Abstract
DNA repair defects are frequently encountered in human cancers. These defects are utilized by traditional therapeutics but also offer novel cancer treatment strategies based on synthetic lethality. To determine the consequences of combined Fanconi anemia and mismatch repair pathway inactivation, defects in Fancd2 and Mlh1 were combined in one mouse model. Fancd2/Mlh1 double mutant embryos displayed growth retardation resulting in embryonic lethality and significant under-representation among progeny. Additional inactivation of Trp53 failed to improve the survival of Fancd2/Mlh1 deficient embryos. Mouse fibroblasts were obtained and challenged with crosslinking agents. Fancd2-deficient cells displayed the FA-characteristic growth inhibition after mitomycin C exposure. In primary fibroblasts, absence of Mlh1 did not greatly affect the mitomycin C sensitivity of Fancd2-deficient and proficient cells. However, in Trp53 mutant immortalized fibroblasts Mlh1-deficiency reduced the growth-inhibiting effect of mitomycin C in Fancd2 mutant and complemented cells. Similar data were obtained using psoralen/UVA, signifying that MLH1 influences the cellular sensitivity to DNA interstrand crosslinks. Next, the effect of MLH1-deficiency on the formation of chromosomal aberrations in response to crosslinking agents was determined. Surprisingly, Mlh1 mutant fibroblasts displayed a modest, but noticeable decrease in induced chromosomal breakage and interchange frequencies, suggesting that MLH1 promotes ICL repair catastrophe. In conclusion, the combined inactivation of Fancd2 and Mlh1 did not result in synthetic lethality at the cellular level. Although, absence of Fancd2 sensitized Mlh1 / Trp53 mutant fibroblasts to mitomycin C, the differential survival of primary and immortalized fibroblasts advocates against systemic inactivation of FANCD2 to enhance treatment of MLH1-deficient tumors.
Keywords: Fanconi anemia, mismatch repair, Fancd2, Mlh1, crosslink
INTRODUCTION
The mammalian cell has assorted DNA repair systems to counteract genomic insults and maintain genomic stability. Failure within the DNA repair network increases mutation frequencies, affects cell cycle regulation and promotes tumorigenesis. At the same time, DNA repair defects provide therapeutic opportunities to treat cancer through DNA damage-inducing radiation and chemotherapies (1). The Fanconi anemia (FA) genes function in a genomic stability pathway required for cellular resistance agents that induce interstrand DNA crosslinks (ICLs) (2). Therefore, the FA proteins are considered to be excellent targets for small molecule inhibitors in order to sensitize FA proficient tumors to the clastogenic effects of chemotherapeutics like cisplatin and mitomycin C (MMC) (3). The discovery of synthetic lethality between FANCD1/BRCA2-deficiency and poly(ADP-ribose) polymerase (PARP)-inactivation has revealed a novel approach to eradicate tumors through concurrent deficiencies in complementary DNA repair functions (4). Besides cells with BRCA2-defects, PARP-inhibitors also inhibit proliferation of human FA-A and FA-D2 mouse fibroblasts (5). Considering the mammalian genome stability network, it is expected that many more synthetic interactions among DNA repair genes which reduce cellular fitness remain to be identified (6, 7). In this report we addressed the functional consequences of combined mismatch repair (MMR) and Fanconi anemia pathway inactivation by using mouse models with Mlh1and Fancd2 defects.
MMR proteins correct single nucleotide mismatches and small misalignments that arise during DNA replication. The MutS complexes, consisting of MSH2 and MSH3 or MSH6, sense DNA mismatches and recruit the MutLα dimer composed of MLH1 and PMS2. The MLH1/PMS2 dimer can introduce nicks close to the mismatch, recruits proteins to resolve the DNA lesion, and connects MMR to cell cycle checkpoint proteins and apoptosis pathways (8).
Within the FA pathway FANCD2 is suggested to function upstream of the FANCD1/BRCA2 protein, which operates in the homology-directed repair of double strand breaks (2). Together with FANCI, FANCD2 forms the ID-complex, and in response to DNA damage both proteins are mono-ubiquinated by the FANCL subunit of the FA core complex that also includes FANCA,-B, -C, -E, -F, -G and -M. Upon activation by mono-ubiquitination, the ID complex localizes in chromatin-associated nuclear foci and is suggested to interact with BRCA1 and FANCD1/BRCA2. After FANCD2 and FANCI have performed their unidentified function, both proteins are deubiquitinated by USP1 (9, 10). The modification of the ID complex by ubiquitination is a key target for existing FA pathway inhibitors to sensitize cells to crosslinking agents or to mediate probable synthetic lethal interactions with other DNA repair defects.
Recently, molecular cross-talk between the FA and MMR pathways has been identified through the interaction between FANCJ and MutLα (11). Moreover, MLH1 and the FA core-complex proteins were found in the so-called BLM-associated protein complex BLAP (12). The functional relevance of the cross-talk between FA and MMR repair proteins remains unclear. However, it is noteworthy that loss of MMR function, generally by MSH2 or MLH1-deficiency, has been correlated with resistance to alkylating agents like cisplatin (13). In part this resistance to cisplatin may be explained by a failure to detect DNA monoadducts. It has been shown that MutS acts as a damage sensor in response to DNA mono-adducts, and recruits and activates ATR/ATRIP (14).
Alternatively, it has been reported that the repair of monoadducts by MMR proteins ends in a futile cycle, resulting in a persistent DNA strand break that initiates damage signaling. This futile cycling does not take place in absence of MMR proteins and consequently abrogates DNA damage signaling (15). Nevertheless, the role of MMR proteins in crosslinker resistance is not undisputed. In vitro absence of MLH1 or MSH2 in tumor cells provides only an approximate 2 fold resistance to cisplatin (16). Moreover, loss of MMR by Msh2-inactivation in primary mouse ES cells did not alter cellular sensitivity to cisplatin (17). The characteristic hypersensitivity of FA cells may give an opportunity to better address differences in crosslinker sensitivity between MMR proficient and deficient cells.
