immunodiffusion analysis in any tissues of the CA II-deficient mice, suggesting a nonsense or a missense mutation at the Car-2 locus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourke E., Delaney V. B., Mosawi M., Reavey P., Weston M. Renal tubular acidosis and osteopetrosis in siblings. Nephron. 1981;28(6):268–272. doi: 10.1159/000182216. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conroy C. W., Maren T. H. The determination of osteopetrotic phenotypes by selective inactivation of red cell carbonic anhydrase isoenzymes. Clin Chim Acta. 1985 Nov 15;152(3):347–354. doi: 10.1016/0009-8981(85)90110-x. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Stern R. H., Womack J. E., Davisson M. T., Roderick T. H., Reynolds S. C. Evolution of mammalian carbonic anhydrase loci by tanden duplication: close linkage of Car-1 and Car-2 to the centromere region of chromosome 3 of the mouse. Biochem Genet. 1976 Aug;14(7-8):651–660. doi: 10.1007/BF00485843. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Guibaud P., Larbre F., Freycon M. T., Genoud J. Ostéopétrose et acidose rénale tubulaire. Deux cas de cette association dans une fratrie. Arch Fr Pediatr. 1972 Mar;29(3):269–286. [PubMed] [Google Scholar]
- Jilka R. L., Rogers J. I., Khalifah R. G., Vaananen H. K. Carbonic anhydrase isozymes of osteoclasts and erythrocytes of osteopetrotic microphthalmic mice. Bone. 1985;6(6):445–449. doi: 10.1016/8756-3282(85)90222-4. [DOI] [PubMed] [Google Scholar]
- Johnson F. M., Lewis S. E. Electrophoretically detected germinal mutations induced in the mouse by ethylnitrosourea. Proc Natl Acad Sci U S A. 1981 May;78(5):3138–3141. doi: 10.1073/pnas.78.5.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. E., Barnett L. B., Felton C., Johnson F. M., Skow L. C., Cacheiro N., Shelby M. D. Dominant visible and electrophoretically expressed mutations induced in male mice exposed to ethylene oxide by inhalation. Environ Mutagen. 1986;8(6):867–872. doi: 10.1002/em.2860080609. [DOI] [PubMed] [Google Scholar]
- Lewis S. E., Johnson F. M., Skow L. C., Popp D., Barnett L. B., Popp R. A. A mutation in the beta-globin gene detected in the progeny of a female mouse treated with ethylnitrosourea. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5829–5831. doi: 10.1073/pnas.82.17.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks S. C., Jr Morphological evidence of reduced bone resorption in osteopetrotic (op) mice. Am J Anat. 1982 Feb;163(2):157–167. doi: 10.1002/aja.1001630205. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Markham A. F., Orkin S. H. Isolation and DNA sequence of a full-length cDNA clone for human X chromosome-encoded phosphoglycerate kinase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):472–476. doi: 10.1073/pnas.80.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson A., Stark G., Sakati N. Marble brain disease: recessive osteopetrosis, renal tubular acidosis and cerebral calcification in three Saudi Arabian families. Dev Med Child Neurol. 1980 Feb;22(1):72–84. doi: 10.1111/j.1469-8749.1980.tb04307.x. [DOI] [PubMed] [Google Scholar]
- Peters J., Andrews S. J., Loutit J. F., Clegg J. B. A mouse beta-globin mutant that is an exact model of hemoglobin Rainier in man. Genetics. 1985 Aug;110(4):709–721. doi: 10.1093/genetics/110.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phromphetcharat V., Jackson A., Dass P. D., Welbourne T. C. Ammonia partitioning between glutamine and urea: interorgan participation in metabolic acidosis. Kidney Int. 1981 Nov;20(5):598–605. doi: 10.1038/ki.1981.182. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G., Skow L. C., Johnson F. M., Lewis S. E. Analysis of a mouse alpha-globin gene mutation induced by ethylnitrosourea. Genetics. 1983 Sep;105(1):157–167. doi: 10.1093/genetics/105.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B., Russell W. L., Popp R. A., Vaughan C., Jacobson K. B. Radiation-induced mutations at mouse hemoglobin loci. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2843–2846. doi: 10.1073/pnas.73.8.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Hewett-Emmett D., Whyte M. P., Yu Y. S., Tashian R. E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A. 1983 May;80(9):2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Whyte M. P., Sundaram V., Tashian R. E., Hewett-Emmett D., Guibaud P., Vainsel M., Baluarte H. J., Gruskin A., Al-Mosawi M. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med. 1985 Jul 18;313(3):139–145. doi: 10.1056/NEJM198507183130302. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Stoward P. J., Tashian R. E. The immunohistolocalization of carbonic anhydrase in rodent tissues. J Histochem Cytochem. 1979 Apr;27(4):820–831. doi: 10.1177/27.4.109495. [DOI] [PubMed] [Google Scholar]
- Sundaram V., Rumbolo P., Grubb J., Strisciuglio P., Sly W. S. Carbonic anhydrase II deficiency: diagnosis and carrier detection using differential enzyme inhibition and inactivation. Am J Hum Genet. 1986 Feb;38(2):125–136. [PMC free article] [PubMed] [Google Scholar]
- Tashian R. E., Hewett-Emmett D., Dodgson S. J., Forster R. E., 2nd, Sly W. S. The value of inherited deficiencies of human carbonic anhydrase isozymes in understanding their cellular roles. Ann N Y Acad Sci. 1984;429:262–275. doi: 10.1111/j.1749-6632.1984.tb12346.x. [DOI] [PubMed] [Google Scholar]
- Vainsel M., Fondu P., Cadranel S., Rocmans C., Gepts W. Osteopetrosis associated with proximal and distal tubular acidosis. Acta Paediatr Scand. 1972 Jul;61(4):429–434. doi: 10.1111/j.1651-2227.1972.tb15859.x. [DOI] [PubMed] [Google Scholar]
- Venta P. J., Montgomery J. C., Hewett-Emmett D., Wiebauer K., Tashian R. E. Structure and exon to protein domain relationships of the mouse carbonic anhydrase II gene. J Biol Chem. 1985 Oct 5;260(22):12130–12135. [PubMed] [Google Scholar]
- Whitney J. B., 3rd, Russell E. S. Linkage of genes for adult alpha-globin and embryonic alpha-like globin chains. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1087–1090. doi: 10.1073/pnas.77.2.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte M. P., Murphy W. A., Fallon M. D., Sly W. S., Teitelbaum S. L., McAlister W. H., Avioli L. V. Osteopetrosis, renal tubular acidosis and basal ganglia calcification in three sisters. Am J Med. 1980 Jul;69(1):64–74. doi: 10.1016/0002-9343(80)90501-x. [DOI] [PubMed] [Google Scholar]