Abstract
In old and even middle age, there are associations between physical health and both intelligence and education. This may occur because intelligence and/or education exert effects on lifestyle choices that, in turn, affect later health. Substance use is one aspect of lifestyle choice in young adulthood that could play such a role. The effects of intelligence and/or education on substance use could be direct and environmental, or indirect due to the presence of confounding genetic and shared family influences. We used the Minnesota Twin Family Study to distinguish these effects in males and females at age 24. In contrast to prevailing expectations, there were moderately negative direct nonshared environmental effects of both IQ and education on both smoking and drinking in both males and females. That is, controlling for positive family background effects in the form of both genetic and shared environmental influences, both higher IQ and greater education were associated with greater alcohol and nicotine use. These effects were accounted for by alcohol and nicotine use at age 17. Our results suggest that genetic and family-culture variables confound the associations between intelligence and education and substance use in young adults, rendering them indirect. Further research is needed to understand the roles of IQ and education in alcohol and nicotine use and their relative impacts on physical health throughout the lifespan.
Keywords: IQ, education, health-related behaviors, alcohol use, smoking, substance use, young adulthood
In middle and old age, there are associations between physical health and both intelligence and education (Carlson, Fried, Ave, Bandeen-Roche, Zeger, & Brandt, 1999; Deary, Whalley, Batty, & Starr, 2006; Gottfredson & Deary, 2004; Malmstrom, Wolinsky, Andresen, Miller, & Miller, 2005; Tabbarah, Crimmins, & Seeman, 2002), but how these associations develop is not understood. Physical health problems are major sources of economic expense, anxiety, and deterioration in quality of life. Understanding the ways in which intelligence and education contribute to physical health would be an important epidemiological advance, and might lead to new approaches to help people better manage their own health, leading to more effective disease prevention and reduced medical care costs.
One possible reason for the associations between physical health and intelligence and education is that intelligence and/or education may act to support lifestyle choices and the development of habits that over time maintain or undermine physical health. Education is generally the variable more readily available to researchers, and many epidemiological studies include adjustment for it as a possible confounding, causally prior environmental factor in studies of the determinants of health outcomes. There is, however, evidence that intelligence functions in this manner as well and may lie earlier on the causal pathways (Gottfredson, 2004; Hart, et al., 2003). The effects of the two variables are rarely compared head to head in epidemiological studies. Moreover, if intelligence and/or education do have their effects on physical health through the emergence of lifestyle choices and habits such as smoking and drinking that maintain or undermine health in later life, we should see their effects on these choices and habits in earlier life. Few studies have examined the influences of intelligence or education on specific lifestyle behaviors such as smoking and drinking in younger adults that might contribute to their later physical health. One study that did, however, found an association between higher IQ and reduced smoking behavior in young adults that was attenuated by education (Batty, Deary, Schoon, & Gale, 2007).
The authors of this study (Batty et al., 2007) were careful not to draw causal conclusions about the nature of the association between earlier measured intelligence and later smoking behavior, but they did note that the range of other risk factors for which they were able to control helped to establish the specificity of the association to IQ. They also pointed out that adjusting for education may have overcorrected since IQ also contributes to educational attainment. But however long, any list of statistical controls is necessarily arbitrary and incomplete. There is always the possibility that some unmeasured variable confounds the association. For example, Batty et al. (2007) controlled for parental and participant social class and participant annual gross earnings. The operative variable, however, could actually be a personality trait like conscientiousness, which could lead to more careful completion of the survey’s IQ test and thus higher scores. Conscientiousness has also been associated with both better health-related behaviors and higher educational attainment (Roberts, Kuncel, Shiner, Caspi, & Goldberg, 2007).
The variables at issue here, particularly IQ but also education, suggest an additional potentially confounding complication. Traditional epidemiological studies are incapable of controlling for genetic or family cultural selection. This is the tendency for genetic or family cultural influences on one trait to influence another as well, so that the association between risk and outcome reflects at least some origin of the risk in the individual rather than merely effect of the risk in its own right (Rutter, 2007). IQ is well known to be subject to such familial influences, particularly genetic ones (e.g., Bouchard & McGue, 1981), as are health-related behaviors such as substance use (e.g., Prescott, Madden, & Stallings, 2006) and physical health itself (e.g., Reed & Dick, 2003). But education is subject to these kinds of familial influences as well (e.g., Behrman, Pollak, & Taubman, 1995; Johnson, Deary, & Iacono, 2008). We are not aware of any studies investigating whether these familial influences on IQ and/or education overlap with those on health-related behaviors such as smoking and drinking, but evidence that intelligence and/or education may be involved in lifestyle choices that affect physical health suggests that they might be. To the extent that they are, we would not consider the effects of intelligence and/or education on health-related behaviors to be direct because these effects would not hold up after controlling for the genetic and family cultural variables that distinguish among but not within families; instead these effects would be indirect because confounded by genetic and shared environmental familial selection processes.1 It makes sense to distinguish direct from indirect familial selection effects to the extent possible, as the policy options for dealing with them differ.
It is thus important that epidemiological research include study designs capable of distinguishing direct effects of intelligence and/or education on health-related behaviors from indirect effects operating through familial selection processes. Behavior genetic twin designs can provide an important element of quasi-experimental control because, to the extent that monozygotic twins differ in intelligence and/or education, associated differences in their health-related behaviors such as smoking and drinking cannot be attributed either to genetic confounds or to any aspect of the family environment that they share such as socioeconomic status, family structure, or family relationships, whether such variables are assessed in the analysis or not. It is still not possible to draw strictly causal conclusions of course, as even differences within pairs may result from factors lther than those contributing to the variables actually considered, but the range of such possibilities is much more limited. In addition, articulation of the kinds of selection processes involved in common genetic and/or family environmental influences can help to develop hypotheses about the specific influences they entail.
In young adulthood, smoking and drinking behaviors may be particularly good examples of the kinds of lifestyle choices and personal habits that may affect later physical health outcomes. Smoking is a well-known risk factor for a host of cardiovascular and respiratory diseases, and excessive alcohol consumption also contributes to later health problems. Moreover, the disinhibitory behavior involved in smoking and drinking behaviors may indicate a lack of personal conscientiousness that may be associated with other lifestyle choices that impact later health. Before any effects of smoking and drinking on physical health can be manifest, however, the choices to drink to excess and to smoke must be made and the habits of doing so instilled. In this study, we focused on the emergence of these health-related behavior choices and habits in young adulthood. We explored the associations between intelligence and education and alcohol use and smoking in young adult male and female twin samples in order to distinguish direct environmental effects of education and intelligence from indirect effects involving genetic and family environmental selection processes. Our study design also allowed us to distinguish the effects of intelligence from those of education.
METHOD
Sample
Participants were male and female twin pairs in the ongoing Minnesota Twin Family Study (MTFS). The MTFS is a population-based accelerated longitudinal study of same-sex twin pairs and their parents. It includes two cohorts, recruited at ages 17 and 11. We made use of the older male and female cohorts in this study. The cohorts were recruited by using publicly available databases to determine the location of more than 90% of the same-sex twin pairs born in Minnesota in the targeted years of 1972–1978 for males and 1975–1979 for females. Located twins living with at least one biological parent within a day’s drive of Minneapolis were invited to complete a day-long, in-person assessment at our labs at the University of Minnesota. Those with significant mental or physical handicap were excluded. Less than 20% of located, eligible families declined participation. Both samples have been consistently sampled for the smoking and drinking behaviors at ages 17, 20, and 24, though we did not make use of the age 20 data here.
The twins and their parents were generally representative of the Minnesota population during the period of the twins' birth. Consistent with the demographics of Minnesota for the birth years sampled, over 98% of the twins were Caucasian. The average Hollingshead (1957) occupational level for the families was about 4. This indicates jobs at the skilled "blue collar" level, commensurate with an average level of education slightly beyond high school. Some parents worked in highly professional occupations while others were unemployed or worked in semi-skilled jobs, however, so the full range of occupations was represented. The standard deviation was just under 2 Hollingshead levels. Fathers reported a little more than 14.5 years of education on average, mothers about 1 year less. More than 80% of the families who did not participate still completed a brief mail or telephone survey. This made it possible to compare participants and non-participants on some measures. Parents in non-participating families were significantly but only modestly less educated than those in participating families. The mean difference was less than .3 years of education. Non-participating families did not differ significantly from participants in self-reported mental health (e.g., treatment or acknowledged problems with depression or substance use), indicating that there was little reason to suspect that non-participating families differed from participants in this very general measure of personal stability that might have some influence on the associations of interest in this study. Iacono, Carlson, Taylor, Elkins, and McGue (1999) provide a complete analysis of non-participants and description of the ascertainment and assessment procedures used in the MTFS.