In this study knockout mice were used to combine targeted defects in Fancd2 and Mlh1 to analyze the consequences of joint FA and MMR defects on embryonic survival, cellular resistance to crosslinking agents, and induced chromosomal aberrations.
MATERIAL & METHODS
Animal husbandry
C57BL/6j or 129S4 Fancd2 heterozygous mice carrying a deletion of exons 26 and 27 were crossed with C57BL/6j mice carrying a deletion of exon 4 in the Mlh1 gene (18, 19). Triple mutant Trp53/Fancd2/Mlh1 mice were generated by introducing targeted disruptions of Fancd2 and Mlh1 from a C57BL/6j genetic background into Trp53 null mice in the 129S4 background (20). Next, Trp53 null, Fancd2/Mlh1 double heterozygous mice were crossed. Consent was obtained from OHSU IACUC for all animal handling procedures following protocol A765. Genotypes of newborn mice were determined by PCR as described. Statistical analysis was performed using χ2 test on birth frequencies.
Mouse fibroblasts
Fibroblasts from ears of Fancd2/Mlh1/Trp53 triple mutant, Fancd2 heterozygous / Mlh1 heterozygous, Fancd2 mutant / Mlh1 heterozygous, Fancd2 heterozygous / Mlh1 mutant and Fancd2 mutant / Mlh1 mutant mice were established as previously described (21). Primary fibroblasts of passage 2 and 3 were used in chromosomal breakage and clonal survival assays. Triple mutant cells immortalized spontaneously by culturing cells until a homogeneous growing cell population was obtained at passage 10. Single cell-derived clones were isolated and expanded to generate isogenic cells which were complemented by retroviral transduction using and pQCLIXH (Clontech, Mountain View, CA) human MLH1 and pMMP-PURO encoding mouse or human FANCD2 (21, 22). The coding region of human MLH1 was amplified from pCMV-MLH1 using Pfx polymerase and forward primer AAAACCATGGGCTAGAAAATGTCGTTCGTGGCAGG, and reverse primer AAAAGGATCCTTAACACCTCTCAAAGACTTTGTATAG (23). The PCR product was blunt-end TOPO cloned (Invitrogen, Carlsbad, CA), and polymerase artifacts were excluded by double stranded sequencing. The MLH1 ORF was cloned into the multiple cloning site of retroviral expression vector pQCLIXH using NotI and BamHI (New England Biolabs, Ipswich, MA). Standard retroviral production and transduction assays were used and stable expression of human FANCD2 or mouse Fancd2 and human MLH1 was obtained by applying selection media with 2 μg/ml puromycin (Sigma-Aldrich, St Louis, MO) and 200 μg/ml hygromycin (Cell-Gro, Manassas, VA) respectively. Uncorrected cells were transduced by empty pMMP-PURO and pQCLIXH vectors and subjected to identical selection procedures. All cells tested negative for mycoplasma infection using the MycoSensor™ PCR Assay Kit (Stratagene, La Jolla, CA) following manufacturer's procedures. Clonal survival, chromosomal breakage assays and exposure to DNA damaging agents MMC, 6-thioguanine (6-TG), diepoxybutane (DEB), 4'-hydroxymethyl-4,5',8-trimethylpsoralen (HMT) and angelicin were performed as described (24, 25, 26). The results of all clonal survival and chromosomal breakage assays were collected using data encryption to exclude observer bias and statistical analysis was performed using a Chi2 test.
RESULTS
Simultaneous inactivation of Fancd2 and Mlh1 results in embryonic lethality
C57BL/6j mice heterozygous for Fancd2 and Mlh1 were interbred and the genotypes of 300 newborn pups were recorded (Table 1). No Fancd2/Mlh1 double mutant mice were observed among these newborn mice indicating a full embryonic lethal phenotype in mice with combined FA and MMR defects. Data presented in table 1 also indicate that inactivation of just Fancd2 already significantly impaired embryonic survival of C57BL/6j mice. Considering the severe impact of Fancd2 disruption on embryonic survival, we set out to also generate Fancc/Mlh1 double mutant mice. Fancc mutant mice display a less severe phenotype in comparison to Fancd2 mutant mice (18). Therefore, the analysis of birth ratios of Fancc/Mlh1 double mutants may further substantiate the observed synthetic lethal interaction between the FA and MMR pathways. As seen in table 1, a consistent embryonic lethal phenotype was observed for Fancc/Mlh1 double mutants in the C57BL/6j strain background. Moreover, embryos with bi-allelic mutations in Fancc only, displayed clearly enhanced survival frequencies in comparison to Fancd2 mutants. This signifies full embryonic lethality was only observed when both FA and MMR pathways were inactivated.
Table 1.
Birth frequencies from breeding pairs with combined heterozygosity for Fancd2 and Mlh1.
| Strain background | Fancd2 Mlh1 C57BL/6j | Fancc Mlh1 C57BL/6j | Fancd2 Mlh1 129S4 C57BL/6j | Fancd2 Mlh1 Trp 53−/− 129S4 C57BL/6j | Expected Birth Frequencies |
|---|---|---|---|---|---|
| Number of mice | 300 | 194 | 240 | 160 | |
| Genotypes | |||||
| Fanc-Mlh1 | |||||
| HET-HET | 0.313 | 0.361 | 0.254 | 0.244 | 0.250 |
| HET-MUT | 0.103 | 0.144 | 0.117 | 0.106 | 0.125 |
| HET-WT | 0.140 | 0.160 | 0.179 | 0.200 | 0.125 |
| MUT-HET | 0.027 | 0.052 | 0.096 | 0.063 | 0.125 |
| MUT-MUT | 0.000A | 0.000B | 0.021C | 0.013D# | 0.063 |
| MUT-WT | 0.017 | 0.036 | 0.058 | 0.069 | 0.063 |
| WT-HET | 0.187 | 0.082 | 0.138 | 0.131 | 0.125 |
| WT-MUT | 0.090 | 0.082 | 0.071 | 0.075 | 0.063 |
| WT-WT | 0.123 | 0.082 | 0.067 | 0.100 | 0.063 |
Chi2 test on observed birth frequency of double mutant mice versus expected birth frequency of double mutant mice:
p<0.0001
p<0.0003
p=0.0055
p=0.0081
Included in this analysis is one perinatal lethal 129S4 + C57BL/6j Trp53 triple mutant newborn.
