Abstract
Twenty-seven schizophrenia spectrum patients and 25 healthy controls performed a probabilistic version of the serial reaction time task (SRT) that included sequence trials embedded within random trials. Patients showed diminished, yet measurable, sequence learning. Postexperimental analyses revealed that a group of patients performed above chance when generating short spans of the sequence. This high-generation group showed SRT learning that was similar in magnitude to that of controls. Their learning was evident from the very 1st block; however, unlike controls, learning did not develop further with continued testing. A subset of 12 patients and 11 controls performed the SRT in conjunction with positron emission tomography. High-generation performance, which corresponded to SRT learning in patients, correlated to activity in the premotor cortex and parahippocampus. These areas have been associated with stimulus-driven visuospatial processing. Taken together, these results suggest that a subset of patients who showed moderate success on the SRT used an explicit stimulus-driven strategy to process the sequential stimuli. This adaptive strategy facilitated sequence learning but may have interfered with conventional implicit learning of the overall stimulus pattern.
Keywords: implicit sequence learning, schizophrenia, positron emission tomography (PET), serial reaction time task (SRT), visuomotor
Implicit learning characterizes the way that people acquire information about regularly occurring events in their environment. This type of learning is unintentional and occurs without explicit awareness that learning has taken place (A. S. Reber, 1989; Seger, 1994). Implicit learning of patterns, or sequences, is a fundamental aspect of human activity that underlies the ability to perceive sounds in speech, ride a bicycle, or interpret unspoken “rules” of social behavior. By contrast, explicit learning involves a deliberate attempt to use information gained from prior experience to predict subsequent outcomes. A fundamental deficit in implicit sequence learning could give rise to a number of difficulties in processes that require the linkage of events, including motor coordination (Manschreck et al., 2000), planning, strategic thinking (Hallett & Grafman, 1997), and social cognition (Lieberman, 2000). One psychiatric population that experiences these types of difficulties is schizophrenia (Calarge, Andreasen, & O’Leary, 2003; Gold, Carpenter, Randolph, Goldberg, & Weinberger, 1997; Marvel, Schwartz, & Rosse, 2004; Pantelis et al., 1997; Sullivan et al., 2001). Recent studies have reported deficits of implicit sequence learning in schizophrenia (Exner, Weniger, Schmidt-Samoa, & Irle, 2006; Green, Kern, Williams, McGurk, & Kee, 1997; Kumari et al., 2002; Marvel, Schwartz, Howard, & Howard, 2005; Schwartz, Howard, Howard, Hovaguimian, & Deutsch, 2003), yet few studies have examined the neural correlates of these impairments.
Implicit sequence learning is often measured by researchers using the serial reaction time task (SRT; Nissen & Bullemer, 1987). The SRT is a visuomotor learning paradigm in which stimuli are displayed in a repeated sequence, and participants press keys that correspond to the respective stimuli. After many presentations of the sequence, random stimuli are suddenly introduced toward the end of the test. The introduction of random stimuli slows the participant’s response time (RT) if the sequence has been learned well. The RT difference between sequence and random trials is the measure of implicit sequence learning. Learning on the SRT is considered to be implicit because participants often cannot verbally describe the sequential nature of the stimuli after the test.
A popular variation of the SRT involves a probabilistic paradigm in which sequential stimuli are embedded with a small percentage of random stimuli in every block (J. H. Howard & Howard, 1997). Thus, random stimuli are presented throughout the entire test instead of at the end. This paradigm is advantageous for several reasons. First, it allows the experimenter to measure trial type differences (i.e., sequence learning) throughout the entire test session. The development of sequence learning over time can be charted, and sequence learning can be distinguished from general motor learning. Second, participants can be given extensive exposure to elements of the sequence without developing explicit awareness of the sequence (Feeney, Howard, & Howard, 2002; D. V. Howard et al., 2004; J. H. Howard & Howard, 1997; Japikse, Negash, Howard, & Howard, 2003; Negash, Howard, Japikse, & Howard, 2003). Finally, this design simulates a “real world” environment in which relevant stimuli must be filtered from a steady stream of background noise.
Studies that have used the standard nonprobabilistic SRT in schizophrenia have reported no significant sequence learning in their patient samples (Exner, Boucsein, Degner, & Irle, 2006; Green et al., 1997; but see also Reiss et al., 2006). These studies relied on the end-test comparison of RT performance between the final block of random trials and the penultimate block of sequence trials. By contrast, two studies that used a probabilistic SRT in schizophrenia demonstrated that patients were able to develop sensitivity to embedded sequences, but the magnitude of their sequence learning was less than that of controls (Marvel et al., 2005; Schwartz et al., 2003). Results of these latter two studies suggest that the patients’ capacity to learn sequences is diminished but measurable. These findings also suggest that patients rely on inefficient cognitive and/or neural strategies to detect complex patterns within their environment.
