Abstract
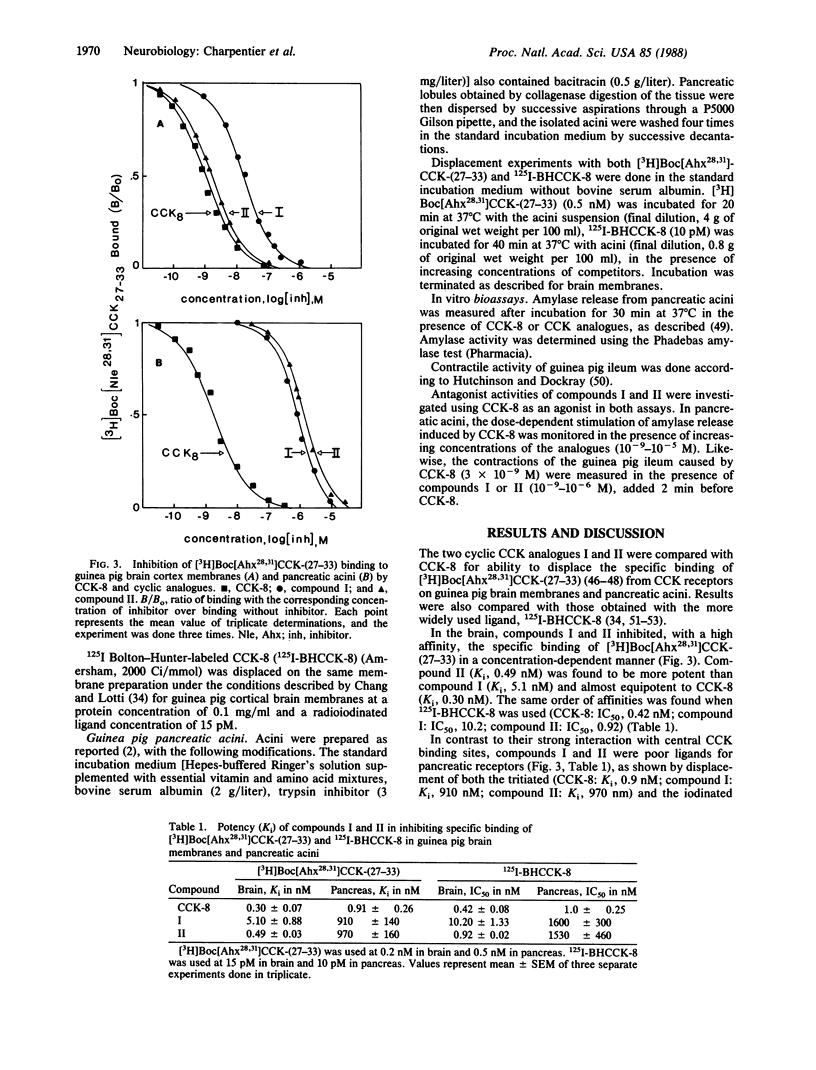
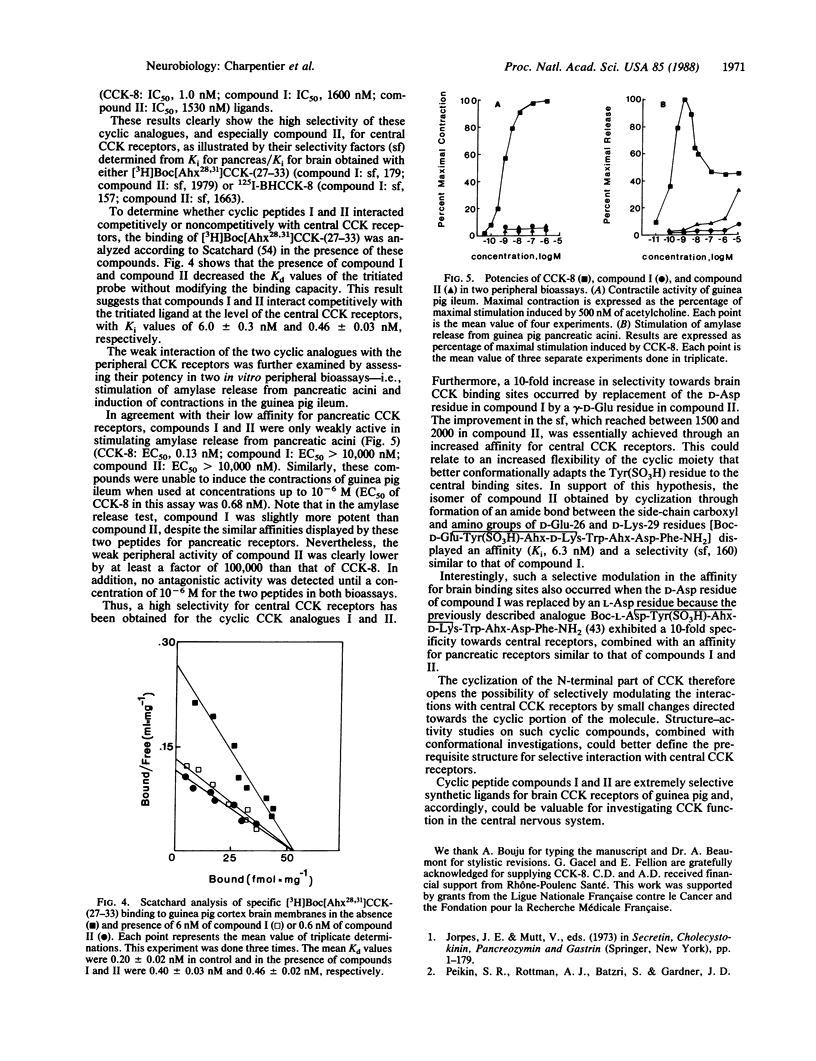
Taking as a model the N-terminal folding of the cholecystokinin tyrosine-sulfated octapeptide [CCK-8; Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2] deduced from conformational studies, two cyclic cholecystokinin (CCK) analogues were synthesized by conventional peptide synthesis: Boc-D-Asp-Tyr(SO3H)-Ahx-D-Lys-Trp-Ahx-Asp-Phe-NH2 [compound I (Ahx, 2-aminohexanoic acid)] and Boc-gamma-D-Glu-Tyr(SO3H)-Ahx-D-Lys-Trp-Ahx-Asp-Phe-NH2 (compound II). The binding characteristics of these peptides were investigated on brain cortex membranes and pancreatic acini of guinea pig. Compounds I and II were competitive inhibitors of [3H]Boc[Ahx28,31]CCK-(27-33) binding to central CCK receptors and showed a high degree of selectivity for these binding sites (compound I: Ki for pancreas/Ki for brain, 179; compound II: Ki for pancreas/Ki for brain, 1979). This high selectivity was associated with a high affinity for central CCK receptors (compound I: Ki, 5.1 nM; compound II: Ki, 0.49 nM). Similar affinities and selectivities were found when 125I Bolton-Hunter-labeled CCK-8 was used as a ligand. Moreover, these compounds were only weakly active in the stimulation of amylase release from guinea pig pancreatic acini (EC50 greater than 10,000 nM) and were unable to induce contractions in the guinea pig ileum (to 10(-6) M). The two cyclic CCK analogues, therefore, appear to be synthetic ligands exhibiting both high affinity and high selectivity for central CCK binding sites. These compounds could help clarify the respective role of central and peripheral receptors for various CCK-8-induced pharmacological effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus M., von Gellhorn K., Münch U., Naber D., Ackenheil M. A double-blind study with ceruletide in chronic schizophrenic patients: biochemical and clinical results. Psychiatry Res. 1986 Sep;19(1):1–7. doi: 10.1016/0165-1781(86)90086-7. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier B., Durieux C., Menant I., Roques B. P. Investigation of peripheral cholecystokinin receptor heterogeneity by cyclic and related linear analogues of CCK26-33: synthesis and biological properties. J Med Chem. 1987 Jun;30(6):962–968. doi: 10.1021/jm00389a002. [DOI] [PubMed] [Google Scholar]
- Clark C. R., Daum P., Hughes J. A study of the cerebral cortex cholecystokinin receptor using two radiolabelled probes: evidence for a common CCK 8 and CCK 4 cholecystokinin receptor binding site. J Neurochem. 1986 Apr;46(4):1094–1101. doi: 10.1111/j.1471-4159.1986.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Crawley J. N. Clarification of the behavioral functions of peripheral and central cholecystokinin: two separate peptide pools. Peptides. 1985;6 (Suppl 2):129–136. doi: 10.1016/0196-9781(85)90145-7. [DOI] [PubMed] [Google Scholar]