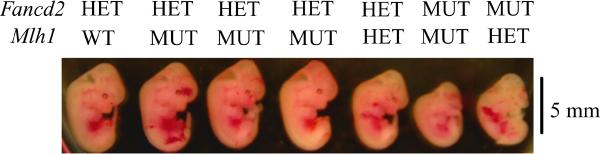
Timed pregnancies were then initiated with Fancd2/Mlh1 double heterozygous mice, and embryos were harvested between 9–14 days of gestation. This resulted in the identification of four underdeveloped double mutant embryos. Close inspection of these double mutant embryos suggested that a general growth retardation is causing embryonic lethality around 10 dpc. (Fig. 1).
Figure 1. Embryonic lethality in Fancd2/Mlh1 double mutant mice.
Image of embryos around 12 dpc depicting a representative litter from Fancd2/Mlh1 double heterozygous breeding pairs. The severely underdeveloped Fancd2/Mlh1 double mutant is undergoing absorption. In this litter the Fancd2 mutant Mlh1 heterozygous embryo also shows developmental defects. Respective genotypes are indicated; HET: heterozygous, MUT: mutant.
The phenotypic consequences of Fancd2-deficiency have been shown to vary among mice with distinct genetic backgrounds, with developmental defects being less prominent in mice from the 129S4 strain (18). To test the effect of the genetic background on the survival of Fancd2/Mlh1 double mutant embryos, we combined the C57BL/6j and 129S4 mouse strains. In this mixed background the genotyping of 240 newborn mice resulted in the identification of 5 Fancd2/Mlh1 double mutant mice, indicating a partial rescue of the synthetic lethality (Table 1). Still, Fancd2/Mlh1 double mutant mice were significantly underrepresented as among 240 newborns 15 double mutant mice were expected following Mendelian ratios. Previously, it was shown that embryonic lethality resulting from Fancd1/Brca2 inactivation can be delayed through additional ablation of p53 (27). To see whether the observed embryonic lethality is a consequence of p53-induced apoptosis, Fancd2/Mlh1heterozygous Trp53 mutant mice were generated in a C57BL/6j and 129S4 mixed genetic background. Among 160 new born mice 1 viable and 1 perinatally lethal triple mutant were identified. These results indicate that inactivation of Trp53 did not improve the survival of Fancd2/Mlh1 double mutant embryos (Table 1). Instead, embryonic lethality may be a result of a diminished cellular proliferative ability, or alternatively, follows p53-independent apoptosis.
Mlh1 and Fancd2 defects in primary mouse fibroblasts challenged with MMC
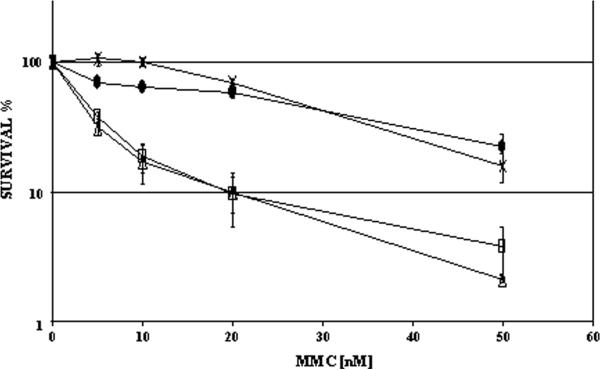
To determine the effect of Mlh1 and Fancd2 function on the cellular response to crosslinking agents, primary mouse ear fibroblasts were generated from C57BL/6j/129S4 mice with appropriate genotypes. At passage 2 and 3 these cells were continuously exposed to various concentrations of MMC in clonal survival assays. The number of clones observed in the control culture plates without MMC was set as 100% growth. A slight but significantly reduced cloning efficiency (CE) was observed for fibroblasts with combined Fancd2/Mlh1 defects (1.5 fold, p<0.01). Diminished cell adherence after plating could result in lower CEs. More likely however, the reduced cloning efficiency of double mutant fibroblasts is a consequence of a compromised proliferative capacity, which is consistent with the observed growth retardation during embryogenesis. As shown in figure 2, fibroblasts heterozygous for Fancd2 and Mlh1 were most resistant to the clastogenic effects of MMC. Fibroblasts mutant for Mlh1 only displayed a slight but noticeable proliferative decrease at low MMC concentrations in comparison to double heterozygous cells proficient for Fancd2 and Mlh1. Absence of Fancd2 clearly resulted in the FA-characteristic crosslinker hypersensitivity, as shown by the poor clonal survival of primary Fancd2-deficient fibroblasts in the presence of MMC. Fancd2/Mlh1 double mutant primary fibroblast displayed a similar proliferative decline as Fancd2 mutant fibroblasts after exposure to MMC, indicating absence of Mlh1 did not alter the MMC sensitivity of primary FA fibroblasts (Fig. 2).
Figure 2. Clonal survival of primary fribroblasts in addition of MMC.
Fancd2 mutant fibroblasts display a characteristic FA-like hypersensitivity to MMC. Additional inactivation of Mlh1 does not alter the MMC sensitivity of Fancd2 mutant primary fibroblasts. Fancd2/Mlh1 double heterozygous (-×-), double mutant (-□-), Fancd2 heterozygous Mlh1 mutant (-•-), and Fancd2 mutant Mlh1 heterozygous (-Δ-), error bars indicate standard error.