The male sample consisted of 578 twins (289 pairs). Of these, 376 were monozygotic (MZ) and 202 were dizygotic (DZ). The female sample consisted of 674 twins (337 pairs), including 446 MZ and 228 DZ twins. At age 24, 532 (92%; 342 MZ, 190 DZ) males and 631 (93%; 410 MZ, 221 DZ) females returned for assessment. For male twins, there were no significant differences between those who returned at age 24 and those who did not in family occupational level or any of the substance use variables. For female twins, those who returned at age 24 had somewhat greater IQ’s at age 17 (standardized mean difference of .36) than those who did not return.
Measures
IQ
The twins were assessed at age 17 using an abbreviated version of the WAIS consisting of 2 verbal (Vocabulary and Information) and 2 performance (Block Design and Picture Arrangement) subtests. These subtests were selected for their high correlation (.90) with total IQ based on all subtests. The variable was approximately normally distributed in both the male and female samples. We standardized it with a mean of 0 and standard deviation of 1 for use in these analyses.
Educational Attainment
At age 24, participants reported both educational attainment to date and their current enrollment in educational programs, if relevant. We used this information to compile a 12-point scale of educational attainment. Failure to complete high school was coded as 0. Participants who dropped out of high school but returned to complete a general equivalency degree were coded as 1; completion of high school in the usual manner with no education beyond that was coded as 2. Enrollment in and completion of a post-high school vocational training program were coded as 3 and 4, respectively; Enrollment in community college through completion of a 4-year college degree were coded as 5 to 8. Participants who were enrolled in master’s degree programs were coded as 9; those who had completed master’s degrees but were no longer enrolled in school were coded as 10, and enrollment in and completion of professional degree programs (PhD, JD, MD, etc.) were coded as 11 and 12. The variable was approximately normally distributed. We standardized it with a mean of 0 and standard deviation of 1 for use in these analyses. We refer to this as Education in the remainder of the paper.
Substance Use Variables
The MTFS incorporates an extensive assessment of substance use and abuse including symptoms of alcohol and nicotine dependence as defined by the Diagnostic and Statistical Manual of Mental Disorders 3rd Edition Revised (DSM-III-R, which was the current diagnostic system when the study began; American Psychiatric Association, 1987). Symptoms of substance use disorders were based on structured interviews by trained interviewers with bachelor-level degrees, using the Substance Abuse Module of the Composite Diagnostic Interview (Robins, Baber, & Cottler, 1987), and derived using a consensus process between two trained graduate-level clinicians. This is the typical way of establishing the validity of such mental disorders, and, in MTFS, generates kappa reliabilities in excess of .91. The assessment intervals were lifetime at age 17 and the last 3–4 years at age 24.
Because clinical substance use disorder symptoms provide only one perspective on clearly multifaceted substance use behaviors, we also made use of the other variables involving substance use behavior that were available in our sample to develop composite measures of alcohol and nicotine use that reflected various aspects of normative and non-normative use. The alcohol use composite included symptoms of alcohol abuse/dependence, number of intoxications, frequency of use in the past 12 months (10 point scale from < 1× a year to >3× a day), average quantity (i.e., number of drinks) in the past 12 months, and maximum number of drinks consumed in 24 hours. The nicotine use composite included symptoms of nicotine dependence, the number of days the participant smoked in a typical month, and the amount smoked (i.e., number of cigarettes) in a typical day during the past 12 months. Test-retest and convergent validity for self-reports of this kind has been established in both clinical and nonclinical groups (Teitelbaum & Carey, 2000), and the approach of assessing both frequency and severity of drinking behaviors and combining them into a single quantitative dimension is now considered relatively standard within the field of substance use research, and particularly in alcohol research (Room, 2000). We computed the composites by summing the standardized (mean 0 and standard deviation of 1) scores for each variable, log transformed to reduce positive skew. We also reversed the variables so that higher scores indicated healthy absence of substance use.
Analytical Approach
The standard quantitative genetic model for a single trait is based on the assumption that the observed variance (Vp) in the trait of interest is a linear additive function of independent genetic (A) and shared (C) and non-shared (E) environmental components of variance. The shared environmental variance represents experiential factors common to the members of a twin pair that operate to make them similar, but distinguish them from members of other families. These factors may include experiences such as growing up with the same religious traditions and parental socioeconomic status. Non-shared environmental variance represents those experiential factors unique to each member of a twin pair that operate to make them different. Such experiences may include having different teachers and friends, participating in different activities, and receiving different parental treatment. Thus if one twin went to college and the other did not, the effects that this had to differentiate their substance use patterns would be captured by the E component. The non-shared environmental component also includes variance attributable to measurement error. The non-shared environmental component was the primary focus of interest in this paper, as it captures the extent to which individuals differ from each other controlling for all family background variables, both genetic and environmental.
Symbolically, the model for a single trait is expressed as,
MZ twin pairs have the same genomes, while DZ twin pairs have, on average, 50% of their segregating genes in common, and members of both kinds of twin pairs are assumed to share environments to the same extent. Thus, the covariance for MZ twin pairs can be expressed symbolically as,
and that for DZ twin pairs can be expressed as,
In this study we were not interested in the magnitudes of genetic or environmental influences on any single trait as these are well documented in other sources (e.g. Plomin, De Fries, McClearn, & McGuffin, 2007). Instead, we were interested in understanding how IQ and Education influenced substance use and, in particular, whether they had direct effects after controlling for family background. This meant considering the genetic and environmental influences on these traits in a multivariate context. The standard model for a single trait can be extended to such multivariate situations by modeling the covariance between one twin's score on one variable and the other twin's score on another variable in the same manner as for a single trait. The model is effectively a regression that recounts the contributions of the variable specified first to variance in all the variables following it in the model, the contributions of the variable specified second to variance in all the variables following it, and so on, while simultaneously decomposing those contributions into their genetic and shared and nonshared environmental components. Like a regression model, therefore, the variables should be placed in appropriate temporal or conceptual order, and the effects of the last contributing variable on the outcome are controlled for the presence of all variables preceding it in the model. We implemented the model in the structural modeling program Mx (Neale, Boker, Xie, & Maes, 2003).
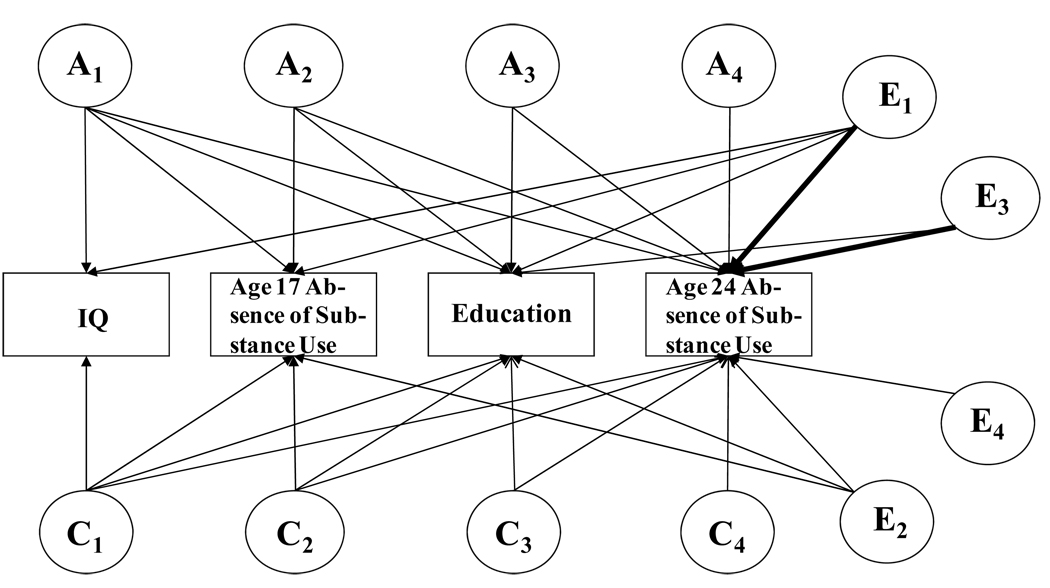
Figure 1 diagrams the genetic, shared, and nonshared environmental paths influencing each of the variables contributing to substance use, with Age 24 Substance Use at the end because it was the outcome variable. The latent genetic and environmental influences are labeled with subscripts 1–4 to emphasize that these influences were not specific to the contributing variables. The nonshared environmental paths from IQ and Education to Age 24 Substance Use that were of particular interest are shown in bold. In our conceptualization of the effects of IQ and Education on later substance use, the causal role of earlier substance use was unclear. We therefore modeled the effects of IQ and Education both with and without control for Age 17 Substance Use.
