Abstract
Earlier studies of androgen-receptor (AR) expression using frozen prostate tissue, and later studies using archived specimens, produced the consensus that ligand-stabilized AR is nuclear, AR expression is similar in benign epithelia and stroma, AR expression is greater in secretory epithelia than basal cells, and AR expression is more variable in prostate cancer (CaP) than in benign prostatic hyperplasia (BPH). Accurate measurement of AR expression remains technically challenging but necessary to evaluate the relevance of ARs to clinical CaP. Recent studies demonstrated that AR expression in epithelia and stroma may be prognostic in clinically localized CaP, and AR expression may play a role in racial differences in CaP mortality and predict response to androgen deprivation therapy. High levels of AR and AR-regulated gene expression indicate a central role for AR in growth regulation of castration-recurrent CaP. New treatments for the lethal phenotype of CaP require better understanding of AR transactivation during androgen deprivation therapy.
Keywords: androgen receptor, prostate cancer, quantitative video image analysis, immunohistochemistry
Androgens are necessary for the development and growth of the prostate and for the development, growth and progression of prostate cancer (CaP). An American man is diagnosed with CaP every 3 minutes,[1] and the worldwide incidence of CaP is increasing an estimated 1.1% annually.[2] Despite increased use of digital rectal examination and serum prostate-specific antigen (PSA) measurement for early detection, approximately 30% of men treated with curative intent suffer CaP recurrence. These men, and those who present with locally advanced or metastatic CaP, can be palliated by androgen deprivation therapy (ADT), which causes regression of androgen-dependent CaP through programmed cell death.[3] Although there are many methods for medical and surgical ADT, the results of ADT remain unimproved since its discovery more than 60 years ago.[4] More than 80% of men with disseminated CaP demonstrate clinical or biochemical response that is associated with a mean life expectancy of approximately 3.5 years, in contrast to non-responders or untreated patients who live an average of 9 months. Regardless of the androgen responsiveness of incurable CaP, almost all patients succumb to castration-recurrent CaP because it responds poorly to all known therapies, and approximately 27,050 American men, or one man every 17 minutes, will die from CaP this year.[1]
Discovery of the AR simultaneously by two groups at University of Chicago[5] and University of North Carolina[6] and development of AR-specific antibodies[7,8] made the study of AR protein expression in prostate tissue practical. Although AR expression is diminished immediately after ADT that induces clinical remission in most patients, castration-recurrent CaP expresses levels of AR protein similar to those found in androgen-stimulated benign prostate and CaP. This observation suggests that the AR may be as important for growth regulation in castration-recurrent CaP as it is in androgen-stimulated benign prostatic hyperplasia (BPH) and CaP. The clinical relevance of inter-individual and population variation of AR protein expression cannot be understood unless AR protein expression can be assessed accurately and correlated with the development and progression of benign and malignant prostate diseases. Since molecular and biochemical studies of AR using human benign CaP cell lines probably more often confuse than inform, this chapter examines the measurement of AR protein expression in clinical specimens and determines whether AR protein expression has clinical relevance to patients suffering from clinically localized or advanced CaP.
AR IN CLINICALLY LOCALIZED PROSTATE CANCER
AR is unique among the steroid hormone receptors in that ligand binding stabilizes AR rather than earmarking it for degradation. AR ligand binding demonstrates a threshold effect whereby non-castrate levels of testicular androgens produce transactivation, and levels above the threshold produce little additional AR activity. By logical extension, normal androgen levels in an adult male should allow AR transactivation and expression of AR-regulated genes. However, the level of AR protein varies within epithelial and stromal nuclei of benign and malignant prostate tissue, and the biologic effects of this variation within the microenvironment of the prostate remain unknown. Relatively few data have been reported that allow one to reach conclusions about the role of AR in the development or progression of CaP. The scarcity of work in this area results from many factors. First, the discovery of AR occurred less than 20 years ago.[5, 6] Second, quantitation of AR protein expression is technically difficult and labor-intensive. Third, the heterogeneity of CaP renders study of diagnostic prostate biopsies and even radical prostatectomies problematic. Fourth, archival CaP tissue must be stored a long time to link it to clinical outcome endpoints due to the long natural history of clinically localized CaP. Time to clinical endpoints is lengthened further due to PSA early detection programs (PSA allows detection of CaP approximately 10 years before palpable changes occur on prostate exam.)[9] Finally, CaP treatment outcomes are confused by a high rate of deaths from other causes and inclusion of incidental CaP in more recent series.
ANDROGEN-RECEPTOR EXPRESSION IN THE EPITHELIA OF CLINICALLY LOCALIZED PROSTATE CANCER
The first study that examined the relationship between AR concentration and Gleason grade and clinical stage was reported in 1986.[10] Since AR antibodies had not yet been developed, biochemical measurement of AR content was required. Tumor specimens were obtained by transurethral resection (TURP) and clinical staging was by digital rectal exam. AR content and histological grade did not correlate, but early stage CaP (n = 5, clinical stage <T3 but one man was M1) had significantly lower AR levels than CaP of higher stage (n = 5, all men T3M1). The authors suggested that greater androgenic stimulation due to higher levels of AR protein provided a growth advantage that resulted in higher stage of tumor at presentation. These seminal observations remain supported many years and studies later.
Perhaps the first study of AR using immunohistochemistry was reported in 1990.[11] Frozen diagnostic prostate needle biopsies from 63 cases of untreated CaP, eight cases of castration-recurrent CaP, and nine cases of BPH were studied using a monoclonal AR antibody. BPH was characterized by AR immunostaining of nuclei (but not cytoplasm) of epithelial and stromal cells, but basal cells did not immunostain for AR. In CaP, AR immunostaining also was confined to nuclei; however, greater variation was described: AR-positive and AR-negative cells intermingled more in CaP than in BPH. In castration-recurrent CaP, the population of AR-positive cells was decreased. Although this study was the first to report an association between AR expression and histological grade of CaP, one contemporaneous study[12] was consistent with this report, but seven other studies[10,13–18] showed no correlation between Gleason grade and AR immunostaining.