Mlh1-deficiency reduces MMC sensitivity in immortalized fibroblasts
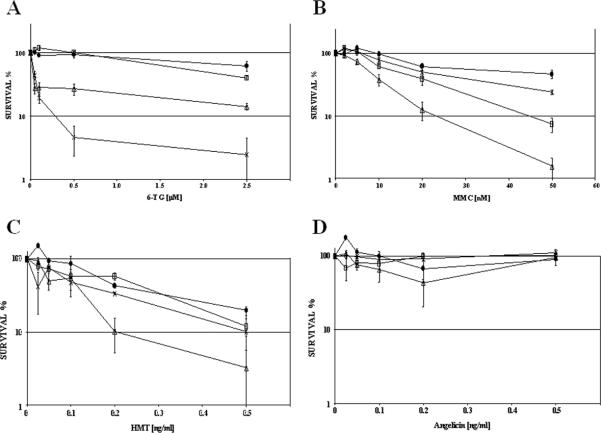
Data implying MMR-deficiency in resistance to alkylating agents have been obtained in immortal tumor-derived cells. Therefore, immortal fibroblasts were generated from Fancd2/Mlh1/Trp53 triple mutant mice. Single cell-derived clones were expanded and isogenic cell lines were established in which stable expression of human FANCD2 and/or MLH1 was restored by retroviral transduction. FANCD2 and MLH1 protein expression was confirmed by western blot (supplementary figures S1, S2), and MLH1 complementation resulted in the stabilization of endogenous Pms2 protein levels (data not shown). Human MLH1 has been demonstrated to functionally complement mouse cells with defects in Mlh1 (23). In our experiments human and mouse Fancd2 equally complemented the MMC hypersensitivity of Fancd2 mutant immortalized fibroblasts, further establishing the functional conservation of FA pathway genes between human and mouse (data not shown) (28, 29). To further address the functional properties of the retroviral-mediated MLH1 complementation, the isogenic cell lines were exposed to 6-TG in clonal survival assays. Cell lines with mismatch repair defects are known to display resistance to DNA damage induced by 6-TG (23). Accordingly, mock transduced Mlh1-deficient immortal fibroblasts showed resistance to 6-TG in clonal survival assays, but reverted into 6-TG sensitive cells upon stable expression of MLH1. Surprisingly, full complementation with MLH1 and FANCD2 made the isogenic fibroblasts even more sensitive to the clastogenic effects of higher 6-TG concentrations in comparison to cells proficient for MLH1 but deficient for Fancd2 (Fig. 3A). This observation could be a result of the slight difference in expression levels of MLH1 (supplementary figure S2). Alternatively, FANCD2 may be involved in the resolution of 6-TG induced lesions. In parallel to 6-TG exposure, clonal survival assays were performed in which the isogenic cell lines were exposed to MMC. In contrast to the data obtained with primary cells, MLH1 expression had very clear effects on the crosslinker sensitivity of the immortal cells. In comparison to MLH1-proficient cells, the MMC-induced growth inhibition was less severe in Mlh1 mutant fibroblasts. In the presence of MMC, double mutant cells showed an increased proliferative ability in comparison to Fancd2 mutant fibroblasts complemented with MLH1. In addition, FANCD2-proficient Mlh1 mutant fibroblasts were less sensitive to MMC than fully complemented FANCD2 and MLH1 expressing cells (Fig. 3B).
Figure 3. Clonal survival of immortalized fibroblasts with Fancd2 and Mlh1 defects in addition of 6-TG, MMC, psoralen or angelicin plus UVA.
A) MLH1 complementation restores 6-thioguanine sensitivity in immortalized double mutant Fancd2/Mlh1 cells. Concurrent expression of FANCD2 made cells even more sensitive to 6-TG at concentrations of 0.5 and 2.5 μM (p≤ 0.005). B) Fancd2/Mlh1 double mutant fibroblasts show a remarkable resistance to MMC displaying clonal survival frequencies close to FANCD2 and MLH1complemented cells. While FANCD2 complementation mediates MMC resistance, expression of MLH1 greatly enhances MMC sensitivity of Fancd2-deficient immortalized fibroblasts, p<0.05 at 5 and 10 nM MMC, p=0.01 at 20 and 50 nM MMC, when compared to clonal survival of double mutant fibroblasts. C) Clonal survival after exposure to psoralen plus UVA irradiation. FANCD2 complemented and Fancd2/Mlh1 double mutant cells are resistant to psoralen/UVA ICL damage. In contrast, Fancd2 mutant fibroblasts expressing functional MLH1 displayed significantly reduced clonal growth after psoralen UVA exposure at concentration of 0.2 and 0.5 ng/ml in comparison to double mutant cells (p<0.05). D) In parallel with psoralen/UVA, cells were exposed to angelicin/UVA and subsequent clonal survival was determined. No clear differences were observed among the clonal survival of the isogenic cell lines. FANCD2 / MLH1 complemented (-×-), double mutant mock complemented (-□-), FANCD2 complemented, Mlh1-deficient (-•-), and Fancd2-deficient MLH1 complemented (-Δ-), error bars indicate standard error.
Mlh1-deficiency reduces the sensitivity of immortalized fibroblasts to interstrand crosslinks
Mitomycin C has been shown to generate mono- and bifunctional DNA adducts (30). To discriminate the growth-inhibition properties of interstrand crosslink damage specifically, the established isogenic fibroblasts cell lines were exposed to HMT or angelicin followed by UVA irradiation in parallel clonal survival assays. Exposure to HMT plus UVA initially generates DNA monoadducts which are converted into DNA interstrand crosslinks upon a second exposure to UVA. In contrast, angelicin and sequential UVA radiation only generates DNA monoadducts (31). This provides an excellent setting to document the effects of MLH1 and FANCD2 activity on these distinct DNA lesions. Figure 3C shows that MLH1 expression has direct consequences specifically for the HMT UVA sensitivity of Fancd2-deficient cells. Absence of MLH1 attenuates the HMT UVA hypersensitivity of Fancd2 mutant fibroblasts, resulting in similar proliferative capacities as FANCD2 complemented cells after HMT UVA exposure (Fig. 3C). In contrast, no apparent toxicity was observed after treatment of the isogenic cell lines with angelicin plus UVA irradiation (Fig. 3D). This indicates that the levels of induced DNA intrastrand crosslinks were insufficient to inhibit cell proliferation. As a result, the observed proliferation inhibition after HMT UVA exposure must be a consequence of induced DNA interstrand crosslinks. All together these experiments show that MLH1 is able to influence the ICL sensitivity of immortalized cells.