Figure 1.
Basic Cholesky model. A refers to genetic, C and E to shared and nonshared environmental influences. The paths in bold represent direct effects of IQ and education on age 24 healthy absence of substance use, controlling for their genetic and shared environmental influences.
Because some participants were missing data for some variables, we read raw data into the Mx program, using full-information maximum likelihood to estimate the model parameters allowing for the absence of small amounts of data. This method relies on the assumptions that the variables are reasonably normally distributed and that the data not present are missing at random (Little & Rubin, 1987). These assumptions were reasonable for our variables.
RESULTS
Descriptive statistics
Table 1 shows descriptive statistics for the composite substance use, IQ, and education variables. To indicate the levels of use reflected by the raw variables (before transformation and standardization) contributing to the composites, we also show their descriptive statistics. Though the means of the variables contributing to the composites did not indicate high levels of usage, the sample included both participants who had never used alcohol or smoked and participants who had high levels of substance dependence. Participants also spanned broad ranges of IQ and Education. Females had significantly lower mean levels of substance use and less variability than did males.
Table 1.
Descriptive Statistics of Composite and Raw Study Variables in Males and Females
| Measure | Males |
Females |
||||
|---|---|---|---|---|---|---|
| Observed Values | Mean | Standard Deviation | Observed Values | Mean | Standard Deviation | |
| Alcohol Use Composite at Age 17 | −.85 to 3.72 | .18 | 1.07 | −.97 to 4.66 | −.15 | .91 |
| Alcohol Use Composite at Age 24 | −1.87 to 3.91 | .43 | 1.06 | −2.12 to 3.03 | −.36 | .79 |
| Nicotine Use Composite at Age 17 | −.55 to 3.60 | .00 | 1.02 | −.55 to 3.20 | .00 | .98 |
| Nicotine Use Composite at Age 24 | .76 to 2.90 | .19 | 1.09 | −.78 to 2.18 | −.16 | .89 |
| WAIS IQ at Age 17 | 69 to 148 | 102.90 | 13.97 | 69 to 151 | 96.93 | 13.59 |
| Education at Age 24 | 0 to 11 | 5.93 | 2.44 | 0 to 11 | 6.53 | 2.34 |
| Alcohol Dependence Symptoms at 17 | 0 to 8 | .62 | 1.38 | 0 to 9 | .42 | 1.25 |
| Number of Intoxications at Age 17 | 0 to 125 | 8.52 | 21.55 | 0 to 999 | 9.27 | 63.21 |
| Frequency of Alcohol Use at Age 17 | 5 to 11 | 9.02 | 1.64 | 2 to 11 | 9.20 | 1.67 |
| Average Number of Drinks at Age 17 | 1 to 13 | 2.92 | 3.29 | 0 to 15 | 1.39 | 2.40 |
| Maximum Drinks in 24 Hours at 17 | 0 to 48 | 7.71 | 9.02 | 0 to 48 | 4.87 | 6.12 |
| Nicotine Dependence Symptoms at 17 | 0 to 7 | .87 | 1.94 | 0 to 7 | .73 | 1.69 |
| Frequency of Smoking at Age 17 | 0 to 30 | 6.17 | 10.99 | 0 to 30 | 6.36 | 11.25 |
| Quantity Smoked per Day at Age 17 | 0 to 35 | 2.06 | 5.25 | 0 to 30 | 2.11 | 4.43 |
| Alcohol Dependence Symptoms at 24 | 0 to 9 | 1.45 | 1.79 | 0 to 8 | .46 | 1.15 |
| Number of Intoxications at Age 24 | 0 to 999 | 119.98 | 242.85 | 1 to 999 | 65.13 | 192.69 |
| Frequency of Alcohol Use at Age 24 | 3 to 11 | 6.72 | 1.65 | 0 to 10 | 6.96 | 2.07 |
| Average Number of Drinks at Age 24 | 0 to 13 | 4.82 | 3.57 | 0 to 15 | 2.58 | 2.03 |
| Maximum Drinks in 24 Hours at 24 | 0 to 60 | 16.50 | 10.02 | 0 to 60 | 8.39 | 5.82 |
| Nicotine Dependence Symptoms at 24 | 0 to 7 | 1.75 | 2.31 | 0 to 7 | .95 | 1.64 |
| Frequency of Smoking at Age 24 | 0 to 30 | 12.38 | 13.72 | 0 to 30 | 8.34 | 12.82 |
| Quantity Smoked per Day at Age 24 | 0 to 40 | 5.31 | 8.24 | 0 to 30 | 3.26 | 5.69 |
Note: Substance use composites were log-transformed and standardized. All sex differences in means for the study variables used were significant at p<.001 with the exception of the nicotine use composite at age 17. Sex differences in the raw variables contributing to the composites were significant at p<.01 for all but the number of intoxications and frequency of alcohol use at age 17, the smoking variables at age 17, and frequency of alcohol use at age 24. For maximum number of intoxications, 999 was used as 'too many to count.'
Table 2 shows the zero-order correlations among the variables we used, separately for males and females. As might be expected, some of the most substantial positive correlations were between the same substance use variables across time, but the positive correlations between contemporaneous substance use variables were similarly substantial. Most of the positive correlations between the healthy substance use variables and Education were stronger than those between IQ and healthy substance use. The differences were significant at nominal p values less than .01 (using Fisher’s transformation with no correction for multiple testing) in both males and females. Though the differences were at best marginally significant, the correlations between Education and healthy Nicotine Use were greater than the correlations between Education and healthy Alcohol Use in both males and females. There were no clear differences in the magnitudes of the correlations between IQ and healthy Nicotine Use and Alcohol Use in either males or females. There were also no clear patterns of sex differences in the magnitudes of the correlations.
Table 2.
Correlations Among Study Variables; Substance Use Variables Reversed to Reflect Healthy Absence of Use
| Measure | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Alcohol Use Composite at Age 17 | 1.00 | .34 | .58 | .44 | .17 | .24 |
| 2. Alcohol Use Composite at Age 24 | .49 | 1.00 | .22 | .37 | −.03 | −.02 |
| 3. Nicotine Use Composite at Age 17 | .59 | .27 | 1.00 | .61 | .15 | .34 |
| 4. Nicotine Use Composite at Age 24 | .40 | .37 | .56 | 1.00 | .15 | .33 |
| 5. WAIS IQ at Age 17 | .19 | .13 | .20 | .16 | 1.00 | .39 |
| 6. Education at Age 24 | .32 | .13 | .42 | .39 | .28 | 1.00 |
Note: Males are below the diagonal; females above. Adjusting for paired observations, correlations of at least .10 were significant at p<.05. Differences in independent correlations (such as those for males and females) were significant if they were in excess of .12. Differences in non-independent correlations (such as those for males or those for females) depended on the correlation between the predicting variables but were generally significant at approximately the same magnitude. See text for further details.