The first study that carefully compared AR immunostaining in CaP and BPH from the same prostatectomy specimen and BPH from patients without CaP was reported in 1993.[19] Tissue preservation of immunoreactivity was enhanced by mounting tissue onto rat-liver homogenate as an adhesive and rapid freezing in isopentane cooled by liquid nitrogen. AR immunostaining was characterized by both intensity of, and variation in, intensity of immunostaining using a visual scoring index. High quality immunostaining and careful visual assessment of immunostaining demonstrated that BPH had similar nuclear AR protein expression whether obtained from CaP patients or patients without CaP, and that CaP had decreased intensity and hence greater heterogeneity of AR immunostaining.
Tilley’s group was the first to report, in 1998, a method for image analysis that sought to improve upon visual assessment of AR immunostaining.[20] Percentage of nuclei immunostained for AR were estimated based upon image analysis of areas of benign or malignant epithelia or stroma; individual nuclei were not evaluated. An affinity-purified polyclonal AR antibody was used to study 30 patients with clinically localized CaP (10 stage A and 20 stage B according to the Whitmore–Jewett system) and frozen research samples were obtained by TURP (n = 24) or radical prostatectomy (n = 6). AR immunostaining in malignant or benign epithelia within the same section appeared similar, with heterogeneous immunostaining composed of weakly to strongly positive nuclear immunoreactivity in the majority of cells. In contrast, stromal cells displayed focal immunoreactivity with regions of AR-immunopositive stromal cells interspersed with regions of negatively immunostained stromal cells. These authors were the first to report that AR immunostaining appeared similar in early CaP and benign prostate tissue. These results were contrasted with studies of advanced CaP from their own laboratory[21] and from others[22–25] where reduced AR immunoreactivity and increased heterogeneity of cellular staining was observed. They attributed the similar findings in early CaP and benign prostate tissue to the lack of development of greater genetic instability and altered androgen sensitivity, which they believed a characteristic of advanced CaP, especially after administration of ADT.[6]
The first careful study of AR expression using archival prostate specimens was reported in 1999.[16] Sixteen radical prostatectomy specimens from patients with clinically localized CaP and 16 TURP specimens from men with BPH were double-labeled with monoclonal AR antibody F39.4.1 and anti-cytokeratin monoclonal 13bE12. The basal epithelial cell layer was visualized in BPH and high-grade prostatic intraepithelial neoplasia (HGPIN), whereas no basal cells were seen in malignant glands. Epithelial cells of CaP glands showed higher AR mean optical density (MOD) than BPH (0.11 ± 0.01 versus 0.07 ± 0.01, P < 0.05). HGPIN identified in ten radical prostatectomy specimens showed AR immunostaining similar to that in BPH (0.05 ± 0.02). BPH from TURP specimens (0.25 ± 0.02) showed significantly higher AR immunostaining intensity compared to BPH from radical prostatectomy specimens (P < 0.01). Differences in AR protein preservation due to differences in tissue procurement and/or tissue fixation preclude study of different sources of tissues – biopsy versus TURP versus radical prostatectomy and frozen versus archival.
ANDROGEN-RECEPTOR EXPRESSION IN THE STROMA OF CLINICALLY LOCALIZED PROSTATE CANCER
In 1996, our group reported that AR immunostaining of frozen radical prostatectomy specimens was diminished in stroma compared to epithelia in both BPH and CaP, and that the decreased AR expression described previously in epithelial nuclei of CaP compared to BPH was also characteristic of the stroma of both tissue types.[14] In 1998, the Tilley laboratory was the first to call attention to variation in AR immunostaining within the stroma; AR immunostaining was diminished in the stroma adjacent to malignant glands.[20] These clinical observations were supported by preclinical data using the Cunha model,[26,27] where tumor stroma supported carcinogenesis of the overlying epithelium. Furthermore, Rowley[28] and Arnold and Isaacs[29] reviewed the data that supported the hypothesis that stroma produces a reciprocal inhibition of epithelia and that the suppressive effect of the stroma is lost in CaP and contributes to CaP progression. AR immunostaining in stroma was more carefully evaluated by Olapade-Olaopa et al[30] using a monoclonal AR antibody and archival TURP and radical prostatectomy specimens from 17 patients with BPH and 39 age-matched patients with CaP. Another new method for visually assessing AR expression was introduced where the number of cells strongly positive within a given area was expressed as a percentage. A final score was given as negative if no cells stained, weak if 1–33% of cells immunostained, moderate if 34–66% of cells immunostained, and strong if >66% of cells immunostained. CaP epithelium had greater heterogeneity and a lower overall level of expression of AR compared to benign prostate tissue. In HGPIN, nuclear immunostaining in the epithelia was intermediate between that of benign and malignant glands. AR immunostaining was more heterogeneous in CaP compared to BPH. AR immunostaining was absent in the nuclei of stromal cells surrounding CaP glands, and this absence was most pronounced in poorly differentiated CaP. AR immunostaining of stroma surrounding HGPIN was reduced similarly. AR was depleted from stroma adjacent to benign glands that were close to CaP glands and was expressed to a greater extent the more distant the benign epithelia was from adjacent cancer.
A third and independent group confirmed the observations that AR immunostaining was reduced in the ‘tumor stroma’.[15] Henshall et al studied 96 radical prostatectomies from patients treated for clinically localized CaP using the same monoclonal AR antibody. They proposed another scoring system for AR immunostaining: 500 epithelial cells and 200–250 stromal cells were evaluated in each tissue sample by three individuals, which included one pathologist. AR expression was categorized in the epithelia and periepithelial stroma as ≤ or >30% AR-positive. They reported that concurrent high AR expression in CaP and low AR expression in the periepithelial stroma was associated with higher clinical stage, higher pretreatment PSA, and earlier CaP recurrence. These data provided the first ever evidence for an association between AR expression in the stromal microenvironment and clinical outcome in CaP patients treated by radical prostatectomy. Both groups[15,30] reported that the stroma surrounding HGPIN also lacked AR expression, which suggested that altered AR expression in the stroma is an early event in CaP development and progression.