MLH1 promotes crosslinker-induced chromosomal aberrations
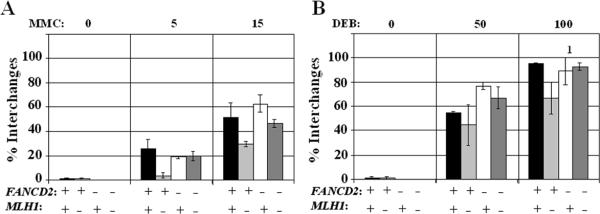
The apparent effect of MLH1 on the survival of Fancd2-deficient immortalized fibroblasts in response to ICLs, raised the question whether MLH1 would also influence the formation of FA-characteristic chromosomal aberrations after the exposure to alkylating agents. Therefore, immortalized isogenic fibroblasts cell lines were exposed to MMC or DEB. Next, metaphases were analyzed to assess chromosomal breakage and the formation of chromosomal interchanges. As shown in figure 4, Mlh1-deficient cells revealed a tendency to display reduced levels of chromosomal interchanges after exposure to crosslinking agents. Similarly, chromosomal breakage frequencies were attenuated in mock transduced Fancd2/Mlh1double mutant cells in comparison to isogenic Fancd2-deficient cells in which MLH1 expression was reconstituted (Supplementary Fig. S3). Notably, ectopic expression of MLH1 also resulted in an increase of chromosomal aberrations in FANCD2 proficient cells (p≤0.005). Therefore, the increased chromosomal damage after crosslinker exposure in Fancd2-deficient fibroblasts complemented with MLH1 could be a result of MLH1 over-expression.
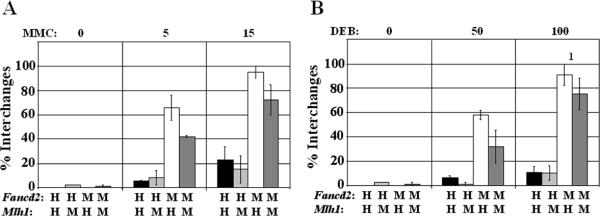
Figure 4. MlH1 increases chromosomal aberrations in immortal fibroblasts after exposure to MMC or DEB.
A, B) Frequencies of chromosomal interchanges after 48 hr of continuous exposure to 0, 5 or 15 ng/ml MMC or 0, 50 or 100 ng/ml DEB. 1 At 100 ng/ml DEB excessive chromosomal damage was observed in one of the averaged experiments and the outcome was set to 100% chromosomal aberrations which is an underrepresentation of the actual damage level. +: complemented with FANCD2 or MLH1, -: mock complemented, error bars indicate standard error.
For that reason, chromosomal breakage assays were also performed on primary fibroblasts with endogenous Mlh1 expression levels. Compared to Fancd2/Mlh1 double mutant cells, expression of endogenous Mlh1 resulted in modestly elevated frequencies of chromosomal interchanges in Fancd2 mutant primary fibroblasts after exposure to MMC or DEB (p=0.05 5 ng/ml MMC, p=0.03 50 ng/ml DEB) (Fig. 5). Also, endogenous expression of Mlh1 increased crosslinker-induced chromosomal breakage frequencies in Fancd2 mutant primary fibroblasts (Supplementary Fig. S4). All together, these data clearly demonstrate that loss of Fancd2 results in the FA-characteristic increase of chromosomal aberrations in response to crosslinking agents, while the additional loss of Mlh1 remarkably attenuates chromosomal breakage and interchange frequencies.
Figure 5. Mlh1 increases chromosomal damage in primary fibroblasts after exposure to MMC or DEB.
A, B) Frequencies of chromosomal interchanges after 48 hr of continuous exposure to 0, 5 or 15 ng/ml MMC or 0, 50 or 100 ng/ml DEB. 1 At 100 ng/ml DEB excessive chromosomal damage was observed in one of the averaged experiments and the outcome was set to 100% aberrations for breaks and interchanges which is an underrepresentation of the actual damage level. H: heterozygous for Fancd2 or Mlh1, M: mutant for Fancd2 or Mlh1, error bars indicate standard error.
DISCUSSION
Inactivation of the mismatch repair pathway is frequently encountered in hereditary and sporadic human cancers and has been correlated with tumor resistance to cisplatin. Nevertheless, the role of mismatch repair in recognition and repair of crosslinker-induced DNA damage requires clarification as in vitro studies have yielded confusing results (13, 17). Defects in the Fanconi anemia genomic maintenance pathway underlie a unique cellular hypersensitivity to crosslinking agents (2). As a result, FA cells offer an experimental opportunity to evaluate the role of MMR in crosslink repair. In this study we describe the consequences of combined inactivation of Fancd2 and Mlh1 using knockout mouse models.
Double mutant Fancd2/Mlh1 mice were severely underrepresented among the off-spring of double heterozygous carriers and showed a full embryonic lethal phenotype in the C57BL/6j genetic background. It remains to be established which factors reduce the penetrance of Fancd2/Mlh1 defects in the 129S4 genetic background. A marked decrease in birth frequencies for Fancd2 null mice was observed compared to a previous report (18). This augmented phenotype may be a consequence of the continuous back-crossing of the Fancd2 mutation into the C57BL/6j genetic background. The combined inactivation of Fancc and Mlh1 also interfered with successful completion of embryogenesis. This suggests that C57BL/6j embryonic lethality is a universal consequence when mutations in FA genes are combined with Mlh1-deficiency. Additional ablation of Trp53 did not improve the embryonic survival frequency of Fancd2/Mlh1 double mutants, indicating that double mutant embryos do not succumb following p53-mediated apoptosis in response to elevated spontaneous DNA damage.