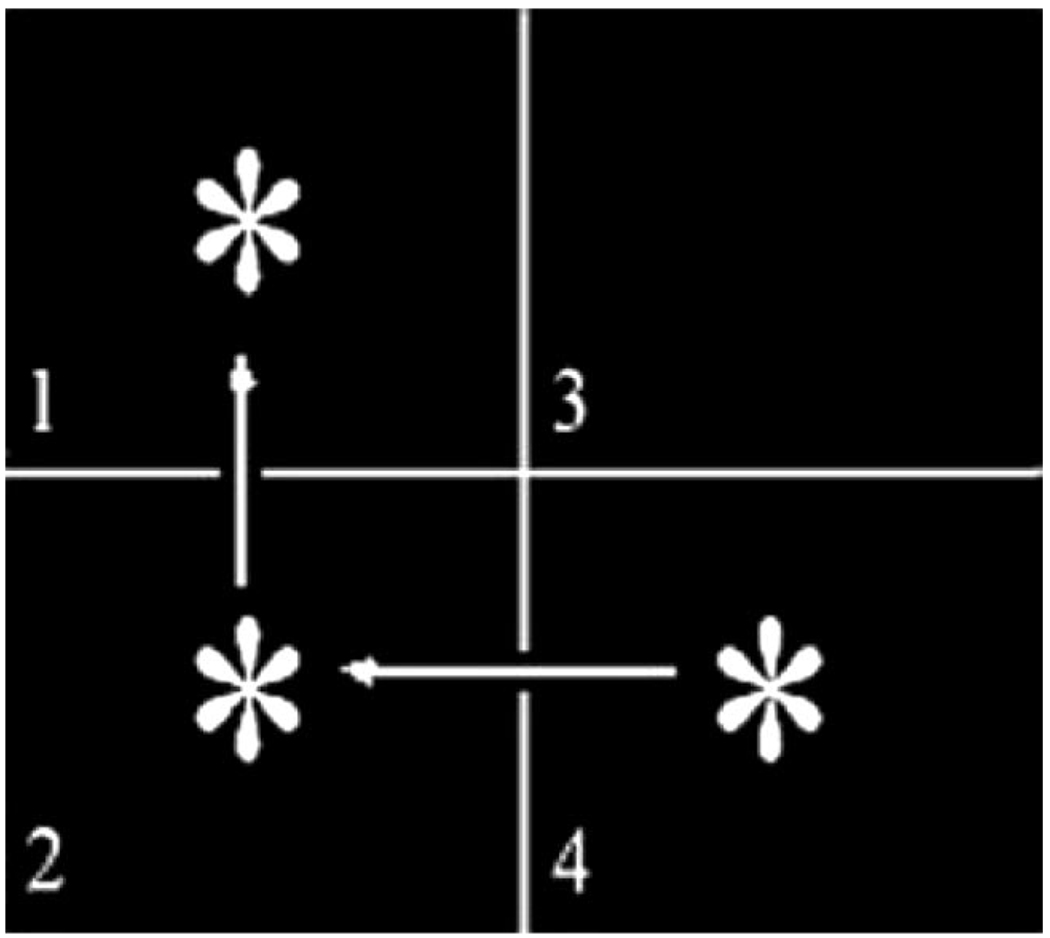
To our knowledge, only one neuroimaging study to date has published findings on the neural correlates of impaired implicit sequence learning in schizophrenia using a probabilistic SRT paradigm. Using functional magnetic resonance imaging (fMRI), Kumari et al. (2002) tested medicated schizophrenia patients and healthy controls on a three-item spatial sequence (similar to that shown in Figure 1), with every fourth location determined randomly. Blocks of sequence trials alternated with blocks of random trials. The primary measure of learning was the RT difference between sequence blocks (which contained a low percentage of random trials) and random blocks (which contained 100% random trials). Over time, healthy participants demonstrated learning with faster RTs on the sequence blocks relative to the random blocks. However, patients failed to show evidence of sequence learning because their RTs on the sequence and random blocks were equivalent.
Figure 1.