Correlations between members of twin pairs for the study variables are given in Table 3. These correlations provide background information from the raw data about the presence of genetic and environmental influences on each variable as preliminary indications of the likely results from the multivariate model. If there were shared environmental influences but no genetic influences on the variables we would expect that the phenotypic correlations in Table 3 would be the same in MZ and DZ twin pairs. In contrast, if there were genetic but no shared environmental influences, we would expect that the phenotypic correlations in Table 3 would be twice as large in MZ as in DZ twin pairs. The correlations thus provided evidence for both genetic and shared environmental influences on the variables.
Table 3.
Twin Correlations of Study Variables; Substance Use Variables Reversed to Reflect Healthy Absence of Use
| Males |
Females |
|||
|---|---|---|---|---|
| Measure | MZ | DZ | MZ | DZ |
| Alcohol Use Composite at Age 17 | .74 | .51 | .68 | .51 |
| Alcohol Use Composite at Age 24 | .56 | .40 | .44 | .07 |
| Nicotine Use Composite at Age 17 | .64 | .60 | .59 | .36 |
| Nicotine Use Composite at Age 24 | .63 | .33 | .51 | .30 |
| WAIS IQ at Age 17 | .80 | .50 | .79 | .48 |
| Education at Age 24 | .69 | .60 | .67 | .50 |
Note: Twin correlations are double-entered Pearsons.
Estimates from the Multivariate Model
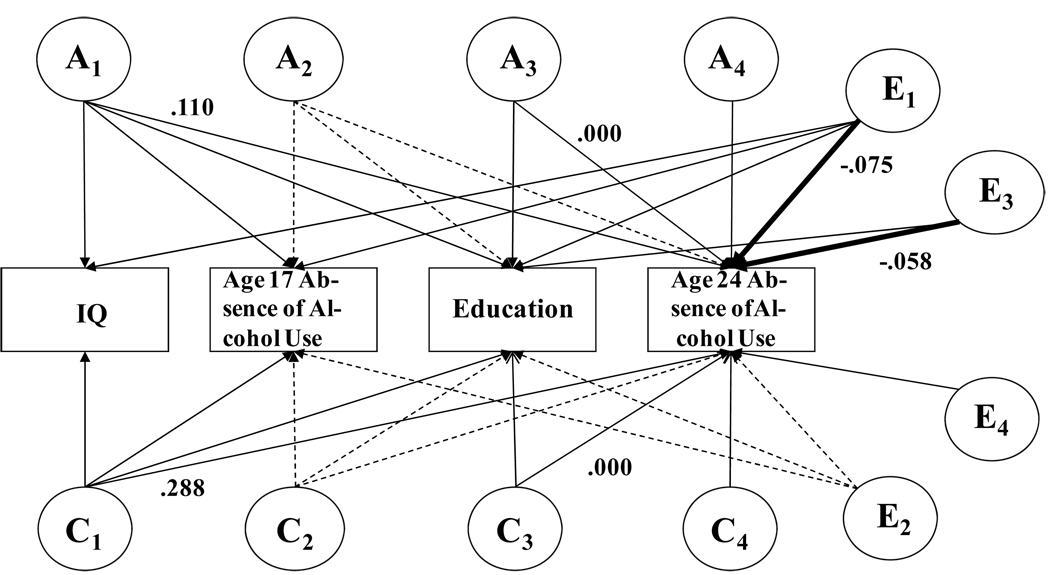
Table 4 shows the effect sizes (proportions of variance) involved in the path coefficients from IQ, substance use at age 17, and Education to substance use at age 24 in males, as allocated to genetic and shared and nonshared environmental influences, along with their 95% confidence levels. As noted above, we show these effects with and without control for earlier substance use. To place them in the context of the full model we used, Figure 2 shows the effect sizes for the results for Alcohol Use at age 24, and the results for Nicotine Use fit into the model analogously. In the figure, the paths estimated only when controlling for earlier absence of use are shown with dashed lines. Without control for earlier substance use, there were direct, nonshared environmental effects of both IQ and Education on later substance use. In contrast to prevailing expectations, these effects were negative. That is, young men with higher IQ’s and greater Education were more likely to use both alcohol and nicotine at age 24, after controlling for genetic and shared environmental influences. For example, for Alcohol Use, Table 4 shows that there were direct nonshared environmental effects totaling 7.5% of the variance in Alcohol Use at age 24 with a 95% confidence interval of 3.6–12.8%, and that these effects were negative. At the same time, IQ had significant protective genetic and shared environmental effects on both Alcohol and Nicotine Use: genetic and shared environmental influences contributing to higher IQ also contributed to healthy absence of substance use. For example, again for Alcohol Use, Table 4 shows that genetic effects shared with IQ accounted for 11.0% of the variance in Alcohol Use at age 24 with a confidence interval of 1.3 to 28.6%, and that these effects were positive. Education showed no family effects of either the genetic or shared environmental kind.
Table 4.
Sizes and Directions of Effects of IQ and Education on Healthy Absence of Substance Use - Males
| Genetic | Shared Environmental | Non-Shared Environmental | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect Size | Direction | Confidence Interval | Effect Size | Direction | Confidence Interval | Effect Size | Direction | Confidence Interval | |
| Alcohol at Age 24 | |||||||||
| Without Control for Earlier Absence of Use | |||||||||
| IQ | .110 | + | .013–.286 | .288 | + | .117–.460 | .075 | − | .036–.128 |
| Education | .000 | + | .000–.069 | .000 | − | .000–.078 | .058 | − | .022–.109 |
| With Control for Earlier Absence of Use | |||||||||
| IQ | .090 | + | .001–.337 | .021 | − | .000–.261 | .002 | − | .000–.023 |
| Use at 17 | .029 | + | .000–.214 | .299 | + | .037–.601 | .035 | + | .008–.084 |
| Education | .048 | − | .000–.382 | .001 | + | .000–.245 | .004 | + | .000–.033 |
| Nicotine at Age 24 | |||||||||
| Without Control for Earlier Absence of Use | |||||||||
| IQ | .080 | + | .013–.242 | .350 | + | .191–.427 | .033 | − | .010–.068 |
| Education | .000 | + | .000–.070 | .000 | + | .000–.066 | .041 | − | .012–.085 |
| With Control for Earlier Absence of Use | |||||||||
| IQ | .001 | − | .000–.093 | .071 | + | .000–.425 | .013 | + | .001–.047 |
| Use at 17 | ,088 | + | .000–.340 | .161 | + | .000–.457 | .062 | + | .023–.122 |
| Education | .057 | + | .000–.532 | .000 | + | .000–.147 | .000 | − | .000–.013 |
Note: Effect size is proportion of total variance. Confidence intervals are at 95%. Noted direction of effect refers to the sign of the estimated parameter. Where the parameter was not significant, its confidence interval had one positive and one negative endpoint so the actual direction of effect was not certain.
Figure 2.
Results for healthy absence of Alcohol Use at age 24, without control for earlier absence of use, from the second section of Table 4. Coefficients are proportions of total variance, and the signs reflect the directions of the effects. Labels are as in Figure 1. Only coefficients for the paths of primary interest are shown. Paths estimated only when controlling for earlier use are shown with dashed lines.
After controlling for Alcohol Use at age 17, the shared and nonshared environmental effects of IQ on age 24 Alcohol Use were no longer significant. That is, the direct negative nonshared environmental effects of IQ were almost completely accounted for by shared environmental influences on use at age 17 (see the results in Table 4 with control for earlier absence of use). Genetic influences on IQ remained significant when age 17 Alcohol Use was controlled, though the magnitude of their effect was somewhat reduced. There was also a significant direct nonshared environmental effect of age 17 use, indicating either a protective effect of avoiding adolescent alcohol use or some stability of behavior across time.
For Nicotine Use, after accounting for age 17 Nicotine Use, the genetic and shared environmental effects of IQ on age 24 Nicotine Use were no longer significant. This indicated that age 17 use accounted for the negative effects of IQ and Education that we observed before control for age 17 use. Age 17 Nicotine Use had moderately large genetic and shared environmental effects on Age 24 Nicotine Use, but these failed to reach statistical significance. Age 17 Nicotine Use also had a direct nonshared environmental effect on Age 24 Nicotine, again reflecting either a protective effect of avoiding nicotine use in adolescence or stability of smoking behavior.