A more precise characterization of AR immunostaining by the Tilley group[18] extended the observations of Henshall et al.[15] Fifty-three patients underwent radical prostatectomy and were followed for a minimum of 3 years. AR was recognized using an affinity-purified rabbit polyclonal AR antibody and current antigen retrieval techniques. AR immunostaining was assessed visually and using video image analysis. Almost all CaP demonstrated nuclear AR immunoreactivity. In contrast to most observers and prior studies from this group, visual assessment indicated that the percentage of AR-positive cells was significantly lower in benign (85%) than malignant (94%) epithelial cells. In the stroma, the percentage of AR-positive cells in the peritumoral stroma was reduced compared to the stroma adjacent to benign glands. Image analysis confirmed the visual assessment that AR-positive nuclear area was lower in benign than in malignant epithelia. The surprising finding was that lower AR immunostaining was associated with significantly fewer PSA relapses (6%) compared to higher AR immunostaining (42%). High AR-positive epithelial nuclear area was associated with extra-prostatic cancer (pT3) but not higher Gleason scores or preoperative serum PSA levels. Relapse became even more likely when AR positivity was <45% in the peritumoral stroma. Thus, two groups acting independently, using different specimens and different methods of analysis, associated poor treatment outcome with high levels of AR expression in CaP epithelia and low levels of AR in peritumoral stroma. These findings suggest that increased androgenic stimulation of epithelia associated with reduced apoptotic influence coming from the stroma may produce a more aggressive phenotype of CaP. However, these findings lack clinical relevance until pretreatment biopsies are studied instead of radical prostatectomy specimens. These findings may also be relevant to racial differences in AR levels between African-American and Caucasian-American men, which is described more fully below. Increased AR levels in CaP epithelia, especially in more advanced CaP, may be associated with increased expression of AR-regulated genes and increased tumor growth and/or metastasis.
ANDROGEN RECEPTOR AND RACIAL DIFFERENCES IN PROSTATE CANCER
Although the frequency of incidental CaP is similar between races,[31,32] African Americans have a higher incidence of, and greater mortality from, CaP than Caucasian Americans. Data from the SEER database (1993–1997) show that the incidence of invasive CaP is greater in African Americans than Caucasian Americans: 1.9 times greater in men <65 years of age and 1.6 times greater in men ≥65 years of age.[33] In men <65 years of age, the CaP mortality rate for African Americans is 3.1 times that of Caucasian Americans. In men ≥65 years of age, the CaP mortality rate for African Americans is 2.3 times that of Caucasian Americans. [33] Difference in CaP incidence between men of different geographic origin is not unique to the United States; for example, in Sao Paulo, Brazil, CaP is 1.8 times more common in Brazilians of African than European origin.[34]
AR protein expression was evaluated in malignant and benign prostate tissue from African Americans and Caucasian Americans who underwent radical prostatectomy for clinically localized CaP.[35] Archived radical prostatectomy specimens obtained from 25 Caucasian Americans and 25 African Americans had AR protein antigen retrieved and immunostained. AR protein expression from CaP and benign tissue was assessed using two methods. Visual scoring suggested that AR immunostained more intensely in both malignant and benign epithelial nuclei in African Americans than Caucasian Americans. Automated digital color video image analysis was used to measure percent positive nuclei and the intensity of expression (MOD) in each nucleus. In African Americans compared to Caucasian Americans, malignant nuclei were 27% more likely immunostained for AR (P = 0.005), and among immunopositive nuclei, AR protein expression was 81% greater (P = 0.002). Among immunopositive benign nuclei, AR protein expression was 22% greater in African Americans compared to Caucasian Americans (P = 0.027). Racial differences in AR protein expression were not explained by age, pathologic grade or stage. CaP may occur at a younger age and progress more rapidly in African Americans than Caucasian Americans due to racial differences in AR, but these findings must be confirmed by others, and tissue expression of AR-regulated genes must be evaluated in men of both races.
ANDROGEN-RECEPTOR EXPRESSION AND PROGNOSIS IN CLINICALLY LOCALIZED PROSTATE CANCER
In addition to the data presented above on the prognostic significance of AR immunostaining in the epithelia and stroma, two other recent reports more critically evaluated AR as a biomarker of treatment response. The Baylor SPORE created a radical prostatectomy tissue microarray that was studied using a monoclonal AR antibody.[36] AR immunostaining was assessed visually and scored semi-quantitatively. An intensity score was assigned and multiplied by a labeling frequency score and AR expression reduced to a dichotomous variable, either low or high level. AR expression was weakly but significantly correlated with stage and Gleason score but did not correlate with preoperative PSA level or surgical margin status. Of greatest interest is that they reported correlation between cellular proliferation measured using Ki-67 labeling index and AR level of expression. Furthermore, they found that patients with high-level AR expression had significantly reduced biochemical progression-free survival compared to those with low-level AR expression. AR expression remained an independent prognostic indicator of biochemical progression-free survival on multivariate analysis. The relationship between Ki-67 and AR expression and outcome is not surprising, since others have reported a relationship between biochemical progression-free survival and Ki-67.[37]
Most recently, the Tilley group[38] reported that elevated levels of HER-2/neu and AR were associated with adverse outcomes after radical prostatectomy. HER-2/neu and AR immunoreactivity were evaluated using quantitative video image analysis of tissue regions in archived prostatic tissues obtained from 53 men with clinically localized CaP who underwent radical prostatectomy; 70% of tumors exhibited high levels of HER-2/neu immunostaining, and 68% of tumors had elevated AR levels. Patients with high levels of both HER-2/neu and AR had the highest rate of PSA failure (56%, 15/27) compared with no PSA failures among seven patients with low levels of both HER-2/neu and AR (log rank statistic 7.69, P = 0.021). Concurrent high levels of HER-2/neu and AR expression were associated with high pathological stage (P = 0.027) and development of metastatic disease (P = 0.022). These findings suggest that the HER-2/neu and AR signaling pathways may contribute to development of metastatic disease.