Stimuli were presented in one quadrant at a time and remained on the screen until the participant pressed a key that corresponded to the quadrant number (even if incorrect). A response prompted the appearance of the next stimulus. Stimulus locations were determined by a set of rules (e.g., every horizontal “line” was followed by a vertical “line,” which was followed by a diagonal “line”). Arrows are shown here to indicate the sequential movement of the targets, even though arrows were not actually shown during the test. Every fourth location was determined randomly. Random stimuli helped to mask the saliency of the rules governing the stimulus locations. An example of a sequence that followed the horizontal– vertical–diagonal rule set might be as follows: 4-2-1-3-4-1-3-2-4-3-2-4. In this series, every fourth position represents a randomly determined stimulus. Note that the final random position of the series happens to be consistent with the rule set. In data analysis, random-yet-rule-consistent trials were considered as part of the sequence rather than as random trials.
Kumari et al. (2002) reported regional brain activity during sequence learning in the frontal lobe (Brodmann’s Area [BA] 45 and 10), anterior cingulate, insula, caudate nucleus, thalamus, and cerebellum in controls. By contrast, patients showed brain activity during sequence blocks in the inferior frontal gyrus (BA 45) only. Kumari et al.’s fMRI results were generally consistent with the literature on sequence learning. Regional brain activity associated with implicit sequence learning has been reported in motor-related areas of the frontal lobe, working memory areas of the prefrontal cortex (BA 9/46), the basal ganglia, the medial temporal lobe, and the cerebellum. The role of each of these areas in implicit sequence learning is described below.
Motor-related frontal lobe areas that have been implicated in implicit sequence learning include the “hand” portion of the primary motor cortex, the premotor cortex, and the supplementary motor area (SMA). The primary motor cortex tends to increase activation during early implicit sequence learning and then decrease activation if the participant achieves explicit sequence awareness (Grafton, Hazeltine, & Ivry, 1995; Pascual-Leone, Grafman, & Hallett, 1994). The premotor cortex also increases activity as implicit sequence learning develops (Hazeltine, Grafton, & Ivry, 1997). The premotor cortex is thought to be involved with externally (e.g., visually) guided movements (Hazeltine & Ivry, 2002; Mushiake, Inase, & Tanji, 1991). Specifically, this region may contribute to the formation of appropriate motor acts in response to sensory stimuli (di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992). By contrast, the SMA is thought to be involved with internally guided sequential movements (Mushiake et al., 1991), such as movements that are well-learned and predictable (i.e., explicit).
Activation of the prefrontal cortex has been reported in several SRT-type studies. Notably, the right dorsolateral prefrontal cortex (DLPFC) tends to increase activation with sequence learning (Grafton et al., 1995; Hazeltine & Ivry, 2002; Jueptner et al., 1997). It has been suggested that rising DLPFC activity accompanied by falling RTs reflects increased maintenance load for contextual information as progressively longer parts of the sequence are learned (Berns, Cohen, & Mintun, 1997). Disruption of DLPFC activity via repetitive transcranial magnetic stimulation prevented implicit sequence learning of spatial cues but spared implicit sequence learning of color cues (Robertson, Tormos, Maeda, & Pascual-Leone, 2001). This functional dissociation suggests that the DLPFC facilitates sequence learning by retaining spatial information over the short term. In this view, the DLPFC does not play a necessary role in the general aspects of implicit sequence learning, but it is important for spatial sequence learning.
Neuroimaging studies of implicit SRT performance have shown increased caudate and putamen activity associated with the degree of sequence learning (Grafton et al., 1995; Rauch et al., 1997). Further evidence of basal ganglia involvement in implicit sequence learning comes largely from studies of patients with basal ganglia pathology. Such patients are impaired on SRT-type tasks, even when motor performance deficits are taken into account (Siegert, Taylor, Weatherall, & Abernethy, 2006). For example, Huntington’s patients have shown normal motor learning (i.e., normal rate of RT decrease across blocks) throughout testing, but they exhibited diminished RT slowing with the introduction of random trials (Knopman & Nissen, 1991). Patients with Parkinson’s disease have also shown diminished implicit sequence learning, although performance normalized when patients were provided with explicit information about the sequence (Pascual-Leone et al., 1993) or were taking dopaminergic medications (Shohamy, Myers, Grossman, Sage, & Gluck, 2005). It has been suggested that dopaminergic activity in the basal ganglia provides a means for reinforcing associations among relevant stimuli, thereby enabling the formation of context (Graybiel, 2005). Therefore, it is possible that dopamine (DA) disruption within the basal ganglia interferes with the formation of stimulus–stimulus associations.