- De Witte P., Swanet E., Gewiss M., Goldman S., Roques B., Vanderhaeghen J. J. Psychopharmacological profile of cholecystokinin using the self-stimulation and the drug discrimination paradigms. Ann N Y Acad Sci. 1985;448:470–487. doi: 10.1111/j.1749-6632.1985.tb29941.x. [DOI] [PubMed] [Google Scholar]
- Denavit-Saubié M., Hurlé M. A., Morin-Surun M. P., Foutz A. S., Champagnat J. The effects of cholecystokinin-8 in the nucleus tractus solitarius. Ann N Y Acad Sci. 1985;448:375–384. doi: 10.1111/j.1749-6632.1985.tb29932.x. [DOI] [PubMed] [Google Scholar]
- DiMaio J., Schiller P. W. A cyclic enkephalin analog with high in vitro opiate activity. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7162–7166. doi: 10.1073/pnas.77.12.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux C., Belleney J., Lallemand J. Y., Roques B. P., Fournie-Zaluski M. C. 1H NMR conformational study of sulfated and non-sulfated cholecystokinin fragment CCK27-33: influence of the sulfate group on the peptide folding. Biochem Biophys Res Commun. 1983 Jul 29;114(2):705–712. doi: 10.1016/0006-291x(83)90838-0. [DOI] [PubMed] [Google Scholar]
- Durieux C., Coppey M., Zajac J. M., Roques B. P. Occurrence of two cholecystokinin binding sites in guinea-pig brain cortex. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1167–1173. doi: 10.1016/0006-291x(86)90348-7. [DOI] [PubMed] [Google Scholar]
- Evans B. E., Bock M. G., Rittle K. E., DiPardo R. M., Whitter W. L., Veber D. F., Anderson P. S., Freidinger R. M. Design of potent, orally effective, nonpeptidal antagonists of the peptide hormone cholecystokinin. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4918–4922. doi: 10.1073/pnas.83.13.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie-Zaluski M. C., Gacel G., Maigret B., Premilat S., Roques B. P. Structural requirements for specific recognition of mu or delta opiate receptors. Mol Pharmacol. 1981 Nov;20(3):484–491. [PubMed] [Google Scholar]
- Fournié-Zaluski M. C., Belleney J., Lux B., Durieux C., Gérard D., Gacel G., Maigret B., Roques B. P. Conformational analysis of cholecystokinin CCK26-33 and related fragments by 1H NMR spectroscopy, fluorescence-transfer measurements, and calculations. Biochemistry. 1986 Jul 1;25(13):3778–3787. doi: 10.1021/bi00361a008. [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Igano K., Watanabe K., Irie I., Inouye K., Okabayashi T. Effects of caerulein-related peptides on cholecystokinin receptor bindings in brain and pancreas. Biochem Pharmacol. 