ANDROGEN RECEPTOR IN ADVANCED PROSTATE CANCER
Androgen receptor as a predictor to response to androgen deprivation therapy
A consensus has been reached by investigators who have reported upon the use of AR immunostaining to predict prognosis of patients with advanced CaP treated with ADT. Barrack’s group first postulated that lack of response to ADT would be indicated by reduced levels of expression of AR. However, they reported that the mean AR concentration per nucleus and the mean percentage of AR-positive nuclei did not differ between responders and non-responders to ADT.[39] However, they subsequently reported that the heterogeneity of expression of AR predicted response to ADT.[25] A series of studies that took advantage of the ability to examine archival tissue and to more precisely quantitate AR expression using image analysis techniques have confirmed these results. Tilley’s group used video image analysis and an affinity-purified polyclonal antibody directed against the N terminus of AR to predict perfectly the outcome after ADT of 21 patients with advanced CaP.[21] They found that more precise measurement of AR expression allowed development of a classification algorithm, but this algorithm has not been evaluated in another patient group or by others. Another group examined 63 frozen CaPs using a polyclonal AR antibody and found that 48% of samples had at least 10% AR-positive cells, and these patients responded better to ADT and survived longer, whereas Gleason grade, clinical stage, and ethnicity were not prognostic.[17]
Takeda et al. studied visually frozen prostate biopsy specimens using a polyclonal AR antibody and calculated AR content as the ratio of positively stained cells to total number of cells.[40] They reported that AR content was related to the presence of bone metastasis. Patients whose tumors were composed of cells that exceeded the mean AR content of 48% all responded to therapy. Careful examination of the paper reveals that the distribution of AR positivity was so great and the non-responders so few that it is unlikely that prognosis of individual patients could be predicted on the basis of AR content. Finally, Prins et al[41] and Nabi et al[42] reported similar results in 45 patients from New York and 85 patients from India, respectively, using archived specimens, antigen retrieval, and similar image analysis and receptograms. Almost all patients who had a high percentage of cells immunonegative for AR had no response, or only a short-term response, to ADT. A consensus seems to have been reached that the presence of a large number of cells that lack AR expression or have a low level of AR creates sufficient heterogeneity in the tumor that response to ADT is poor. Such patients may benefit from early application of chemotherapy.
Androgen-receptor immunostaining in castration-recurrent prostate cancer
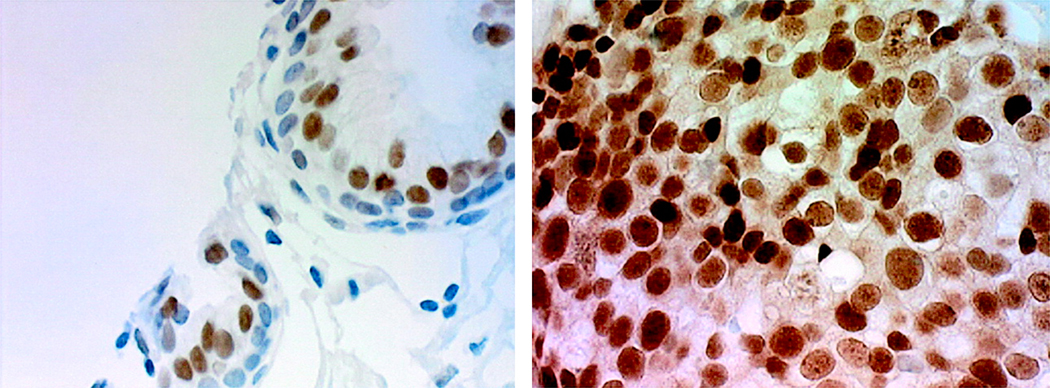
The frequency of low-level expression of AR in androgen-stimulated advanced CaP suggested the hypothesis that castration-recurrent CaP is composed of CaP cells that lack AR expression, since it was thought that castration-recurrent CaP did not depend on androgens and hence did not require AR for growth. However, immunohistochemistry suggested that AR expression was similar in castration-recurrent CaP and androgen-stimulated BPH and CaP.[43] AR immunostaining of 19 specimens of castration-recurrent CaP (% positive nuclei, 83.7 ± 11.6, and MOD, 0.284 ± 0.115) was similar to 16 specimens of benign prostate (% positive nuclei, 77.3 ± 13.0, and MOD, 0.315 ± 0.044) (P = 0.25 for % positive nuclei and 0.48 for MOD) (Figure 1).[3] The finding that AR protein levels were similar in archived specimens of castration-recurrent CaP and benign prostate when measured using a monoclonal AR antibody and automated image analysis was similar to earlier reports that used qualitative methods.[44,45] High levels of AR expression in castration-recurrent CaP in the absence of testicular androgens provides the potential for enhanced AR sensitivity to available androgens or alternate mechanisms of activation that would allow AR to remain central to growth regulation of castration-recurrent CaP.[46–49] These surprising findings suggested to many that AR plays a critical role in castration-recurrent CaP.[46–48,50] A critical role for AR in castration-recurrent CaP was supported by three critical preclinical observations. First, intracellular injection of AR ribozyme decreased the growth of androgen-independent LNCaP cells.[48] Second, AR over-expression by amplification was common to all castration-recurrent CaP xenografts studied. [51] Finally, preventing AR nuclear translocation or ligand binding delayed CaP recurrence in xenograft models.[51]
Figure 1.
Androgen-receptor protein expression is similar in androgen-stimulated benign prostate (left) and castration-recurrent prostate cancer (right).
The mechanisms demonstrated for castration recurrence in preclinical studies have been found to be relatively rare causes of castration recurrence in studies of clinical specimens. For example, in contrast to early reports where AR mutations were common, [52] the true frequency of AR mutations in castration-recurrent CaP is approximately 5%, but may be as high as 30% in bone-marrow metastasis from patients having prolonged treatment with anti-androgens.[53] Although AR gene amplification is common[54,55] in castration-recurrent CaP, and AR amplification was associated with approximately a 50% increase in the amount of AR protein expressed per cell, survival of CaP patients with amplification was similar to those without amplification. Finally, several laboratories continue to investigate preclinically ligand-independent AR activation and even AR-independent androgen-regulated gene expression although little data suggests that either of these mechanisms for growth occur clinically.