Until recently, it had generally been accepted that medial temporal lobe structures were not essential to SRT-type learning. This notion was based on findings that patients with declarative memory impairments (e.g., amnesics) could learn sequential information on the SRT (Hopkins, Waldram, & Kesner, 2004; Nissen & Bullemer, 1987; Nissen, Willingham, & Hartman, 1989; P. J. Reber & Squire, 1994). However, recent evidence suggests that the hippocampus, entorhinal cortex, and parahippocampus may be important for learning what are known as higher order associations. Higher order associations within the SRT require learning three or more successive stimuli. Curran (1998) demonstrated that amnesic patients performed normally overall on the SRT, but closer examination revealed that the patients did not learn as much higher order information as controls did. Using fMRI in healthy participants, Schendan, Searl, Melrose, and Stern (2003) found medial temporal lobe activation in early learning of higher order information for both implicit and explicit sequences. These results suggest that the role of the medial temporal lobe in sequence learning may be important for creating higher order stimulus–stimulus associations but need not be tied to awareness.
Several neuroimaging studies that used SRT-type paradigms have shown that cerebellar activity changes in association with sequence learning; however, the reported pattern of activity has been mixed. Hazeltine et al. (1997) found decreased cerebellar activation across learning blocks. Yet, Doyon, Owen, Petrides, Sziklas, and Evans (1996) found that cerebellar activity increased for a new sequence relative to a well-learned sequence. It is difficult, however, to make direct comparisons of cerebellar activity across studies when different designs have been used. For example, Hazeltine et al. used a dual-task distractor and a fixed interstimulus interval (ISI) of 1,500 ms. By contrast, Doyon et al. used a single-task paradigm with a fixed ISI interval of 800 ms. Task distractors and lengthy ISIs can influence SRT learning by affecting attention and working memory capability, and presumably their underlying neural correlates (Frensch & Miner, 1994; Nissen & Bullemer, 1987). Further evidence for the role of the cerebellum in SRT learning comes from the neuropsychological literature. In one study, when patients with cerebellar atrophy were tested on a standard SRT, implicit sequence learning was impaired (Pascual-Leone et al., 1993). Even when patients were given explicit knowledge of the sequence, they were unable to use this information to improve their performance. In another study, patients with focal lesions isolated to the cerebellar hemispheres were tested on a nonspatial SRT (Gomez-Beldarrain, Garcia-Monco, Rubio, & Pascual-Leone, 1998). In this paradigm, cerebellar patients showed impaired sequence learning but only when using the hand ipsilateral to the site of the lesion. When they were able to use their hand contralateral to the lesion, they performed normally. Moreover, transient disruption of the cerebellum on the side ipsilateral to the responding hand in healthy participants has been shown to interfere with SRT learning (Torriero, Oliveri, Koch, Caltagirone, & Petrosini, 2004). Thus, the cerebellum appears closely tied to visuomotor aspects of implicit sequence learning in the SRT paradigm.
In schizophrenia, structural and functional abnormalities have been reported in many of the areas relevant to SRT learning: the prefrontal cortex, basal ganglia, medial temporal lobe, and cerebellum (see Shenton, Dickey, Frumin, & McCarley, 2001, for review). Researchers have argued that higher level cognitive deficits in schizophrenia, such as planning, problem-solving, and abstraction, are attributed to dysfunction of the frontal lobes (Zakzanis & Heinrichs, 1999). Accordingly, diminished blood flow to the prefrontal cortex has been demonstrated in patients during functional imaging studies of executive function (Berman, Torrey, Daniel, & Weinberger, 1992; Weinberger, Berman, & Zec, 1986). Because DA blockers have been a primary treatment in schizophrenia, the DA-enriched basal ganglia and its projections to the prefrontal cortex have also become a focus of research. Structural imaging has shown that medicated patients tend to have enlarged basal ganglia volumes relative to their nonmedicated patient counterparts (Chakos et al., 1994; Corson, Nopoulos, Miller, Arndt, & Andreasen, 1999). Furthermore, functional neuroimaging in schizophrenia has revealed abnormal basal ganglia activity during cognition and at rest (Manoach et al., 2000; Menon, Anagnoson, Glover, & Pfefferbaum, 2001). Disruption of dopaminergic pathways could affect the patients’ ability to become sensitive to the relationships among stimuli in their environment (Braver, Barch, & Cohen, 1999) or lead to motor abnormalities, such as slowed responses and difficulty completing complex voluntary movements (Manschreck, Maher, Rucklos, & Vereen, 1982). Researchers have also implicated medial temporal lobe structures by reporting morphological abnormalities in the hippocampus (Breier et al., 1992) and parahippocampus (McCarley et al., 1993). It has been proposed that a disconnection between the prefrontal cortex and the hippocampus contributes to patients’ declarative memory deficits (Weiss & Heckers, 2001). Finally, abnormal cerebellar structure and function has been reported in schizophrenia (Andreasen et al., 1997; Andreasen, Paradiso, & O’Leary, 1998; Nopoulos, Ceilley, Gailis, & Andreasen, 1999; Okugawa et al., 2002). Ho, Mola, and Andreasen (2004) examined 155 neuroleptic-naïve schizophrenia patients on a comprehensive cognitive battery and rated patients for cerebellar soft signs (motor impairments referable to the cerebellum). Patients with measurable cerebellar soft signs also exhibited specific deficits of attention, working memory, problem solving, and figure copying in addition to having overall smaller cerebellar volume relative to their patient counterparts who did not display cerebellar soft signs. These results suggest that cerebellar dysfunction in schizophrenia is related to impairments of motor coordination and higher order cognitive skills. There have not been consistent reports of abnormalities in the primary motor area or premotor cortex, although the SMA has been implicated recently (Exner, Weniger, et al., 2006). These motor regions of the frontal lobe that are relevant to SRT learning, therefore, would be less likely than the aforementioned regions to contribute to sequence learning deficits in schizophrenia.