1985 Apr 1;34(7):1103–1107. doi: 10.1016/0006-2952(85)90616-1. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Agnati L. F., Benfenati F., Cimmino M., Algeri S., Hökfelt T., Mutt V. Modulation by cholecystokinins of 3H-spiroperidol binding in rat striatum: evidence for increased affinity and reduction in the number of binding sites. Acta Physiol Scand. 1981 Dec;113(4):567–569. doi: 10.1111/j.1748-1716.1981.tb06942.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Jackson M. J. Regulation of amylase release from dispersed pancreatic acinar cells. J Physiol. 1977 Sep;270(2):439–454. doi: 10.1113/jphysiol.1977.sp011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner P. Effect of the C-terminal octapeptide of cholecystokinin on guinea pig ileum and gall-bladder in vitro. Acta Physiol Scand. 1970 Feb;78(2):232–235. doi: 10.1111/j.1748-1716.1970.tb04658.x. [DOI] [PubMed] [Google Scholar]
- Hommer D. W., Pickar D., Roy A., Ninan P., Boronow J., Paul S. M. The effects of ceruletide in schizophrenia. Arch Gen Psychiatry. 1984 Jun;41(6):617–619. doi: 10.1001/archpsyc.1984.01790170091010. [DOI] [PubMed] [Google Scholar]
- Hutchison J. B., Dockray G. J. Inhibition of the action of cholecystokinin octapeptide on the guinea pig ileum myenteric plexus by dibutyryl cyclic guanosine monophosphate. Brain Res. 1980 Dec 8;202(2):501–505. doi: 10.1016/0006-8993(80)90164-x. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Skirboll L., Rehfeld J. F., Goldstein M., Markey K., Dann O. A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contains a cholecystokinin-like peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience. 1980;5(12):2093–2124. doi: 10.1016/0306-4522(80)90127-x. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Bunney B. S., Charney D. S., Price L. H., Glazer W. M., Sternberg D. E., Rubin A. L., Heninger G. R. Does the cholecystokinin antagonist proglumide possess antipsychotic activity? Psychiatry Res. 1986 May;18(1):1–7. doi: 10.1016/0165-1781(86)90056-9. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Corrêa F. M., Uhl G. R., Schneider B., Snyder S. H. Cholecystokinin octapeptide-like immunoreactivity: histochemical localization in rat brain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):521–525. doi: 10.1073/pnas.76.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Snyder S. H. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S., Katsuura G. Behavioral effect of cholecystokinin octapeptide in vagotomized rats. Can J Physiol Pharmacol. 1986 Jun;64(6):745–747. doi: 10.1139/y86-125. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interactions of COOH-terminal fragments of cholecystokinin with receptors on dispersed acini from guinea pig pancreas. J Biol Chem. 1982 May 25;257(10):5554–5559. [PubMed] [Google Scholar]