The observation of p53-independent embryonic lethality as a consequence of simultaneous Fancd2/Mlh1 inactivation suggests that this genetic interaction may prove useful to eradicate specific tumors through a synthetic lethal approach. Therefore, mouse ear fibroblasts were generated to determine functional consequences of combined Fancd2 and Mlh1 inactivation at the cellular level. Our data showed that mismatch repair inactivation by Mlh1 mutations did not result in cellular resistance to MMC in primary cells, which corresponds to the observations made by Claij et al using primary mouse ES cells deficient for Msh2 (17). These data are in contrast with the concept that loss of MMR mediates resistance to alkylating agents like cisplatin. However, primary fibroblasts with functional cell cycle checkpoints are a poor representation of tumor-derived cells. Hence, immortalized fibroblasts were established from Fancd2/Mlh1/Trp53 triple mutant mice to mimic tumor-like cell lines. In addition, the complementation by retroviral transduction of single-cell-derived fibroblasts clones with human MLH1 and human FANCD2 allowed us to study MMR and FA defects using isogenic controls. In our experiments human MLH1 readily reverted the 6-TG tolerance of Mlh1-deficient cells and expression of FANCD2 reverted the crosslinker hypersensitivity of Fancd2 mutant cells, providing direct evidence for functional complementation. Based on our data obtained with immortalized cells exposed to MMC, HMT and angelicin, we conclude that loss of mismatch repair function by Mlh1-inactivation can mediate cellular resistance to interstrand crosslinks. However, loss of Mlh1 alone is not sufficient to acquire cellular crosslinker resistance as additional changes that take place during cellular immortalization are essential to bring about Mlh1-dependent MMC resistance. In agreement with our data, a recent study by Wu et al. also concluded that MLH1-deficiency mediates cellular resistance to HMT/UVA- induced ICLs, which correlated with reduced apoptosis and attenuated levels of phosphorylated ATR, CHK1 and CHK2, indicating a decreased DNA damage response (32). Previously, c-Abl, p73 and cyclin D have been implied in the signaling cascade that is affected in MMR-deficient cells upon exposure to cisplatin (33, 34). It remains to be determined whether the MMR signaling response is similar for mono-alkylating and bi-functional DNA damage (35). The attenuation of the DNA damage response is likely to contribute to the enhanced proliferative ability of crosslinker resistant cells.
In FA cells hypersensitivity to bifunctional alkylating agents correlates with elevated frequencies of chromosomal aberrations. Since loss of Mlh1 function attenuated the crosslinker hypersensitivity of immortalized FA cells, we assessed the effect of MLH1 on the formation of chromosomal breaks and interchanges. Upon exposure to MMC or DEB primary and immortal fibroblasts deficient for Fancd2 and Mlh1 displayed fewer chromosomal aberrations than Fancd2 mutant cells that were proficient for MLH1. This clearly demonstrates that endogenous Mlh1 and complemented MLH1 promote mitotic catastrophe in Fancd2-deficient cells in response to crosslinking agents. In addition, a notable increase in chromosomal damage was observed in FANCD2 complemented cells in which MLH1 expression was reconstituted. Chromosomal instability in response to DNA damage due to ectopic MLH1 expression could be a result of abnormal MLH1 protein complex ratios, potentially deregulating PMS2 endonuclease or EXO1 exonuclease function (36, 37, 38). As a result, chromosomal instability and aberrant MLH1 expression should be considered in human cancer.
Our results with primary and immortalized fibroblasts question the correlation between crosslinker-induced levels of chromosomal aberrations and cellular survival. Since our primary fibroblasts were derived from p53 proficient mice and the immortal cells were obtained from Trp53 mutant mice, p53 status has likely affected the outcome of cell survival and chromosomal breakage assays. Previously, p53 was shown to be involved in cell cycle arrest after ICL induction by psoralen/UVA (39). Accordingly, primary Fancd2/Trp53 double mutant embryonic fibroblasts showed S-phase progression after ICL induction, while primary Fancd2 mutant p53 proficient cells did not show DNA replication (30). In addition, loss of p53 attenuated the MMC hypersensitivity of primary Fancc-deficient bone marrow progenitors (40). These findings suggest that in primary fibroblasts p53 will mediate a robust cell cycle arrest and/or induce programmed cell death due to inflicted DNA damage, which is likely to override the proliferative gain mediated through loss of Mlh1 after ICL exposure. In contrast, p53-deficient cells display a higher DNA damage tolerance and fail to halt DNA replication, which apparently emphasizes Mlh1 function in cell cycle progression and DNA damage resolution. Loss of DNA damage sensing and futile cycling are models to explain enhanced survival of MMR-deficient cells after exposure to monoalkylating agents (15). Moreover, these resistance models could be applicable to ICL repair after crosslink unhooking and translesion synthesis (TLS) have taken place (41). Conversely, recent data imply the FA pathway promotes the repair of ICLs through TLS and the resolution of DSB-intermediates by homology-directed repair (42). The replication blocking characteristics of the ICL implies a sister chromatid is not available as a template for homologous recombination. Therefore, error-free repair by homologous recombination can only occur by using the homologous chromosome. Loss of MMR function has been shown to increase recombination frequencies between divergent sequences (43, 44). Accordingly, homeologous recombination as a result of Mlh1-deficiency may increase the frequency of successful but error-prone ICL repair, resulting in an attenuation of DNA damage signaling, reduced levels of chromosomal abnormalities, and enhanced survival of immortal cells.