Newer theories for the development of castration-recurrent CaP require examination. First, AR responds to castration with molecular and biochemical alterations that cause hypersensitivity to low levels of ligand. Our group was the first to demonstrate that AR in androgen-independent CaP cell lines was hypersensitive. AR was approximately four logs more sensitive to androgens. [56] A biochemical explanation was offered: the AR coactivator profile changed from SRC-1 toward TIF-2, a finding that was true both in vitro and in clinical specimens.[57] Further clinical relevance to this finding was suggested recently by Agoulnik et al[58]who reported that levels of TIF-2 expression were prognostic when combined with levels of AR expression in men with clinically localized CaP treated by radical prostatectomy. A newer biochemical mechanism for hypersensitization of the AR in the face of low ligand availability has been suggested by Guo et al[59] who reported that phosphorylation of Y534 by a Sarc tyrosine kinase hypersensitized AR to low levels of androgen and was a common characteristic of clinical specimens of castration-recurrent CaP. Similar findings were found at Y267 and Y363, which were phosphorylated by ACK1 tyrosine kinase, which was also increased in clinical samples of castration-recurrent CaP.[60]
ACCURATE MEASUREMENT OF ANDROGEN-RECEPTOR EXPRESSION IN CLINICAL SPECIMENS
Immunohistochemistry for androgen receptor
The preceding sections should demonstrate that the relevance of AR to clinical CaP is difficult to determine due to variation (and often errors) in clinical samples chosen for analysis, immunostaining methodological details, antibodies, assessment of AR content, and outcome uncertainty. Biochemical measurement of AR protein content in clinical specimens is impractical due to variation in the relative contribution of epithelia, endothelia and stroma; the increasingly small size of CaP; multifocality of CaP; and heterogeneity of AR expression among cells, glands and tumors. Immunohistochemistry upon fresh or frozen prostate tissues is made difficult by the small specimen size and the difficulty of gross recognition of CaP. The first study of AR protein expression in prostate tissue used frozen sections, visual scoring, and a polyclonal antibody.[19]
The discovery of methods for antigen retrieval from archived clinical specimens[61] and the development of a highly specific monoclonal antibody allowed correlation between AR protein expression and clinical parameters of interest. Antigen retrieval is best performed using Reveal Citra buffer (Biocare Medical, Walnut Creek, CA, USA) and a pressurized antigen-decloaking chamber for 2 minutes at 120°C and 21 PSI. Monoclonal antihuman AR antibody F39.4.1 (Biogenex, San Ramon, CA, USA) has consistently yielded superior results. Other AR antibodies should be used only after careful optimization, and results using other AR antibodies should be interpreted with caution. Benign and malignant tissues and appropriate controls should be immunostained in a single batch. Special care must be taken when studying tissue microarrays. Tissue microarrays constructed from 2 mm cores can be placed on standard slides, but 1.0 mm or 0.6 mm cores require polymerized slides. Use of polymerized slides allows 1000-fold dilution of primary antibody but increases background, which makes pathologists less comfortable interpreting histology. Image analysis is unaffected since background is subtracted.
AR immunostaining in individual tissue samples cannot be evaluated properly using the standard 0 to 3+ ‘gestaldt’ assigned by clinical pathologists or laboratory technicians. A visual scoring technique was developed that assessed intensity of AR immunostaining on a scale of 0 (none) to intense (3+) for each of 100 randomly selected nuclei. This scoring system attempted to capture overall immunostaining intensity and heterogeneity of AR protein expression. However, recognition of malignant nuclei required a skilled pathologist or a highly trained technician and visual assignment of immunopositivity is subjective, tedious, and poorly reproducible. Mean optical density (MOD) measured using image analysis was found to be more accurate and reproducible for measuring AR expression, but object identification remained difficult. Investigators used a light pen to encircle[25] or a sampling window[41] to select areas of malignant nuclei for optical density measurement. However, these interactive image analysis methods were tedious, and user bias influenced the measurements. Tilley et al[21] used an automated color video image analysis system to measure MOD of each positively stained area in visually marked malignant tissue. MOD was calculated as the total integrated optical density divided by the area. Such measurements tend to be less accurate when compared to MOD measured from individual nuclei. In addition, MOD depends on variability of immunostaining intensity among tissue sections and tissue thickness.[21,25,41] However, the need to capture relatively small differences in levels of AR protein expression made precise measurement of AR protein expression essential for proper investigation of the clinical relevance of AR to CaP.
Quantitative image analysis for androgen receptor
In order to quantify AR accurately, primary antibody concentration, DAB concentration and DAB incubation time (5.25 minutes) were optimized to achieve a linear relationship between AR protein content and optical density of immunoperoxidase–DAB reaction product.[16] Next, prostatic epithelial nuclei are segmented from complex histological images and nuclei classified as malignant or benign. This step presents the primary obstacle to accurately quantifying AR expression using image analysis. The barrier was overcome by combining segmentation algorithms and nuclear morphometry.[62]
Images are acquired at total magnification of at least 400×. Each image consists of 640 × 480 pixels collected in 24-bit color mode (16.7 million colors) and is stored in an uncompressed tagged image format file (TIFF). The resolution for digital images is 0.13 µm/pixel (1500×). The image analysis system was developed on networked DEC 5000 workstations, and uses programs written in C++, Windows-based Statgraphics 4.1 and Optimas 6.0.[16,63,64] The C++ programs incorporated graphics and image processing libraries (IGLOO). [65] The image analysis programs were later ported to a PC-compatible system on a Linux platform. However, images were collected using Windows-based systems that required image transfer across platforms. The current image-analysis software was developed on a Java platform (Java Runtime Environment, Sun Microsystems, Santa Clara, CA, USA) and incorporates all features of previous versions. The software eliminates the need for Statgraphics and Optimas and can be deployed on a variety of platforms with several versions of the software running at the same time without conflict.[66] The program is associated with a Web browser and can be downloaded freely from our website.[67] Once downloaded, Web connection is no longer required. Nuclear boundaries are extracted automatically using our segmentation algorithms.[65,68] A segmented object is classified as ‘malignant epithelial nucleus’ or ‘object of no interest’ using a classification algorithm according to hue, size, and shape.[62] The optical density of immunoperoxidase–DAB product is measured using an image subtraction technique at the maximum absorption wavelengths.[69] AR immunostaining is described by optical density and per cent AR positivity.[16] Twenty images (approximately 1500–2000 nuclei) provided an adequate sample size, since the average deviations from the MOD for a specimen remained within 5% upon collection of additional images.
Human intervention is only required to create a set of classification parameters. These parameters are used to reduce the effect of local variations in slide preparation and image acquisition on nuclear measurements. This system is superior to commercially available image analysis software, such as Adobe Photoshop,[70] Optimas,[42] and Image Pro Plus,[71] which contain macros that allow semi-automated image analysis. User interaction is required for object (nuclei) selection, modification of object boundaries, and/or selection of thresholds. Dedicated image analysis systems such as CAS-200,[72] ACIS[73] and Autocyte[74] consist of hardware and proprietary software which typically require user interaction and cannot be modified for this application.