Table 5 shows the information analogous to Table 4 for females. As for males, there were direct negative nonshared environmental effects of both IQ and Education on both Alcohol and Nicotine Use, indicating that those with higher IQ’s and greater Education drank and smoked more. Again as for males, there were protective positive genetic and shared environmental effects of IQ. For Alcohol Use, control for age 17 use accounted for these effects, yet the relatively small direct protective nonshared environmental effects on earlier Alcohol Use did not appear sufficient to account for the full association but no other effects were significant. For Nicotine Use, the genetic, and shared and nonshared environmental effects for IQ on age 24 Nicotine were no longer significant after controlling for age 17 Nicotine Use. Age 17 Nicotine Use had significant genetic and nonshared environmental effects on Age 24 Nicotine Use.
Table 5.
Sizes and Directions of Effects of IQ and Education on Healthy Absence of Substance Use - Females
| Genetic | Shared Environmental | Non-Shared Environmental | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect Size | Direction | Confidence Interval | Effect Size | Direction | Confidence Interval | Effect Size | Direction | Confidence Interval | |
| Alcohol at Age 24 | |||||||||
| Without Control for Earlier Absence of Use | |||||||||
| IQ | .118 | + | .018–.266 | .181 | + | .053–.313 | .039 | − | .016–.072 |
| Education | .000 | + | .000–.052 | .000 | + | .000–.045 | .013 | − | .001–.038 |
| With Control for Earlier Absence of Use | |||||||||
| IQ | .002 | − | .000–.041 | .000 | + | .000–.068 | .001 | + | .000–.011 |
| Use at 17 | .047 | + | .000–.195 | .012 | + | .000–.092 | .024 | + | .006–.055 |
| Education | .030 | − | .000–.260 | .000 | + | .000–.082 | .002 | + | .000–.037 |
| Nicotine at Age 24 | |||||||||
| Without Control for Earlier Absence of Use | |||||||||
| IQ | .086 | + | .013–.242 | .282 | + | .128–.427 | .065 | − | .035–.105 |
| Education | .000 | + | .000–.070 | .000 | + | .000–.062 | .039 | − | .012–.076 |
| With Control for Earlier Absence of Use | |||||||||
| IQ | .018 | + | .000–.114 | .004 | + | .000–.178 | .001 | + | .000–.014 |
| Use at 17 | .277 | + | .062–.531 | .011 | + | .000–.244 | .058 | + | .026–.106 |
| Education | .000 | + | .000–.176 | .019 | − | .000–.178 | .004 | + | .000–.021 |
Note: Effect size is proportion of total variance. Confidence intervals are at 95%. Noted direction of effect refers to the sign of the estimated parameter. Where the parameter was not significant, its confidence interval had one positive and one negative endpoint so the actual direction of effect was not certain.
The confidence intervals around the genetic and shared environmental path coefficients were much larger than those around the nonshared environmental path coefficients, and some of the apparently nonsignificant path coefficients were reasonably substantial in size. This suggested that we lacked sufficient sample size to distinguish between genetic and shared environmental confounds. To test this, we estimated a combined familial influence consisting of effectively equal genetic and shared environmental influences by setting the similarity of DZ twins relative to MZ twins to .75 (rather than the .5 used in the standard behavior genetic model) and dropping the separate paths for shared environmental influences. This was preferable to dropping the genetic and shared environmental paths, which would effectively shift all the common variance between IQ and/or Education and the substance use outcomes to the nonshared environmental paths. As anticipated, this substantially narrowed the confidence intervals of the estimates for the path coefficients representing the combined familial influences.
The results for the combined family effects are shown in Table 6. The direct, nonshared environmental effects were essentially identical to those shown in Table 4 and Table 5. Combining the familial sources of influence did not shed additional light on the situation for Alcohol Use in males as the pattern of statistical significance of results was the same. For Nicotine Use in males, however, the familial effects of both age 17 use and Education were now significant, thus showing the way in which earlier use accounted for the associations between IQ and Education and age 24 use and indicating that the negative nonshared environmental effects of IQ and Education represented suppressor effects. That is, inclusion of age 17 Nicotine Use reduced the protective familial effects of IQ, accounted for substantial variance in its own right, and caused the negative direct nonshared environmental effects of both IQ and Education to change direction, though the resulting protective effects were not significant. This was the case for Alcohol Use in females as well: combining the familial influences made clear that together their influences on age 17 Alcohol Use accounted for the protective genetic and shared environmental effects of IQ on age 24 use, as well as the negative direct nonshared environmental effects. In addition, we were now able to distinguish a small negative familial effect of Education. Combining the familial sources of influence did not shed additional light on the situation for Nicotine Use in females.
Table 6.
Sizes and Directions of Combined Familial Effects of IQ and Education on Healthy Absence of Substance Abuse
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Effect Size | Direction | Confidence Interval | Effect Size | Direction | Confidence Interval | |
| Alcohol | ||||||
| Without Control for Earlier Absence of Use | ||||||
| IQ | .394 | + | .284–.527 | .300 | + | .215–.403 |
| Education | .000 | + | .000–.077 | .000 | + | .000–.049 |
| With Control for Earlier Absence of Use | ||||||
| IQ | .023 | + | .001–.075 | .002 | − | .000–.016 |
| Use at 17 | .230 | + | .132–.358 | .050 | + | .019–.097 |
| Education | .009 | − | .000–.050 | .011 | − | .001–.039 |
| Nicotine | ||||||
| Without Control for Earlier Absence of Use | ||||||
| IQ | .423 | + | .313–.557 | .360 | + | .269–.469 |
| Education | .000 | + | .000–.070 | .000 | + | .000–.074 |
| With Control for Earlier Absence of Use | ||||||
| IQ | .014 | + | .000–.061 | .022 | + | .003–.062 |
| Use at 17 | .303 | + | .183–.455 | .253 | + | .163–.364 |
| Education | .058 | + | .014–.135 | .003 | + | .000–.021 |
Note: Effect size is proportion of total variance. Confidence intervals are at 95%. Noted direction of effect refers to the sign of the estimated parameter. Where the parameter was not significant, its confidence interval had one positive and one negative endpoint so the actual direction of effect was not certain.
DISCUSSION
In this study, we used male and female young adult twin samples to explore the associations between intelligence and education and Alcohol and Nicotine Use behaviors that may contribute to later-life physical health. Education is often used as an environmental control variable in epidemiological studies, but there is increasing evidence that intelligence, which contributes to educational attainment, may be one of the primary variables that actually drives the association between education and physical health. Because genetic and shared family cultural environmental factors contribute to both education and intelligence yet this is not recognized in common statistical treatments in epidemiology, we were particularly interested in distinguishing direct environmental effects of education and intelligence from indirect effects involving genetic and shared family environmental selection processes. Our study design also allowed us to distinguish the effects of intelligence from those of education. Our results may be surprising to many, as they indicated that the direct effects of both IQ and Education on both Alcohol and Nicotine Use in both males and females were negative. That is, those with higher IQ’s and greater education tended to use more alcohol and nicotine. These effects, however, were offset by genetic and shared environmental family influences on earlier adolescent alcohol and nicotine use patterns.
Limitations of the Study
Our study is subject to several methodological limitations that should be considered before discussing the results in greater detail. First, the sample is representative of the population born in Minnesota in the 1970's, but it is primarily of European-American descent and thus should not be considered representative of the population of the United States as a whole. In particular, it probably does not include sufficient representation of some of the worst concentrations of urban poverty in the United States. This makes it difficult to generalize our results beyond this group, but it has the at least partially off-setting advantage that we did not have to consider the possibility that the overall results we obtained might hide potentially large moderating effects of demographic stratification. Second, our participants only had consistent composite substance use data through age 24. For both males and females, substance use increases throughout adolescence and early adulthood in these data. Alcohol use begins to taper off for females by age 24, but not until later in males, while nicotine dependence remains relatively steady. These patterns are generally typical (Chen & Kandel, 1995) and reflect the well-established disinhibitory and sensation-seeking behaviors common in adolescence (e.g., Chassin, Flora, & King, 2004), which may be only tangentially related to longer-term lifestyle choices associated with physical health in middle and later adulthood. Third, our participants reported education at age 24, when some of them had not yet completed the educational programs in which they were enrolled.