Although our initial results revealed embryonic lethality of FA and Mlh1 double mutant mice, viable mice and cells with Fancd2 and Mlh1 deficiencies were generated using mixed genetic backgrounds, providing evidence against a synthetic lethal interaction. Our data indicate that Mlh1-deficient cells can be sensitized to MMC by loss of Fancd2 function, however, isogenic control fibroblasts proficient for MLH1 displayed even greater MMC hypersensitivity due to Fancd2-deficiency. To what extend immortalized mouse fibroblasts represent human tumor cells is unclear. Nevertheless, our data suggest that the systemic application of FA pathway inhibitors to potentiate the therapeutic effect of alkylating agents in MLH1-deficient tumors may favor tumor cell survival over somatic cell survival.
Acute myeloid leukemia (AML) and squamous cell carcinoma of the head and neck (HNSCC) are malignancies that are frequently encountered in FA patients (2). In the general population loss of MLH1 function is observed in a significant number of AML and HNSCC incidences (45). Therefore, resistance to therapeutic agents due to loss of MLH1 function should be considered when treating FA patients for acute myeloid leukemia or squamous cell carcinoma of the head and neck.
In summary, our data show that mismatch repair deficiency by loss of Mlh1 can reduce the DNA interstrand crosslinker hypersensitivity of Fancd2-deficient cells and attenuates crosslinker induced chromosomal aberrations. As a result, FA mouse models and cells provide a unique model to study the mechanisms of mismatch repair-associated resistance to agents that induce DNA interstrand crosslinks. The functional consequences of combined FA and MMR defects should be considered when treating malignancies, particularly in FA patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Lieneke Bouwman, Adrian Wilson, and Xiaoman Zhu for technical assistance, Laura Roy, Sean Baker, Qingshou Zhang, and Scot Stadler for helpful discussions and comments. We also thank Sietske Bakker and Hein te Riele for supporting experiments and critical reading of the manuscript.
This work was supported by a Dutch Cancer Society fellowship (H.J.V.) and NIH NHLBI program project grant P01 HL048546 (M.G.)
Footnotes
Oregon Health & Science University and M. Grompe have a significant financial interest in the On-Q-ity. This potential individual and institutional conflict of interest has been reviewed and managed by Oregon Health & Science University. The other authors have no conflict of interest.
REFERENCES
- 1.Hoeijmakers JHJ. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Van de Vrugt HJ, Grompe M. Fanconi anemia. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn errors of development. 2nd ed. Oxford University Press; New York: 2008. pp. 1230–6. [Google Scholar]
- 3.Chirnomas D, Taniguchi T, De la Vega M, Vaidya AP, Vasserman M, Hartman AR, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5:952–61. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]
- 4.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 5.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–15. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 6.Pan X, Ye P, Yuan DS, Wang X, Bader JS, Boeke JD. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell. 2006;124:1069–81. doi: 10.1016/j.cell.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Martin SA, Lord CJ, Ashworth A. DNA repair deficiency as a therapeutic target in cancer. Curr Op Gen Dev. 2008;18:1–7. doi: 10.1016/j.gde.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Wimmer K, Etzer J. Constitutional mismatch repair-deficiency syndrome: have we so far seen only the tip of an iceberg? Hum Gen. 2008;124:105–22. doi: 10.1007/s00439-008-0542-4. [DOI] [PubMed] [Google Scholar]
- 9.Grompe M, Van de Vrugt H. The Fanconi family adds a fraternal twin. Dev Cell. 2007;12:661–2. doi: 10.1016/j.devcel.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Smogorzewska A, Matsuoka S, Vinciguerra P, McDonald ER, Hurov KE, Luo J, et al. Identification of the FANCI Protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129:289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng M, Litman R, Xie J, Sharma S, Brosh RM, Jr, Cantor SB. The FANCJ/MutL interaction is required for correction of the cross-link response in FA-J cells. Embo J. 2007;26:3238–49. doi: 10.1038/sj.emboj.7601754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meetei AR, Sechi S, Wallisch M, Yang D, Young MK, Joenje H, et al. A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome. Mol Cell Biol. 2003;23:3417–26. doi: 10.1128/MCB.23.10.3417-3426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–5. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka K, Yoshioka Y, Hsieh P. ATR kinase activation mediated by MutSα and MutLα in response to cytotoxic O6-methylguanine adducts. Mol Cell. 2006;22:501–10. doi: 10.1016/j.molcel.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J J, Edelmann W. Mismatch repair proteins as sensors of alkylation DNA damage. Cancer Cell. 2006;9:417–8. doi: 10.1016/j.ccr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehme A, et al. The Role of DNA Mismatch Repair in Platinum Drug Resistance. Cancer Res. 1996;56:4881–6. [PubMed] [Google Scholar]
- 17.Claij N, Te Riele H. Msh2 deficiency does not contribute to cisplatin resistance in mouse embryonic stem cells. Oncogene. 2004;23:260–6. doi: 10.1038/sj.onc.1207015. [DOI] [PubMed] [Google Scholar]
- 18.Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, et al. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 2003;17:2021–35. doi: 10.1101/gad.1103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–42. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- 20.Houghtaling S, Granville L, Akkari Y, Torimaru Y, Olson S, Finegold M, et al. Heterozygosity for p53 (Trp53+/−) accelerates epithelial tumor formation in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Cancer Res. 2005;65:85–91. [PubMed] [Google Scholar]