Potential pitfalls must be avoided. Commercial reagents used for immunostaining are not standardized, and thus immunostaining patterns differ among research laboratories. When combined with local variations in image acquisition, the resulting automated analysis may produce significant errors. A set of classification parameters must be generated to calibrate the nuclear analysis software with each new dataset that makes the automated image analysis software independent of the type of immunostaining or imaging system used. Discriminant analysis of grayscale histograms of red, green and blue color information determines optimal thresholds for automated segmentation of red, green and blue images that is necessary to segment nuclei from a complex histological image.[75,76] The hue, saturation and intensity component images are multiplied by their corresponding discriminant coefficients from the parameter file and combined to form a single image. A nuclear mask is applied to the image and the resulting nuclear areas are classified as immunopositive or immunonegative depending on their classification score.
Practice points
prostate cancer, compared to benign prostate, exhibits decreased intensity and greater heterogeneity of androgen-receptor protein expression
prostate cancer that is associated with higher levels of androgen-receptor protein expression, and loss of androgen-receptor protein expression within the peritumoral stroma is associated with higher tumor volume at presentation and higher failure rate of potentially curative therapies
androgen-receptor protein expression is higher in the benign prostate and even higher in prostate cancer from African Americans compared to Caucasian Americans, and is the only potentially relevant biological difference found between the races that is likely to contribute to the more than two-fold higher mortality of prostate cancer in African Americans
prostate cancer that recurs during androgen deprivation therapy expresses androgen-receptor protein at levels similar to those expressed in androgen-stimulated benign prostate; these surprising findings have led to intensive investigation of the critical role for the androgen receptor in castration-recurrent prostate cancer
Research agenda
the molecular mechanism responsible for the increased expression of androgen-receptor protein in more aggressive prostate cancers and in the benign and malignant prostate of African Americans requires further study
androgen-receptor mutations, androgen-receptor gene amplification, ligand-independent androgen-receptor activation, and even androgen-receptor-independent androgen-regulated gene expression appear to play limited roles in the progression of prostate cancer to castration-recurrent prostate cancer
intensive investigation of the hypersensitization to the androgen receptor, which enables transactivation in the absence of circulating testicular androgens, should be undertaken
further study is required for the mechanism of production of tissue androgen levels sufficient for androgen-receptor activation in prostate cancer that recurs during androgen-deprivation therapy
androgen-receptor immunohistochemistry is technically difficult. Assessment of androgen receptor, in order to be quantitative, requires proper antigen retrieval, establishment of linear relationships between androgen-receptor expression and immunostaining, and accurate and reproducible measurement of androgen-receptor expression. Further research should establish an optimal method for measurement of androgen-receptor expression, which almost certainly must be performed using video color image analysis
the difficulty of procuring specimens of castration-recurrent prostate cancer for study, especially serially during treatment of patients with advanced prostate cancer, requires a commitment by physicians and patients to research, rapid procurement, and optimal storage of these invaluable specimens
CONCLUSION
One must accept that the AR plays an important role in the development and progression of CaP and appears critical to the growth of castration-recurrent CaP in spite of castrate levels of circulating testicular androgens. Further study of the role of AR is made difficult by several factors. First, the evaluation of AR function in vitro requires highly artificial situations where AR reporter constructs must be cotransfected, and often wild-type AR must be cotransfected. Second, the literature is hopelessly confused by careless use of terminology and difficulties in measuring androgen levels. Androgen-sensitive and androgen-independent growth are often not used carefully, androgen absence and androgen depletion are confused, and different types of androgen deprivation therapy are often used interchangeably when they may not be equivalent. AR is labile and subject to tissue ischemia and fixation differences before one even begins to attempt quantitation using immunohistochemistry. Immmunohistochemical assessment is made difficult by all the different means of quantitatively assessing AR expression. Visual scoring systems are almost as numerous as the number of laboratories assessing AR immunostaining. AR measurement using image analysis is made difficult by the relative lack of programs available to segment nuclei from complex immunohistochemical images. Once segmented, malignant nuclei must be properly segregated from other nuclei. Once malignant epithelial nuclei are segregated, AR protein expression must be measured accurately. In order to more properly establish the role of AR in clinical CaP, a better appreciation must be obtained of the differences between AR expression and function in vitro and in vivo. Unfortunately, in vitro study of AR may more often confuse than inform. Although closer to the clinical situation, study of clinical specimens all too often is done with lack of precision that may make the recognition of subtle differences in AR expression and activation difficult. These difficulties may preclude recognition of subtle differences in AR expression that may provide biomarkers of CaP aggressiveness or treatment failure. Lastly, the preclinical and clinical data all suggest that AR plays a critical central role in castration-recurrent CaP. Difficulty in procuring specimens of castration-recurrent CaP for study, especially serially during treatment of patients with advanced CaP, slows progress in the field.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (CA77739) and Department of Defense Prostate Cancer Research Program (DAMD 17-03-2-0052).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate 1941. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 5.Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 6.Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- 7.Lubahn DB, Joseph DR, Sar M, Tan J, Higgs HN, Larson RE, et al. The human androgen receptor: complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol. 1988;2:1265–1275. doi: 10.1210/mend-2-12-1265. [DOI] [PubMed] [Google Scholar]