Given the structural and functional deficits in schizophrenia within areas of the brain that have been implicated in implicit sequence learning for SRT-type tests, it is not surprising that patients have demonstrated implicit sequence learning deficits in prior studies. However, because there has only been one neuroimaging study of probabilistic sequence learning in schizophrenia to date, little is known about the neural underpinnings of the patients’ impairments in this type of learning. This study aimed to better understand this particular type of cognitive deficit in schizophrenia by following up on the previous neuroimaging study conducted by Kumari et al. (2002). One limitation of their study, however, was that the number of trials was not equated between groups. Completion of trials was self-paced and fixed at 30 s for each block. Controls were faster than patients during the sequence blocks, which enabled them to complete more trials and gain more exposure to the sequence during these 30-s intervals. Another limitation of their study was that RT performance on the sequence and random trials during the sequence blocks were averaged together, and therefore, trial type differences within the sequence blocks were not computed. Patient sensitivity to the sequence, if it existed, may have gone undetected. Finally, it is unclear when sequence learning developed for controls and how their learning improved across blocks. Had these variables been measured, the nature of the patients’ deficits could have been characterized more clearly.
This study was designed to extend the findings of Kumari et al. (2002), with further examination of the neural underpinnings of implicit sequence learning in schizophrenia using positron emission tomography (PET). We implemented a self-paced version of a probabilistic sequence learning paradigm that was similar to the one used by Kumari et al., with two important modifications. First, we included a fixed number of trials per block to ensure that patients and controls would receive equal exposure to the sequence and random stimuli. Second, we included one random block at the beginning and one random block at the end of testing. By eliminating intervening random blocks (as were used by Kumari et al., 2002), we hoped to decrease the overall exposure to distracting stimuli. Random trials remained embedded within the sequence at every fourth trial. These two changes were incorporated so that RT performance between sequence and random trials could be compared directly within each group. This comparison would enable us to monitor sequence learning throughout the entire test session and to characterize group differences.
We hypothesized that patients would demonstrate implicit sequence learning when given adequate exposure to the sequence. Consistent with previous studies that have used probabilistic paradigms, we expected to see that implicit sequence learning would be diminished, relative to controls. In addition, we expected to see group differences in neural activations that corresponded to group differences in implicit sequence learning, particularly in the prefrontal cortex, basal ganglia, medial temporal lobe, and cerebellum.
Method
Participants
Twenty-seven patients and 25 nonpsychiatric control participants were recruited from the University of Iowa hospital community. Twelve patients and 10 controls underwent behavioral testing only. We recruited an additional 15 patients and 15 controls to perform the sequence learning test while their cerebral blood flow was measured using PET. We ultimately decided to exclude left-handed participants (patients = 3, controls = 4) from the PET portion of the study to avoid handedness as a potential confound in the PET data. We did, however, include these left-handed participants in the overall behavioral data set. Table 1 shows the demographic characteristics of each group. This research was approved by the University of Iowa Institutional Review Board. All participants gave their written informed consent prior to inclusion in the study and were paid for their participation.
Table 1.