Our composite substance use variables included measures of use that have clinical implications for mental health, including symptoms of diagnosable psychopathology and obvious indications of abuse such as numbers of intoxications. Many epidemiological studies are based on reported usage patterns such as numbers of alcoholic drinks consumed per week and pack-years of smoking, which were not included here. How these quantitative habit measures relate to our composite use measures is not clear, nor are the implications of potentially clinical mental health impairments in young adulthood on later-life physical health. Still, our measures were obtained during structured interviews and many of the responses included in the composites were evaluated by independent teams in a formal consensus process so they may be less subject to distortions due to social desirability and selective memory than the questionnaire self-reports commonly used in epidemiological studies.
Finally, many epidemiological studies have shown that alcohol abstainers, light/moderate drinkers, and alcohol abusers have different health outcomes, with light/moderate drinkers often showing the best outcomes. Because we treated alcohol use as a single continuous variable in a linear setting we were not able directly to ascertain the existence of such inverted U-shaped associations. To address the possibility of distortions resulting from our approach, we broke our sample into three alcohol use groups at both ages 17 and 24: abstainers, light/moderate drinkers, and abusers. As alcohol use at age 17 is illegal in Minnesota and levels of safe consumption at that age are far from established, we limited membership in the light/moderate drinkers group at this age to those who reported drinking at most a single drink about once a year. We considered all others abusers at this age. At age 24, we included as light/moderate drinkers those who reported no symptoms of alcohol dependence, no more than 10 lifetime intoxications, drinking no more frequently than a couple times per week, drinking no more on average at one time than 4 drinks for females and 6 for males, and no more than 6 drinks for females and 8 for males in any single 24-hour period. We considered those who reported more use at age 24 abusers.
This generated a total of 32 mean comparisons between higher- and lower-IQ co-twins and co-twins with higher and lower levels of education (3 for each drinking group when co-twins fell into the same group × 2 for sex × 2 for age × 2 for education and IQ + 2 for sex × 2 for age group × 2 for education and IQ when co-twins did not fall into the same group). As would be expected by chance, there was only one difference in mean levels of composite use, and it indicated that at age 17 the higher-IQ female twin in the light/moderate use group had higher use than her co-twin (with an effect size of 1.0 SD within the very narrow range of the light/moderate use group at this age). Such an inability to distinguish the effects we observed in our continuous variable quantitative genetic analysis would be expected due to the loss of statistical power associated with separating a continuous variable into categories, and treating IQ and education separately. There was no evidence that our continuous linear analysis had distorted underlying nonlinear effects.
Direct vs. Indirect Effects of Intelligence and Education
The direct negative nonshared environmental effects of IQ and Education that we observed on substance use after control for genetic and shared environmental effects demonstrate rather dramatically that consideration of education as an environmental cause of health-related behaviors and outcomes is overly simplistic. This emphasizes the importance of recognizing that people are not fungible products of stochastic environmental processes; they also create and shape the environments they experience, thus contributing to the outcomes we observe.
Methodologically, this gets at the distinction between ecological correlations (between group means) and individual correlations (between measures of individuals). It is well known that ecological correlations cannot address individual-level mechanisms. One common (and relevant) example is the association between socioeconomic status (SES) and academic achievement (White, 1981), which is very strong across schools because people of high SES tend to purchase good schools for their children (either by buying homes in certain neighborhoods or by paying directly for schooling), but much weaker among students within schools because the factors involved in the ecology of school quality and peer associations that contribute to academic achievement are different from those involved in individual achievement when school ecology is controlled. A study that evaluated the association between SES and academic achievement by sampling one individual from each school would be unable to distinguish between the ecological and individual correlations.
Studies that sample only one individual per family (such as the study by Batty et al., 2007 that we discussed in the introduction which found an association between IQ and smoking behavior in young adults that was attenuated by education) have the same problem when the relevant ecology involves the family. Our results suggest that genetic and shared family environmental influences involving greater intelligence and education act to distinguish among families but to protect members of the same family similarly against substance use in adolescence. In addition, familial influences involving intelligence extend this familial similarity into adulthood. Our analyses did not provide evidence for the specific shared environmental influences involved, but possibilities include parenting styles (such as authoritative vs. other parenting styles; eg., Baumrind, 1991; Reiss, et al., 1995; Steinberg, Mounts, Lamborn, & Dornbusch, 1991), actions involving parental monitoring (e.g., Kerr & Stattin, 2000; Laird, Pettit, Bates, & Dodge, 2003), and family cultural influences such as religious involvement (e.g., Boomsma, de Geus, van Baal, & Koopmans, 1999) and values related to education (e.g., Gottfried, Fleming, & Gottfried, 1994). At the same time, within families, different combinations of influences involving greater intelligence and education have direct nonshared environmental effects that increase the risk of substance use. What might such undermining influences be?
Again our analyses did not provide evidence for any specific influences, but we can speculate about some possibilities. There is evidence that intelligence and education are associated with openness to experience. Experimentation with substance use can be an example of openness to experience, and some of those who experiment also end up abusing. Pressure to maintain academic achievement in pursuit of educational goals or to demonstrate intelligence can also lead to psychological distress (Gadeyne, Ghesquiere, & Onghena, 2004; Stoeber & Rambow, 2007), which is known to be associated with adolescent substance use (Brook, Brook, Zhang, & Cohen, 2004; Ellikson, Tucker, & Klein, 2003). Moreover, substance use, including both alcohol and nicotine, is associated with neurocognitive deficits in adults (e.g., Ernst, Heishman, Spurgeon, & London, 2001; Oscar-Berman & Marinkovic, 2003) and, to a more limited extent, in adolescents (e.g., Brown, Tapert, Grangholm, & Delis, 2000). As we measured IQ at the same time as earlier adolescent substance use, this could explain some of the protective familial effects we observed. That is, adolescent substance use may be particularly damaging to the developing brain (Keyes, Iacono, & McGue, 2007). This could account for the negative direct nonshared environmental effects of intelligence and education on substance use if those who delay experimentation with substances beyond age 17 suffer fewer developmental brain effects. Finally, the college years can be a period of relative freedom from responsibility for many, compared to peers who do not attend college and must work for a living. This could increase the opportunity for substance use among the brighter youth who are more likely to attend college.
Presuming our results are robust and extend beyond the particular habits in the young adult period investigated here (a large presumption at this stage), the suggestion to which we referred in the introduction that intelligence and education may support lifestyle choices and the development of habits that over time maintain or undermine physical health may be too simplistic. Our results suggest the possibility that it may not be any tendency for those with greater intelligence and education to make any particular lifestyle choices that facilitates better health. Instead, it could be the power of intelligence and education in our society to enable individuals to obtain financial and personal resources to manage their lives as they choose that facilitates better health. This would be consistent with the ideas regarding physical responses to stress that undermine health that are proposed by Sapolsky (e.g., 2004), McEwen (e.g., 2007), Marmot, (e.g., Singh,Manoux, Marmot, & Adler, 2005), Adler (e.g., Adler, Marmot, McEwen, & Stewart, 1999), and others. Of course, the two possibilities are in no way mutually exclusive and may interact and/or reinforce each other.
Comparing Our Results Across Substances
The effects we observed were somewhat stronger for Nicotine than Alcohol Use. From one perspective, this is not surprising, as the detrimental health effects of smoking are more strongly documented than those of drinking. For drinking alcohol, beneficial effects of moderate consumption are even noted. From another perspective, however, it is somewhat surprising that intelligence and education would have greater effects on smoking behavior. Its detrimental health effects are at this point so well documented that people who are unaware of them must be extremely rare.
Comparing Our Results in Males and Females
In general, the natures and magnitudes of all the effects we observed were very similar for males and females. The primary difference was that females showed both lower mean levels of substance use and less variance. In fact, even the relatively insensitive Levene test for homogeneity of variance indicated that the variance differences were significant for all but Nicotine Use at age 17. Another way to look at this statistically is that, in the multivariate models, it was not possible to constrain the parameters equal for males and females without very significant deterioration in model fit (all p’s<.001). At the same time, when we standardized the variables within sex to remove the effects of their differences in means and total variances, imposing these constraints across sex did not result in significant deterioration in model fit.
