Abstract
Pregnancy following squamous cell carcinoma of the vulvar is rare. Its rarity is reflected by a paucity of cases reported in the literature. We report two cases of pregnancy following diagnosis and treatment for vulvar squamous cell carcinoma, and review eleven prior reported cases. In successfully treated vulvar cancer subsequent pregnancy is not shown to increase the risk of disease recurrence, and there appears to be no deleterious effects during the antenatal period. It is possible, when considering prior reports, that prior vulvectomy may increase the likelihood of delivery by caesarean section, though modifications in the surgical management of vulvar carcinoma may have decreased this risk.
Keywords: Vulvar cancer, Squamous cell carcinoma, Radical vulvectomy, Pregnancy
INTRODUCTION
Reports of pregnancy following treatment for squamous cell carcinoma of the vulva are rare. In recent years an increased incidence of vulvar cancer in younger women has been observed,1 with a concomitant increase in the incidence of human papillomavirus, vulvar intraepithelial neoplasia (VIN) and human immunodeficiency virus. Obstetricians and gynaecological oncologists are progressively more likely to encounter patients presenting in pregnancy following treatment for vulvar cancer. Given the sparse literature on this subject, we present two cases from our unit highlighting some of the difficulties that arise in patient management.
CASE REPORTS
1. Case 1
A 29 year old parous (G2P1) smoker presented in August 1989 with a twelve-month history of vulvar pruritis. She had a prior history of recurrent genital herpes, genital warts, and VIN 3. Examination revealed an extensive, thick, white lesion with coarse punctuation over the entire right labium minus. Vulvar biopsy confirmed VIN 3 with the presence of early invasion. A radical vulvectomy with bilateral groin node dissection was performed with subsequent vulvar skin grafting. Histology revealed carcinoma in situ on a background of VIN 3 (VIN 3 extending to the excision margin). Lymph nodes were negative and a diagnosis of FIGO stage I squamous cell carcinoma (SCC) of the vulva was made. Seven months later laser treatment and a (later reversed) loop ileostomy were performed for extensive perianal intraepithelial neoplasia.
Two years following primary diagnosis she presented at eleven weeks gestation. In view of extensive vulvar scarring delivery by caesarean section was planned. The antenatal period was routine and a live healthy infant was delivered by elective caesarean section at 38 weeks gestation. She has since undergone two further local excisions for recurrent anal intraepithelial neoplasia (AIN) and remains alive and well and free of recurrence twenty years post primary diagnosis.
2. Case 2
A 36-year old nulliparous, non-smoker presented in January 2000 with a history of vulvar pruritis. She had a prior history of genital herpes and genital warts. Examination revealed a large area of thickened pigmented skin affecting the labia minora, posterior vaginal fourchette, and perineum with extension towards the anal margin. Proctoscopy and mapping biopsies were performed. Proctoscopy was normal. Biopsies revealed extensive VIN, AIN, and well to moderately differentiated squamous cell carcinoma of the vulva.
A wide local excision of the vulva, defunctioning loop ileostomy (due to the tumour site) and reconstructive plastic surgery were preformed. Bilateral groin node dissection and ileostomy reversal were performed two months later. Lymph nodes were negative and a diagnosis of FIGO Ib vulvar SCC was made. Further vulvar excisions were performed at six months, and seven years later revealing recurrent VIN 3.
Twenty months post diagnosis she presented at five weeks gestation. In view of extensive vulvar scarring delivery by caesarean section was planned. Sadly at 29 weeks gestation an intra-uterine foetal death (IUFD) was diagnosed following a six-day history of decreased foetal movements. Labour was induced and a normal vaginal delivery achieved. No obvious clinical cause was identified for the IUFD, and no abnormality detected at post-mortem. At nine years following initial diagnosis the patient is alive and well with no evidence of recurrence.
DISCUSSION
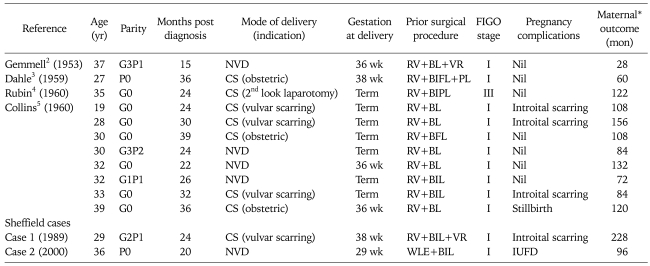
Literature search revealed four prior reports,2-5 including eleven cases of pregnancy following treatment for vulvar squamous cell carcinoma. All eleven women had prior radical vulvectomy and lymphadenectomy performed, with the majority (91%) being FIGO stage I tumours. Of these cases one antenatal complication was documented, and no incidence of disease recurrence was reported.