Characteristics of Patient and Control Groups
| Characteristic | All patients | All controls | PET patients | PET controls |
|---|---|---|---|---|
| Sample size | 27 | 25 | 12 | 11 |
| Sex ratio (male:female) | 19:8 | 12:13 | 7:5 | 4:7 |
| Handedness (right:left:ambidextrous) | 21:3:3 | 20:5:0 | 10:0:2 | 11:0:0 |
| Age (years)a | 32.8 (9.4) | 34.6 (9.2) | 38.1 (10.1) | 29.5 (7.7) |
| Education (years)b | 13.2 (2.2) | 15.8 (1.7) | 13.8 (2.5) | 15.6 (2.0) |
| Parent’s education (years) | 14.4 (2.9) | 13.3 (1.7) | 13.4 (2.8) | 13.7 (1.6) |
| FSIQ | 101.30 (19.27) | 103.92 (15.68) | 104.50 (21.64) | 95.09 (12.81) |
| Duration of illness (years) | 11.93 (8.42) | 15.83 (9.04) | ||
| CPZ | 530.93 (502.51) | 512.50 (403.98) | ||
| Positive symptom severityc | 5.58 (2.92) | 5.40 (3.27) | ||
| Negative symptom severityc | 10.25 (2.94) | 9.50 (3.17) | ||
| Disorganized symptom severityc | 3.67 (2.35) | 3.40 (0.97) | ||
| AIMS | 0.04 (0.20) | 0.08 (0.29) |
Note. Standard deviations are reported in parentheses. Psychiatric symptoms were assessed using the Comprehensive Assessment of Symptoms and History (CASH). Positive, negative, and disorganized symptoms were comprised of the sum of individual symptom categories: positive = delusions and hallucinations; negative = alogia, bluntness, lack of affect, avolition, and anhedonia; disorganized = bizarreness, formal thought disorder, catatonia, and lack of attention. Scores may range from 0 (none) to 5 (severe) on the CASH and from 0 (none) to 4 (severe) on the Abnormal Involuntary Movement Scale (AIMS). PET = positron emission tomography; FSIQ = full-scale IQ from the Wechsler Adult Intelligence Scale (Wechsler, 1997); CPZ = chlorpromazine equivalent of daily dose of neuroleptic medication.
PET patients were older than PET controls, p < .05.
All patients had fewer years of education than all controls, p < .01.
Current data was unavailable for 3 patients: all patients n = 24, and PET patients n = 10.
Trained members of our clinical research team evaluated all patients within 1 month of study participation using the Comprehensive Assessment of Symptoms and History (CASH; Andreasen, 1985). The CASH includes the Scale for the Assessment of Negative Symptoms (Andreasen, 1984a) and the Scale for the Assessment of Positive Symptoms (Andreasen, 1984b). Together, these instruments provided diagnostic information and symptom ratings of the patients at the time of testing. Signs of movement abnormalities were assessed with the Abnormal Involuntary Movement Scale (National Institute of Mental Health, 1976). On the Abnormal Involuntary Movement Scale, 1 patient received a global score of 1 (minimal deficits), and all other patients received a global score of 0 (no deficits). Patients were excluded from the study if they had a history of serious medical or neurologic disorders, head injury, or mental retardation. A prior, but not current, history of substance dependence was found in 7 of our patients, which is consistent with the high rate of substance use reported in the schizophrenia population at large (Kavanagh, McGrath, Saunders, Dore, & Clark, 2002). Three of these patients were included in the PET study. All but 3 patients were medicated at the time of test. Twenty-one were taking second-generation neuroleptics, and 3 were taking a combination of second-generation and conventional neuroleptics (none were taking conventional neuroleptics alone). The 3 nonmedicated patients had been medicated in the past and were not neuroleptic-naïve. Of the 27 patients included in the study, 24 met Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; American Psychiatric Association, 2000) diagnostic criteria for schizophrenia, and 3 met criteria for schizoaffective disorder.
Healthy comparison participants were initially screened by telephone and further evaluated with an abbreviated version of the CASH to exclude participants with medical, neurologic, or psychiatric illnesses, including substance dependence. Recruitment efforts focused on controls with demographic variables consistent with those from our previous studies (e.g., right-handed, around the age of 30 years, with parental education of approximately 12 years) in an effort to match the anticipated characteristics of our patient group.
Design
The design was a 2 × 2 × 9 (Group × Trial Type × Block) mixed factorial with group (control and patient) as a between-subjects variable and trial type (sequence and random) and block (Blocks 2–10) as within-subject variables.
Procedure
Participants viewed stimuli presented on a 15-in. (38.1 cm) video monitor positioned 18 in. (45.72 cm) from the participant’s eyes. Stimuli were presented as white targets (asterisks) on a black background, as depicted in Figure 1, and delivered with MEL 2.0 (Psychology Software Tools, 1995) on a Gateway computer. Two white lines intersected in the center of the screen to create four quadrants. Each quadrant was numbered in the lower left-hand corner. Key presses were made with an ergonomic response box fitted to the participant’s right arm. The layout of the response box consisted of four buttons that ran left to right. Participants responded by pressing the index, middle, ring, or pinky fingers to indicate whether the stimulus appeared in quadrants 1, 2, 3, or 4, respectively.