One sex difference did stand out as potentially worthy of further exploration despite its lack of specific statistical significance in these data. This was the familial effect of Education on Nicotine Use after control for earlier use, which was much stronger in males than in females, though the 95% confidence intervals still overlapped (.058 [.014–.135] in males; .003 [.000–.021] in females; Table 6). This may reflect the fact that the average level of education among females in these data was higher than that among males (Table 1), and that the correlation between IQ and education was marginally significantly stronger in females than in males (.39 in females vs. .28 in males; Table 2). This could be the case if conscientiousness were involved in both the familial effect of Education in males and the higher level of Education in females than in males, but this must be explored in future research.
Comparing the Effects of IQ and Education
In general, the effects of Education that we observed were weaker than those of IQ, in contrast to the observation in at least one other study of smoking (Gale, Johnson, Deary, Schoon, & Batty, 2008) and to the higher correlations between substance use and Education in these data as shown in Table 2. In part, this reflected our treatment, as the effects of Education were measured controlling for the effects of IQ. Our treatment was, however, consistent both with the prior measurement of IQ in our data and with the body of evidence that intelligence contributes more strongly to educational attainment than vice versa (Ceci, 1996). Consistent with a large volume of other data (e.g., Bartels, Reitveld, van Baal, & Boomsma, 2002), IQ contributed to Education both genetically and through shared family environmental influences. Though we did not show these results in our tables as they were not the focus of the analyses, in males, genetic influences on IQ contributed 18.6% of the variance in Education and shared family environmental influences contributed 16.2%. In females, genetic influences on IQ contributed 4.4% of the variance in Education, and shared family environmental influences contributed 20.0%. There was no significant nonshared environmental effect of IQ on Education in either sex. The sex difference in genetic contribution was significant, but not the sex difference in shared environmental contribution. This is consistent with the commonly made observation that females obtain more education than males, but not because they are smarter.
The effects of IQ were almost completely accounted for by substance use at age 17. Thus, the primary reason for the greater effect of intelligence than education appeared to be that brighter adolescents were less likely to get involved in early substance use at age 17, before disparities in level of educational attainment took place. This is consistent with the well-established roles of low academic achievement and cognitive delays and difficulties in delinquent behaviors including substance use (e.g. Fergusson & Horwood, 1995), and suggests that substance use may be a factor that compounds low IQ in impairing later educational attainment. If substance use causes neurocognitive damage, it may also have effects of its own in impairing educational attainment that were not captured in our analysis.
Both the confounding genetic and shared family environmental effects we observed and the difficulties we encountered in distinguishing between genetic and shared family environmental confounding effects would be involved in this likely developmental process. As noted earlier, the methods we used to estimate genetic and environmental effects are based on the assumption that genetic and environmental influences are independent. This means that we assumed that there were no correlations between genetic and either shared or nonshared environmental influences and also no genetically influenced differences in sensitivity to the environment, commonly known as gene-environment interactions. For example, some young adults with particular genetic backgrounds may be more sensitive to substance use than others, producing more serious dependence symptoms and greater neurocognitive impairment. To the extent they existed but were not modeled, gene-environment correlations and interactions acted to create differing degrees of genetic and environmental influences within different subgroups of the sample. This does not invalidate the overall approach. Rather, it renders the estimates applicable only on an overall, average population-level basis, and introduces systematic distortions in the estimates, including the inability to distinguish genetic and shared environmental reasons for familial similarity.
Conclusion and Practical Implications
In conclusion, this study provides evidence that the protective associations between education and intelligence and substance use behaviors in young adults may be primarily indirect, passed through familial selection processes that involve genetic as well as shared family environmental influences. Moreover, it suggests that, when these confounding factors are controlled, intelligence and education may actually be associated with greater substance use. Clearly, our findings should be replicated in other samples and the extent to which they persist beyond early adulthood should be investigated. Whatever the outcome of such future studies, however, these findings have important implications for future research in this area because they highlight the importance of developing research programs to understand how individuals create and shape the environments they experience, thus contributing to the outcomes we observe. The fact that genetic influences appear to be involved in these processes does not mean that the processes and outcomes are deterministic. Rather, it emphasizes that broad social forces have different effects on individuals with different genetic backgrounds, and we should expect that any given environmental circumstance will have different effects on different individuals.
What are the practical implications for public health messages if the overall conclusion that there are negative direct effects of intelligence on health-related behaviors that can be accounted for by early adolescent use is correct and pervasive? The example of smoking may be most relevant. At this point, it certainly does not take high intelligence to know that smoking is not good for health, yet many adolescents continue to take up the habit. Reducing smoking likely involves identifying the family background characteristics and psychological factors involved in early substance use and improving the life conditions associated with them rather than additional public health messages on the subject.
ACKNOWLEDGEMENTS
This research was supported by US Public Health Service Grants #AA00175, DA 13240, and DA 05147. Wendy Johnson holds a Research Council of the United Kingdom Fellowship and is a member of the Medical Research Council Centre for Cognitive Ageing and Cognitive Epidemiology, which is supported by the BBSRC, EPSRC, and MRC as part of the cross-council Health and Well-being Initiative. The University of Edinburgh is a charitable body, registered in Scotland, with registration number SC005336. We thank the twins and their families and the recruiting, interviewing, data management, and lab staffs of the Minnesota Twin Family Study.
Footnotes
Readers may be most familiar with the terms ‘direct’ and ‘indirect effects’ from path analysis/structural equation modeling, in which direct effects are unmediated or not confounded, and indirect effects are mediated or confounded by some intervening variable. Conceptually, we are using these terms in exactly this way throughout this paper. There is a small difference in our usage, however, as our analyses did not specify or make use of measured observations of the intervening genetic and shared environmental influences involved in the family selection processes, so these confounding or mediating genetic and shared environmental influences remain latent. As usual in such analyses, total effects in our analyses are the sums of direct and indirect effects, in this case the sums of the genetic, shared, and nonshared environmental effects in Figure 1. As always, apparently direct effects may actually be indirect if additional relevant intervening variables were to be identified and measured.