- 21.Houghtaling S, Newell A, Akkari Y, Taniguchi T, Olson S, Grompe M. Fancd2 functions in a double strand break repair pathway that is distinct from non-homologous end joining. Hum Mol Genet. 2005;14:3027–33. doi: 10.1093/hmg/ddi334. [DOI] [PubMed] [Google Scholar]
- 22.Timmers C, Taniguchi T, Hejna J, Reifsteck C, Lucas L, Bruun D, et al. Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol Cell. 2001;7:241–8. doi: 10.1016/s1097-2765(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 23.Buermeyer AB, Patten C V, Baker SM, Liskay RM. The Human MLH1 cDNA Complements DNA Mismatch Repair Defects in Mlh1-deficient Mouse Embryonic Fibroblasts. Cancer Res. 1999;59:538–41. [PubMed] [Google Scholar]
- 24.Akkari Y, Noll M, Bateman RL, Reifsteck CA, D'Andrea AD, Olson SB, et al. The 4N Cell Cycle Delay in Fanconi Anemia Reflects Growth Arrest in Late S Phase. Mol Genet Met. 2001;74:403–12. doi: 10.1006/mgme.2001.3259. [DOI] [PubMed] [Google Scholar]
- 25.Newell AH, Hemphill A, Akkari Y, Hejna J, Moses RE, Olson SB. Loss of homologous recombination or non-homologous end joining leads to radial formation following DNA interstrand crosslink damage. Cyto Genome Res. 2008;121:174–80. doi: 10.1159/000138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koomen M, Cheng NC, Van de Vrugt HJ, Godthelp BC, Van der Valk MA, Oostra AB, et al. Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice. Hum Mol Genet. 2002;11:273–81. doi: 10.1093/hmg/11.3.273. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997;11:1226–41. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 28.Van de Vrugt HJ, Cheng NC, De Vries Y, Rooimans MA, De Groot J, Schepers RJ, et al. Cloning and characterization of murine Fanconi anemia group A gene: Fanca protein is expressed in lymphoid tissues, testis, and ovary. Mamm. Genome. 2000;11:326–31. doi: 10.1007/s003350010060. [DOI] [PubMed] [Google Scholar]
- 29.Van de Vrugt HJ, Koomen M, Berns MAD, De Vries Y, Rooimans MA, Van der Weel L, et al. Characterization, expression and complex formation of the murine Fanconi anaemia gene product Fancg. Genes Cell. 2002;7:333–42. doi: 10.1046/j.1365-2443.2002.00518.x. [DOI] [PubMed] [Google Scholar]
- 30.Paz M, Ladwa S, Champeil E, Liu Y, Rockwell S, Boamah EK, et al. Mapping DNA adducts of mitomycin C and decarbamoyl mitomycin C in cell lines using liquid chromatography/electrospray tandem mass spectrometry. Chem Res Tox. 2008;21:2370–8. doi: 10.1021/tx8002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bissonnette L, Arnason JT, Smith ML. Real-time fluorescence-based detection of furanocoumarin photoadducts of DNA. Phytochemical Analysis. 2008;19:342–7. doi: 10.1002/pca.1058. [DOI] [PubMed] [Google Scholar]
- 32.Wu Q, Vasquez KM. Human MLH1 protein participates in genomic damage checkpoint signaling in response to DNA interstrand crosslinks, while MSH2 functions in DNA repair. PLoS Genetics. 2008;4:e1000189. doi: 10.1371/journal.pgen.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong J, Costanzo A, Yang HQ, Melino G, Kaelin WG, Levrero M, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 34.Lan Z, Sever-Chroneos Z, Strobeck MW, Park C, Baskaran R, Edelmann W, et al. DNA Damage Invokes Mismatch Repair-dependent Cyclin D1 Attenuation and Retinoblastoma Signaling Pathways to Inhibit CDK2. J Biol Chem. 2002;277:8372–81. doi: 10.1074/jbc.M108906200. [DOI] [PubMed] [Google Scholar]
- 35.Stojic L, Mojas N, Cejka P, di Pietro M, Ferrari S, Marra G, et al. Mismatch repair-dependent G2 checkpoint induced by low doses of SN1 type methylating agents requires the ATR kinase. Genes Dev. 2004;18:1331–44. doi: 10.1101/gad.294404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadyrov FA, Dzantiev L, Constatin N, Modrich P. Endonucleolytic function of MutLα in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 37.Schmutte C, Sadoff MM, Shim KS, Acharya S, Fishel R. The interaction of DNA mismatch repair proteins with human exonuclease I. J Biol Chem. 2001;276:33011–8. doi: 10.1074/jbc.M102670200. [DOI] [PubMed] [Google Scholar]
- 38.Yang W. Human MutLα the jack of all trades in MMR is also an endonuclease. Mut Res. 2007;6:135–9. doi: 10.1016/j.dnarep.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derheimer FA, Hicks JK, Paulsen MT, Canman CE, Ljungman M. Psoralen-induced DNA Interstrand Cross-Links Block Transcription and Induce p53 in an Ataxia-Telangiectasia and Rad3-Related-Dependent Manner. Mol Pharm. 2009;75:599–607. doi: 10.1124/mol.108.051698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freie BW, Li X, Ciccone SLM, Nawa K, Cooper S, Vogelweid C, et al. Fanconi anemia type C and p53 cooperate in apoptosis and tumorigenesis. Blood. 2004;102:4146–52. doi: 10.1182/blood-2003-03-0971. [DOI] [PubMed] [Google Scholar]
- 41.Niedernhofer LJ, Lalai AS, Hoeijmakers JHJ. Fanconi Anemia (Cross)linked to DNA Repair. Cell. 2005;123:1191–8. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet. 2007;8:735–48. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- 43.Elliott B, Jasin M. Repair of double-strand breaks by homologous recombination in mismatch repair-defective mammalian cells. Mol Cell Bio. 2001;21:2671–82. doi: 10.1128/MCB.21.8.2671-2682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao C, Deng L, Chen Y, Kucherlapati R, Stambrook PJ, Tischfield JA. Mlh1 mediates tissue-specific regulation of mitotic recombination. Oncogene. 2004;23:9017–24. doi: 10.1038/sj.onc.1208148. [DOI] [PubMed] [Google Scholar]
- 45.Sengupta S, Chakrabarti S, Roy A, Panda CK, Roychoudhury S. Inactivation of human mutL homolog 1 and mutS homolog 2 genes in head and neck squamous cell carcinoma tumors and leukoplakia samples by promoter hypermethylation and its relation with microsatellite instability phenotype. Cancer. 2007;109:703–12. doi: 10.1002/cncr.22430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.