Implicit Sequence Learning Test
Participants were asked to press a key that corresponded to each stimulus location as quickly and accurately as possible. They were told nothing about a rule or pattern associated with the stimulus presentation. For 14 patients and 13 controls, stimuli were presented one at a time in a sequence of quadrant locations following three specific rules: a horizontal “line” (created by two consecutively presented targets that appeared in quadrants to the right or left of one another), followed by a vertical “line” (created by two consecutively presented targets that appeared in quadrants above or below one another), followed by a diagonal “line” (created by two consecutively presented targets that appeared in quadrants above or below and contralateral to one another). To counterbalance the design, we presented the remaining 13 patients and 12 controls with a second set of rules: a horizontal “line,” followed by a diagonal “line,” followed by a vertical “line.” Every fourth stimulus location was determined randomly by the computer. The stimulus, however, could not remain in the same quadrant on two consecutive trials. This required the random stimulus to appear in one of the remaining three quadrants, only one of which was, by definition, consistent with the rule set. Thus, two thirds of all random trials were inconsistent with the rule set (i.e., truly random), which represented 17% (2/3 of 25%) of all trials in the task. The remaining 83% of trials, referred to as sequence trials, consisted of both sequence and random-yet-rule-consistent stimuli.
Participants performed 11 blocks of implicit sequence learning. As shown in the study design in Table 2, the task began with a block of 100% random (R) trials. The next nine blocks consisted of stimuli that followed the rule on 83% of trials, designated as sequence (S) blocks. A final R block was given at the end of the test. Blocks that were performed in conjunction with PET contained 180 trials. Shorter blocks performed between injections contained 80 trials. The first stimulus location of each block was randomly generated by the computer. Each subsequent trial was determined by the sequence rule with the exception of every fourth trial. The first sequence trial following a randomly generated trial resumed the rule set based on the location of the random stimulus relative to the previous stimulus (exemplified in Figure 1). The onset of each trial was initiated with a key press in response to the previous trial (response-to-stimulus interval = 0 ms). RTs were recorded by the computer. Prior to the test, all participants performed a practice test of 12 random trials to confirm that they understood the instructions and were comfortable using the response box.
Table 2.
Order of Stimulus Presentation and Bolus Injections by Block
| PET condition | Block no. | Stimulus type (% of random trials) |
|---|---|---|
| Scout injection | 1 | Random (100) |
| Injection 1 EARLY | 2 | Sequence (17) |
| Practice | 3 | Sequence (17) |
| Practice | 4 | Sequence (17) |
| Injection 2 MIDDLE | 5 | Sequence (17) |
| Practice | 6 | Sequence (17) |
| Practice | 7 | Sequence (17) |
| Injection 3 LATE | 8 | Sequence (17) |
| Practice | 9 | Sequence (17) |
| Practice | 10 | Sequence (17) |
| Injection 4 SWITCH | 11 | Random (100) |
| Injection 7 BASELINE | 12 | Random (passive viewing; 100) |
| Injection 8 FIXATION | 13 | Centered stimulus (passive viewing) |
Note. A postexperimental questionnaire was conducted after the SWITCH condition. BASELINE and FIXATION conditions were given to participants undergoing positron emission tomography (PET) only.
Tests for Explicit Knowledge of the Sequence
The following tests were conducted at the end of the implicit sequence learning task:
Verbal report
Immediately following the final R block, participants were asked a series of questions to probe for any knowledge of the rule-based sequence. Specifically, participants were asked to describe regularities they may have noticed during the test.
Generation
Participants were shown two consecutive stimulus locations and asked to indicate (verbally) in which quadrant they thought the stimulus might appear next. They were permitted to practice key pressing on the response box to help them remember. Twelve trials were given, and responses were not time restricted.
Recognition
Participants were shown a split-screen presentation of two squares each divided into four quadrants. Similar to the depiction in Figure 1, arrows indicated three consecutive stimulus locations in each half of the screen. Only one sequence of stimuli followed the participant’s rule set. Participants were asked to select the three-item sequence that they believed occurred more often throughout the test. Responses were self-paced across 12 trials.
Baseline PET Conditions
After completion of the sequence learning task, two additional blocks were administered to those undergoing PET to serve as comparison conditions. In Block 12 (BASELINE), participants passively viewed 180 random stimulus locations. Each stimulus location was presented for 650 ms, which approximated the rate of stimulus presentation (and key pressing) during the implicit learning test. This baseline condition allowed us to identify brain activity related to sequence learning and to key pressing. During Block 13 (FIXATION), participants were presented with a single white asterisk in the center of a black screen that was refreshed every 650 ms. This FIXATION condition was compared with BASELINE to identify brain activity related to visual tracking of moving stimuli.