- 8.Chang C, Chodak G, Sarac E, Takeda H, Liao S. Prostate androgen receptor: immunohistological localization and mRNA characterization. J Steroid Biochem. 1989;34:311–313. doi: 10.1016/0022-4731(89)90099-x. [DOI] [PubMed] [Google Scholar]
- 9.Bartsch W, Klein H, Schiemann U, Bauer HW, Voigt KD. Enzymes of androgen formation and degradation in the human prostate. Ann N Y Acad Sci. 1990;595:53–66. doi: 10.1111/j.1749-6632.1990.tb34282.x. [DOI] [PubMed] [Google Scholar]
- 10.Habib FK, Odoma S, Busuttil A, Chisholm GD. Androgen receptors in cancer of the prostate. Correlation with the stage and grade of the tumor. Cancer. 1986;57:2351–2356. doi: 10.1002/1097-0142(19860615)57:12<2351::aid-cncr2820571219>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Masai M, Sumiya H, Akimoto S, Yatani R, Chang CS, Liao SS, et al. Immunohistochemical study of androgen receptor in benign hyperplastic and cancerous human prostates. Prostate. 1990;17:293–300. doi: 10.1002/pros.2990170405. [DOI] [PubMed] [Google Scholar]
- 12.Brendler CB, Isaacs JT, Follansbee AL, Walsh PC. The use of multiple variables to predict response to endocrine therapy in carcinoma of the prostate: a preliminary report. J Urol. 1984;131:694–700. doi: 10.1016/s0022-5347(17)50585-6. [DOI] [PubMed] [Google Scholar]
- 13.Benson RC, Jr, Utz DC, Holicky E, Veneziale CM. Androgen receptor binding activity in human prostate cancer. Cancer. 1985;55:382–388. doi: 10.1002/1097-0142(19850115)55:2<382::aid-cncr2820550215>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Mohler JL, Chen Y, Hamil K, Hall SH, Cidlowski JA, Wilson EM, et al. Androgen and glucocorticoid receptors in the stroma and epithelium of prostatic hyperplasia and carcinoma. Clin Cancer Res. 1996;2:889–895. [PubMed] [Google Scholar]
- 15.Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, et al. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 2001;61:423–427. [PubMed] [Google Scholar]
- 16.Kim D, Gregory CW, Smith GJ, Mohler JL. Immunohistochemical quantitation of androgen receptor expression using color video image analysis. Cytometry. 1999;35:2–10. doi: 10.1002/(sici)1097-0320(19990101)35:1<2::aid-cyto2>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Pertschuk LP, Macchia RJ, Feldman JG, Brady KA, Levine M, Kim DS, et al. Immunocytochemical assay for androgen receptors in prostate cancer: a prospective study of 63 cases with long-term follow-up. Annals of surgical oncology. 1994;1:495–503. doi: 10.1007/BF02303615. [DOI] [PubMed] [Google Scholar]
- 18.Ricciardelli C, Choong CS, Buchanan G, Vivekanandan S, Neufing P, Stahl J, et al. Androgen receptor levels in prostate cancer epithelial and peritumoral stromal cells identify non-organ confined disease. Prostate. 2005;63:19–28. doi: 10.1002/pros.20154. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto KK, McSherry SA, Dent GA, Sar M, Wilson EM, French FS, et al. Immunohistochemistry of the androgen receptor in human benign and malignant prostate tissue. J Urol. 1993;149:1015–1019. doi: 10.1016/s0022-5347(17)36284-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang SX, Bentel JM, Ricciardelli C, Horsfall DJ, Haagensen DE, Marshall VR, et al. Immunolocalization of apolipoprotein D, androgen receptor and prostate specific antigen in early stage prostate cancers. J Urol. 1998;159:548–554. doi: 10.1016/s0022-5347(01)63981-8. [DOI] [PubMed] [Google Scholar]
- 21.Tilley WD, Lim-Tio SS, Horsfall DJ, Aspinall JO, Marshall VR, Skinner JM. Detection of discrete androgen receptor epitopes in prostate cancer by immunostaining: measurement by color video image analysis. Cancer Res. 1994;54:4096–4102. [PubMed] [Google Scholar]
- 22.Chodak GW, Kranc DM, Puy LA, Takeda H, Johnson K, Chang C. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J Urol. 1992;147:798–803. doi: 10.1016/s0022-5347(17)37389-5. [DOI] [PubMed] [Google Scholar]
- 23.de Winter JA, Trapman J, Brinkmann AO, Boersma WJ, Mulder E, Schroeder FH, et al. Androgen receptor heterogeneity in human prostatic carcinomas visualized by immunohistochemistry. J Pathol. 1990;160:329–332. doi: 10.1002/path.1711600409. [DOI] [PubMed] [Google Scholar]
- 24.Pertschuk LP, Schaeffer H, Feldman JG, Macchia RJ, Kim YD, Eisenberg K, et al. Immunostaining for prostate cancer androgen receptor in paraffin identifies a subset of men with a poor prognosis. Lab Invest. 1995;73:302–305. [PubMed] [Google Scholar]
- 25.Sadi MV, Barrack ER. Image analysis of androgen receptor immunostaining in metastatic prostate cancer. Heterogeneity as a predictor of response to hormonal therapy. Cancer. 1993;71:2574–2580. doi: 10.1002/1097-0142(19930415)71:8<2574::aid-cncr2820710823>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Grossfeld GD, Hayward SW, Tisty TD, Cunha GR. The role of stroma in prostatic carcinogenesis. Endocr Relat Cancer. 1998;5:253–270. [Google Scholar]
- 27.Thompson TC, Timme TL, Kadmon D, Park SH, Egawa S, Yoshida K. Genetic predisposition and mesenchymal-epithelial interactions in ras+myc-induced carcinogenesis in reconstituted mouse prostate. Mol Carcinog. 1993;7:165–179. doi: 10.1002/mc.2940070307. [DOI] [PubMed] [Google Scholar]
- 28.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer metastasis reviews. 1998;17:411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 29.Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell's fault. Endocr Relat Cancer. 2002;9:61–73. doi: 10.1677/erc.0.0090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olapade-Olaopa EO, MacKay EH, Taub NA, Sandhu DP, Terry TR, Habib FK. Malignant transformation of human prostatic epithelium is associated with the loss of androgen receptor immunoreactivity in the surrounding stroma. Clin Cancer Res. 1999;5:569–576. [PubMed] [Google Scholar]
- 31.Guileyardo JM, Johnson WD, Welsh RA, Akazaki K, Correa P. Prevalence of latent prostate carcinoma in two U.S. populations. J Natl Cancer Inst. 1980;65:311–316. [PubMed] [Google Scholar]
- 32.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150:379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 33.Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Bouchardy C, Mirra AP, Khlat M, Parkin DM, de Souza JM, Gotlieb SL. Ethnicity and cancer risk in Sao Paulo, Brazil. Cancer Epidemiol Biomarkers Prev. 1991;1:21–27. [PubMed] [Google Scholar]