- Kopple K. D., Schamper T. J., Go A. Conformation of cyclic peptides. 8. Cyclic hexapeptides containing the L-Pro-D-Phe sequence. J Am Chem Soc. 1974 Apr 17;96(8):2597–2605. doi: 10.1021/ja00815a046. [DOI] [PubMed] [Google Scholar]
- Lin C. W., Miller T. Characterization of cholecystokinin receptor sites in guinea-pig cortical membranes using [125I]Bolton Hunter-cholecystokinin octapeptide. J Pharmacol Exp Ther. 1985 Mar;232(3):775–780. [PubMed] [Google Scholar]
- Lotstra F., Verbanck P., Mendlewicz J., Vanderhaeghen J. J. No evidence of antipsychotic effect of caerulein in schizophrenic patients free of neuroleptics: a double-blind cross-over study. Biol Psychiatry. 1984 Jun;19(6):877–882. [PubMed] [Google Scholar]
- Marley P. D., Rehfeld J. F., Emson P. C. Distribution and chromatographic characterisation of gastrin and cholecystokinin in the rat central nervous system. J Neurochem. 1984 Jun;42(6):1523–1535. doi: 10.1111/j.1471-4159.1984.tb12738.x. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Jardine I., Weissman E., Go V. L., Speicher D. Characterization of cholecystokinin from the human brain. J Neurochem. 1984 Sep;43(3):835–840. doi: 10.1111/j.1471-4159.1984.tb12806.x. [DOI] [PubMed] [Google Scholar]
- Moroji T., Watanabe N., Aoki N., Itoh S. Antipsychotic effects of caerulein, a decapeptide chemically related to cholecystokinin octapeptide, on schizophrenia. Int Pharmacopsychiatry. 1982;17(4):255–273. doi: 10.1159/000468582. [DOI] [PubMed] [Google Scholar]
- Nair N. P., Bloom D., Lal S., Debonnel G., Schwartz G., Mosticyan S. Clinical and neuroendocrine studies with cholecystokinin peptides. Ann N Y Acad Sci. 1985;448:535–541. doi: 10.1111/j.1749-6632.1985.tb29946.x. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Pluscec J., Sabo E. F., Sheehan J. T., Williams N. Synthesis of cholecystokinin-pancreozymin. I. The C-terminal dodecapeptide. J Am Chem Soc. 1970 Jan 14;92(1):195–199. doi: 10.1021/ja00704a033. [DOI] [PubMed] [Google Scholar]
- Pélaprat D., Zajac J. M., Gacel G., Durieux C., Morgat J. L., Sasaki A., Roques B. P. [3H] Boc [Nle28, 31]CCK27-33, a new highly labelled ligand for CCK receptors: binding on brain and on pancreas. Life Sci. 1985 Dec 30;37(26):2483–2490. doi: 10.1016/0024-3205(85)90605-8. [DOI] [PubMed] [Google Scholar]
- Roques B. P., Garbay-Jaureguiberry C., Oberlin R., Anteunis M., Lala A. K. Conformation of Met5-enkephalin determined by high field PMR spectroscopy. Nature. 1976 Aug 26;262(5571):778–779. doi: 10.1038/262778a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gayo M., Daugé V., Menant I., Bégué D., Gacel G., Roques B. P. Synthesis and biological activity of Boc [Nle28, Nle31]CCK27-33, a highly potent CCK8 analogue. Peptides. 1985 May-Jun;6(3):415–420. doi: 10.1016/0196-9781(85)90106-8. [DOI] [PubMed] [Google Scholar]