Including our data a total of eight women (61%) had a caesarean section; five women (38%) had a caesarean section in relation to their prior diagnosis of / or treatment for vulvar cancer; three (23%) had a caesarean section for obstetrical reasons) (Table 1).
Table 1.
Pregnancy outcomes in women following treatment for squamous cell vulvar cancer
BIFL: bilateral inguinofemoral lymphadenectomy, BIL: bilateral inguinal lymphadenectomy, BL: bilateral lymphadenectomy, CS: caesarean section, IUFD: intrauterine foetal death, NVD: normal vaginal delivery, PL: pelvic lymphadenectomy, RV: radical vulvectomy, VR: vulvar reconstruction.
*All women in case reports were alive and well.
Though it appears likely that pregnancy poses no increased risk for disease recurrence, and a prior diagnosis of vulvar carcinoma has no deleterious effects upon the antenatal period, it appears that these women may have an increased rate of delivery by caesarean section.
In recent years modifications in the surgical management of vulvar carcinoma has resulted in a reduction in surgical morbidity. Pelvic lymphadenectomy is no longer recommended,6 triple incision techniques are more frequently employed,7 and in early stage disease (FIGO Ia) wide local excision of the primary tumour alone is advocated as the risk of lymph node metastases in such circumstances are reported as negligible.8 The introduction of sentinel node assessment may also result in a further reduction in lymphadenectomy for node negative disease.
As the majority of these cases (92%) were reported prior to such surgical modifications then it is possible that the incidence of delivery by caesarean section is no longer increased although vulvar scarring, plastic reconstruction, and patient choice may still influence decision making.
In successfully treated vulvar cancer subsequent pregnancy is not shown to increase the risk of disease recurrence, and there appears to be no deleterious effects during the antenatal period. It is possible, when considering prior reports, that vulvectomy may increase the likelihood of delivery by caesarean section, though modifications in the surgical management of vulvar carcinoma may have decreased this risk. It certainly seems pertinent however that those women are advised of this possibility.
The paucity of reported cases and lack of larger data series in the literature has prevented the development of accepted management plans for pregnancy following treated vulvar cancer. The authors feel that data centralisation would be beneficial in identifying optimal management strategies in not only these rare tumours, but also in other malignant tumours diagnosed and treated prior to, and during pregnancy.
References
- 1.Cancer Research UK. London: Cancer Research UK; 2008. [cited 2009 Nov 10]. UK vulva cancer incidence statistics [Internet] Available from: http://info.cancerresearchuk.org/cancerstats/types/vulva/incidence/?a=5441. [Google Scholar]
- 2.Gemmell AA, Haines M. Pregnancy following radical vulvectomy for carcinoma of the vulva. J Obstet Gynaecol Br Emp. 1960;67:199–207. doi: 10.1111/j.1471-0528.1960.tb06979.x. [DOI] [PubMed] [Google Scholar]
- 3.Dahle T. Carcinoma of the vulva and subsequent successful pregnancy. Acta Obstet Gynecol Scand. 1959;38:448–452. doi: 10.3109/00016345909153943. [DOI] [PubMed] [Google Scholar]
- 4.Rubin A, Lewis GC., Jr Pregnancy and vaginal delivery following radical surgery for cancer of the vulva: review of the literature and case report. Am J Obstet Gynecol. 1953;65:1347–1349. doi: 10.1016/0002-9378(53)90378-0. [DOI] [PubMed] [Google Scholar]
- 5.Collins JH, Birch HW, Pailet M, Avent JK. Pregnancy and delivery following extensive vulvectomy. Am J Obstet Gynecol. 1960;80:167–171. doi: 10.1016/s0002-9378(16)36435-3. [DOI] [PubMed] [Google Scholar]
- 6.Homesley HD, Bundy BN, Sedlis A, Adcock L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol. 1986;68:733–740. [PubMed] [Google Scholar]
- 7.Hacker NF, Leuchter RS, Berek JS, Castaldo TW, Lagasse LD. Radical vulvectomy and bilateral inguinal lymphadenectomy through separate groin incisions. Obstet Gynecol. 1981;58:574–579. [PubMed] [Google Scholar]
- 8.Hacker NF, Van der Velden J. Conservative management of early vulvar cancer. Cancer. 1993;71(4 suppl):1673–1677. doi: 10.1002/cncr.2820710436. [DOI] [PubMed] [Google Scholar]