- 35.Gaston KE, Kim D, Singh S, Ford OH, 3rd, Mohler JL. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol. 2003;170:990–993. doi: 10.1097/01.ju.0000079761.56154.e5. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Wheeler T, Dai H, Frolov A, Thompson T, Ayala G. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–934. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Pollack A, DeSilvio M, Khor L, Li R, Al-Saleem T, Hammond M, et al. Ki-67 staining is a strong predictor of patient outcome for prostate cancer patients treated with androgen deprivation plus radiotherapy: An analysis of RTOG 92-02. Int J Radiat Oncol Biol Phys. 2003;57 suppl:200–201. [Google Scholar]
- 38.Ricciardelli C, Jackson M, Choong C, Stahl J, Marshall V, Horsfall D, et al. Elevated levels of HER-2/neu and androgen receptor in clinically localized prostate cancer identifies metastatic potential. Prostate. doi: 10.1002/pros.20747. in press. [DOI] [PubMed] [Google Scholar]
- 39.Sadi MV, Walsh PC, Barrack ER. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer. 1991;67:3057–3064. doi: 10.1002/1097-0142(19910615)67:12<3057::aid-cncr2820671221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Takeda H, Akakura K, Masai M, Akimoto S, Yatani R, Shimazaki J. Androgen receptor content of prostate carcinoma cells estimated by immunohistochemistry is related to prognosis of patients with stage D2 prostate carcinoma. Cancer. 1996;77:934–940. [PubMed] [Google Scholar]
- 41.Prins GS, Sklarew RJ, Pertschuk LP. Image analysis of androgen receptor immunostaining in prostate cancer accurately predicts response to hormonal therapy. J Urol. 1998;159:641–649. [PubMed] [Google Scholar]
- 42.Nabi G, Seth A, Dinda AK, Gupta NP. Computer based receptogram approach: an objective way of assessing immunohistochemistry of androgen receptor staining and its correlation with hormonal response in metastatic carcinoma of prostate. J Clin Pathol. 2004;57:146–150. doi: 10.1136/jcp.2003.010520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 44.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, et al. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48:189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 45.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 46.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 47.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 49.Sadar MD, Hussain M, Bruchovsky N. Prostate cancer: molecular biology of early progression to androgen independence. Endocr Relat Cancer. 1999;6:487–502. doi: 10.1677/erc.0.0060487. [DOI] [PubMed] [Google Scholar]
- 50.Klocker H, Eder IE, Comuzzi B, Bartsch G, Culig Z. Androgen receptor function in prostate cancer progression. In: Chung LW, Isaacs WB, Simons JW, editors. Prostate cancer - Biology, genetics, and the new therapeutics. 2nd ed. Totowa, NJ: Humana Press; 2007. [Google Scholar]
- 51.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 52.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 53.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–2515. [PubMed] [Google Scholar]
- 54.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 55.Ford OH, 3rd, Gregory CW, Kim D, Smitherman AB, Mohler JL. Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J Urol. 2003;170:1817–1821. doi: 10.1097/01.ju.0000091873.09677.f4. [DOI] [PubMed] [Google Scholar]
- 56.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–2898. [PubMed] [Google Scholar]
- 57.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 58.Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WE, 3rd, Erdem H, et al. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66:10594–10602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- 59.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci U S A. 2007;104:8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 62.Kim D, Charlton JD, Coggins JM, Mohler JL. Semiautomated nuclear shape analysis of prostatic carcinoma and benign prostatic hyperplasia. Anal Quant Cytol Histol. 1994;16:400–414. [PubMed] [Google Scholar]
- 63.Gaston KE, Ford IO, Singh S, Gregory CW, Weyel DE, Smith GJ, et al. A novel method for the analysis of the androgen receptor. Curr Urol Rep. 2002;3:67–74. doi: 10.1007/s11934-002-0013-8. [DOI] [PubMed] [Google Scholar]
- 64.Kim D, Gregory CW, French FS, Smith GJ, Mohler JL. Androgen receptor expression and cellular proliferation during transition from androgen-dependent to recurrent growth after castration in the CWR22 prostate cancer xenograft. Am J Pathol. 2002;160:219–226. doi: 10.1016/S0002-9440(10)64365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coggins JM. Image and Graphics Library, Object-Oriented (IGLOO) Manual. Chapel Hill, NC: Department of Computer Science, University of North Carolina; 1993. [Google Scholar]
- 66.Java Web Start Reference Manual. http://java.sun.com/products/javawebstart/developers.htm.
- 67.Roswell Park Cancer Institute Prostate Cancer Research. http://www.roswellpark.org/Site/Research/Research_Programs/Prostate.
- 68.Kim D. Multiscale image analysis of prostatic carcinoma and benign hyperplasia using confocal laser scanning microscope. University of North Carolina. 1993 [Google Scholar]
- 69.Pappolla MA. Computerized image-analysis microspectroscopy of tissue sections. Arch Pathol Lab Med. 1988;112:787–790. [PubMed] [Google Scholar]
- 70.Mofidi R, Walsh R, Ridgway PF, Crotty T, McDermott EW, Keaveny TV, et al. Objective measurement of breast cancer oestrogen receptor status through digital image analysis. Eur J Surg Oncol. 2003;29:20–24. doi: 10.1053/ejso.2002.1373. [DOI] [PubMed] [Google Scholar]
- 71.Blatt RJ, Clark AN, Courtney J, Tully C, Tucker AL. Automated quantitative analysis of angiogenesis in the rat aorta model using Image-Pro Plus 4.1. Computer methods and programs in biomedicine. 2004;75:75–79. doi: 10.1016/j.cmpb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Cell analysis systems: Quantitative estrogen progestoerone user's manual, Application Version 2.0, Catalog Number 201325-00. Cell Analysis Systems, Inc. USA. 1990 [Google Scholar]
- 73.Acis®. Information available from http://www.chromavision.com/product/acis.htm.
- 74.Workstation AP. Information available from http://tripathimaging.com/nonus_ac_quic_immuno.htm. [Google Scholar]
- 75.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Systems Man Cybernetics. 1979;9:62–66. [Google Scholar]
- 76.Gonzalez RC, Woods RE. In: Digital image processing. Addison-Wesley, editor. Reading, MA: 1992. pp. 229–237. [Google Scholar]