- Saito A., Goldfine I. D., Williams J. A. Characterization of receptors for cholecystokinin and related peptides in mouse cerebral cortex. J Neurochem. 1981 Aug;37(2):483–490. doi: 10.1111/j.1471-4159.1981.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto C., Williams J. A., Goldfine I. D. Brain CCK receptors are structurally distinct from pancreas CCK receptors. Biochem Biophys Res Commun. 1984 Oct 30;124(2):497–502. doi: 10.1016/0006-291x(84)91581-x. [DOI] [PubMed] [Google Scholar]
- Schick R. R., Yaksh T. L., Go V. L. Intracerebroventricular injections of cholecystokinin octapeptide suppress feeding in rats--pharmacological characterization of this action. Regul Pept. 1986 Jul;14(4):277–291. doi: 10.1016/0167-0115(86)90170-9. [DOI] [PubMed] [Google Scholar]
- Shioiri T., Ninomiya K., Yamada S. Diphenylphosphoryl azide. A new convenient reagent for a modified Curtus reaction and for the peptide synthesis. J Am Chem Soc. 1972 Aug 23;94(17):6203–6205. doi: 10.1021/ja00772a052. [DOI] [PubMed] [Google Scholar]
- Van Dijk A., Richards J. G., Trzeciak A., Gillessen D., Möhler H. Cholecystokinin receptors: biochemical demonstration and autoradiographical localization in rat brain and pancreas using [3H] cholecystokinin8 as radioligand. J Neurosci. 1984 Apr;4(4):1021–1033. doi: 10.1523/JNEUROSCI.04-04-01021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ree J. M., Gaffori O., De Wied D. In rats, the behavioral profile of CCK-8 related peptides resembles that of antipsychotic agents. Eur J Pharmacol. 1983 Sep 16;93(1-2):63–78. doi: 10.1016/0014-2999(83)90031-6. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Lotstra F., De Mey J., Gilles C. Immunohistochemical localization of cholecystokinin- and gastrin-like peptides in the brain and hypophysis of the rat. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1190–1194. doi: 10.1073/pnas.77.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennogle L. P., Steel D. J., Petrack B. Characterization of central cholecystokinin receptors using a radioiodinated octapeptide probe. Life Sci. 1985 Apr 15;36(15):1485–1492. doi: 10.1016/0024-3205(85)90057-8. [DOI] [PubMed] [Google Scholar]
- Worms P., Martinez J., Briet C., Castro B., Biziere K. Evidence for dopaminomimetic effect of intrastriatally injected cholecystokinin octapeptide in mice. Eur J Pharmacol. 1986 Mar 4;121(3):395–401. doi: 10.1016/0014-2999(86)90260-8. [DOI] [PubMed] [Google Scholar]