REFERENCES
- Adler NE, Marmot MG, McEwen BS, Stewart JE. Socioeconomic Status and Health in Industrial Nations: Social, Psychological, Biological Pathways. New York: New York Academy of Sciences; 1999. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bartels M, Reitveld MJH, van Baal CM, Boomsma DI. Heritability of educational achievement in 12-year-olds and the overlap with cognitive ability. Twin Research. 2002;5:544–553. doi: 10.1375/136905202762342017. [DOI] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Schoon I, Gale CR. Mental ability across childhood in relation to risk factors for premature mortality in adult life: The 1970 British Cohort Study. Journal of Epidemiological and Community Health. 2007;61:997–1003. doi: 10.1136/jech.2006.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumrind D. Cowan PA, Hetherington EM. Family Transitions. Hillsdale, NJ: Erlbaum; 1991. Effective parenting during the early adolescent transition. [Google Scholar]
- Behrman JR, Pollak RA, Taubman P. From Parent to Child. Chicago: University of Chicago Press; 1995. [Google Scholar]
- Boomsma DI, de Geus EJC, van Baal GCM, Koopmans JR. A religious upbringing reduces the influence of genetic factors on disinhibition: Evidence for interaction between genotype and environment on personality. Twin Research. 1999;2:115–125. doi: 10.1375/136905299320565988. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, McGue M. Familial studies of intelligence: A review. Science. 1981;212:1055–1059. doi: 10.1126/science.7195071. [DOI] [PubMed] [Google Scholar]
- Brook JS, Brook DW, Zhang , Cohen P. Tobacco use and health in young adulthood. Journal of Genetic Psychology. 2004;165:310–323. doi: 10.3200/GNTP.165.3.310-323. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcohol Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Carlson MC, Fried LP, Ave QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical function performance in community in-dwelling older women. Journal of Gerontology - Series B - Psychological and Social Sciences. 1999;54:S262–S270. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- Ceci SJ. On Intelligence: A Bioecological Treatise on Intellectual Development. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. American Journal of Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Batty GD, Starr JM. Physical fitness and lifetime cognitive change. Neurology. 2006;67:1195–1200. doi: 10.1212/01.wnl.0000238520.06958.6a. [DOI] [PubMed] [Google Scholar]
- Ellikson PL, Tucker DS, Klein DJ. Ten-year prospective study of public health problems associated with early drinking. Pediatrics. 2003;111:949–955. doi: 10.1542/peds.111.5.949. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Early disruptive behavior, IQ, and later school achievement and delinquent behavior. Journal of Abnormal Child Psychology. 1995;23:183–199. doi: 10.1007/BF01447088. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Schiaffino A, Borrell C, Benach J, Ariza C, Ramon JM, et al. Social class, education, and smoking cessation: Long-term follow-up of patients treated at a smoking cessation unit. Nicotine and Tobacco Research. 2006;8:29–36. doi: 10.1080/14622200500264432. [DOI] [PubMed] [Google Scholar]
- Gadeyne E, Ghesquiere P, Onghena P. Longitudinal relations between parenting and child adjustment in young children. Journal of Clinical Child and Adolescent Psychology. 2004;33:347–358. doi: 10.1207/s15374424jccp3302_16. [DOI] [PubMed] [Google Scholar]
- Gale CR, Johnson W, Deary IJ, Schoon I, Batty GD. Intelligence in girls and their subsequent smoking behaviours as mothers: The 1958 National Child Development Study and the 1970 British Cohort Study. International Journal of Epidemiology. 2008 doi: 10.1093/ije/dyn201. in press. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS. Intelligence: Is it the epidemiologists' elusive "fundamental cause" of social class inequalities in health? Journal of Personality and Social Psychology. 2004;86:174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS, Deary IJ. Intelligence predicts health and longevity, but why? Current Directions in Psychological Science. 2004;13:1–4. [Google Scholar]
- Gottfried AE, Fleming JS, Gottfried AW. Role of parental motivational practices in children’s academic intrinsic motivation and achievement. Journal of Educational Psychology. 1994;86:104–113. [Google Scholar]
- Graham H. When Life's a Drag: Women, Smoking, and Disadvantage. London: HMSO; 1993. [Google Scholar]
- Grano N, Virtanen M, Vahtera J, Elovainio M, Kivimaki M. Impulsivity as a predictor of smoking and alcohol consumption. Personality and Individual Differences. 1996;37:1693–1700. [Google Scholar]
- Hart CL, Taylor MD, Davey-Smith G, Whalley LJ, Starr JM, Hole DJ, et al. Childhood IQ, social class, deprivation, and their relationships with mortality and morbidity risk in later life: Prospective observational study linking the Scottish Mental Survey 1921 and the Midspan studies. Psychosomatic Medicine. 2003;65:877–883. doi: 10.1097/01.psy.0000088584.82822.86. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Johnson W, Deary IJ, Iacono WG. Genetic and environmental selection processes underlying educational attainment: Something for everyone but everything for noone. Psychological Methods. 2008 under review. [Google Scholar]
- Kassel JD, Evatt DP, Greenstein JE, Wardle MC, Yates MC, Veilleux JC. The acute effects of nicotine on positive and negative affect in adolescent smokers. Journal of Abnormal Psychology. 2007;116:543–553. doi: 10.1037/0021-843X.116.3.543. [DOI] [PubMed] [Google Scholar]
- Kerr M, Stattin H. What parents know, how they know it, and several forms of adolescent adjustment: Further support for a reinterpretation of monitoring. Developmental Psychology. 2000;36:366–380. [PubMed] [Google Scholar]
- Keyes MA, Iacono WG, Mcgue M. Early onset problem behavior, young adult psychopathology, and contextual risk. Twin Research and Human Genetics. 2007;10:45–53. doi: 10.1375/twin.10.1.45. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Johnson W. John OP, Robins RW, Pervin LA. Handbook of Personality. New York: Guilford; 2008. Behavioral genetics and personality: A new look at the integration of nature and nurture. [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird RD, Pettit GS, Bates JE, Dodge KA. Parents’ monitoring-relevant knowledge and adolescents’ delinquent behavior: Evidence of correlated developmental changes and reciprocal influences. Child Development. 2003;74:752–768. doi: 10.1111/1467-8624.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJ, Rubin D. Statistical Analysis with Missing Data. New York: Wiley; 1987. [Google Scholar]
- Malmstrom TK, Wolinsky FD, Andresen EM, Miller JP, Miller DK. Cognitive ability and physical performance in middle-aged African Americans. Journal of the American Geriatrics Society. 2005;53:997–1001. doi: 10.1111/j.1532-5415.2005.53318.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: The central role of the brain. Physiological Review. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Michell L, West P. Peer pressure to smoke: The meaning depends on the method. Health Education Research. 1996;1:39–49. [Google Scholar]
- Neale MC, Boker S, Xie G, Maes HH. Mx: Statistical Modeling. Richmond, VA: Medical College of Virginia, Department of Psychiatry; 2003. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcoholism and the brain: An overview. Alcoholism and Health. 2003;27:125–133. [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Geentics. 5th Ed. New York: Worth; 2007. [Google Scholar]
- Prescott CA, Madden PA, Stallings MC. Challenges in genetic studies of the etiology of substance use and substance use disorders: Introduction to the special issue. Behavior Genetics. 2006;36:473–482. doi: 10.1007/s10519-006-9072-9. [DOI] [PubMed] [Google Scholar]
- Reed T, Dick DM. Heritability and validity of healthy physical aging (wellness) in elderly male twins. Twin Research. 2003;6:227–234. doi: 10.1375/136905203765693889. [DOI] [PubMed] [Google Scholar]
- Reiss D, Hetherington EM, Plomin R, Howe GW, Simmens SJ, Henderson SH, O’Connor TJ, Bussell DA, Anderson ER, Law T. Genetic questions for environmental studies: Differential parenting and psychopathology in adolescence. Archives of General Psychiatry. 1995;52:925–936. doi: 10.1001/archpsyc.1995.03950230039007. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Kuncel NR, Shiner R, Caspi A, Goldberg LR. The power of personality: The comparative validity of personality traits, socioeconomic status, and cognitive ability for predicting important life outcomes. Perspectives on Psychological Science. 2007;2:313–345. doi: 10.1111/j.1745-6916.2007.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Baber T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis, MO: Author; 1987. [Google Scholar]
- Room R. Measuring drinking patterns: The experience of the last half century. Journal Substance Use Behaviors. 2000;12:23–31. doi: 10.1016/s0899-3289(00)00038-9. [DOI] [PubMed] [Google Scholar]
- Rutter M. Proceeding from observed correlation to causal inference. Perspectives in Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Fergusson D, Horwood LJ, Goodman R, Maughan B, et al. Sex differences in developmental reading disability: New findings from four epidemiological studies. Journal of the American Medican Association. 2004;291:2007–2012. doi: 10.1001/jama.291.16.2007. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why Zebras Don’t Get Ulcers. New York: Henry Holt; 2004. [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Mounts NS, Lamborn SD, Dornbusch SM. Authoritative parenting and adolescent adjustment across varied ecological niches. Journal of Research on Adolescence. 1991;1:19–36. [Google Scholar]
- Stoeber J, Rambow A. Perfection in adolescent school students: Relations with motivation, achievement, and well-being. Personality and Individual Differences. 2007;42:1379–1389. [Google Scholar]
- Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: The MacArthur Longitudinal Study of Successful Aging. Journal of Gerontology - Series A - Biological and Medical Sciences. 2002;57:M228–M235. doi: 10.1093/gerona/57.4.m228. [DOI] [PubMed] [Google Scholar]
- Teitelbaum LM, Carey KB. Temporal stability of alcohol screening measures in a psychiatric setting. Psychology of Addictive Behaviors. 2000;14:401–404. doi: 10.1037//0893-164x.14.4.401. [DOI] [PubMed] [Google Scholar]
- White KR. The relation between socioeconomic status and academic achievement. Psychological Bulletin. 1981;91:462–481. [Google Scholar]