Magnetic Resonance Imaging (MRI) Data Acquisition and Processing
MRI scans were obtained for each participant with a standard T1-weighted and T2-weighted three-dimensional spoiled gradient recall echo sequence on a 1.5-T GE scanner (T1 parameters: echo time = 6 ms, repetition time = 20 ms, flip angle = 30°, number of excitations = 2, slice thickness = 1.5 mm; T2 parameters: echo time = 90 ms, repetition time = 4,800 ms, number of excitations = 3, slice thickness = 1.8 mm). For both scans, the field of view (FOV) was 26 × 26 cm, and the matrix was 256 × 192. MRI images were analyzed with locally developed BRAINS2 software (Magnotta et al., 2002). All brains were realigned parallel to the anterior commissure–posterior commissure line and the interhemispheric fissure to ensure comparability of head position across participants. Alignment also placed the images in standard Talairach space (Talairach & Tournoux, 1988). Images from multiple participants were coregistered and resliced in three orthogonal planes to produce a three-dimensional data set that was used for visualization and localization of functional activity.
PET Image Data Acquisition and Processing
We obtained and analyzed PET images using methods consistent with our previous studies (see Andreasen et al., 1995, for details). Specifically, imaging was performed on a GE 4096 Plus whole-body tomograph (15 slices, 10-cm axial FOV, with an interslice separation of 6.5 mm). This size FOV precludes total brain coverage. To ensure that brain regions consistent with our a priori hypothesis were included in the FOV, we aligned participants with the most inferior transaxial slice 1 cm below the auditory meatus and the outer canthus of the eye. The resultant FOV included the DLPFC (9/46), basal ganglia, medial temporal lobe, and all but the most inferior aspects of the cerebellum. It is important to note, however, that this FOV excluded other important areas of the brain that have shown activity in previous SRT imaging studies, such as the “hand” portion of the primary motor cortex, the dorsal aspect of the premotor cortex, and the SMA.
Position was verified before each injection. Each participant received one 15 mCi scout (given during Block 1) and four 50 mCi of (15O) water separated by approximately 15-min intervals. For Blocks 2–13, each task was initiated 20 s prior to the bolus arrival, which was determined by the scout injection. Imaging began at the time of the injection (t = 0) and continued for 100 s (20 s × 5 s imaging bins). We created a single static image for each injection by summing the dynamic frames representing the 40 s immediately postbolus transit and reconstructing them into 2-mm pixels (128 × 128 matrix) using filtered backprojection and a Butterworth filter (order = 6, cutoff frequency = 0.35 Nyquist interval).
Images for each participant were normalized by dividing each voxels’ count activity by the global PET count activity within the brain. PET data are reported as normalized values reflecting the redistribution of blood flow within the brain (i.e., fractional change) rather than an absolute increase or decrease in blood flow in the particular region. Automated Image Registration (Woods, Grafton, Watson, Sicotte, & Mazziota, 1998) was used to coregister each individual’s PET and MRI images. We processed each participant’s MRI scans using a combination of automated methods and hand editing, resulting in a brain that was aligned in a standardized coordinate space (Talairach & Tournoux, 1988). An 18-mm Hanning filter was applied to the PET images for each condition.
Activation Analysis
For within-group comparisons, the relative PET count activity during BASELINE was subtracted from activity during the implicit sequencing scans on a participant-by-participant basis. The subtraction images were averaged across participants, and we performed voxel-by-voxel t tests of the relative count changes using a technique that corrects for the large number of voxel t tests, the lack of independence between voxels, and the resolution of the processed PET images (Worsley, Evans, Marrett, & Neelin, 1992). Significant regions of activation were calculated on the t-map images. A t value of 3.61 was considered to be statistically significant (p < .0005, one-tailed, uncorrected), and a minimum size threshold was set at 50 contiguous voxels to omit isolated outlying values. We identified regions of cerebral activation by visual inspection using the Talairach atlas (Talairach & Tournoux, 1988). We identified cerebellar activations using an atlas specific to the cerebellum (Schmahmann et al., 1999).
In addition to image subtraction analyses, correlational analyses were used to examine the relationship between learning and normalized regional cerebral blood flow (rCBF) for each of the test conditions. Locally developed software was used to perform a correlation analysis between learning and normalized PET counts in every voxel across participants (see Schultz et al., 1999, for details of the methodology). We determined significant correlations using a Pearson’s r threshold of .75, which corresponds to p < .005 for 11 participants, and we confirmed these correlations through visual inspection of scatterplots. Minimum cluster size was 50 voxels. On the basis of previous research that associated DLPFC (BA 9/46), basal ganglia, medial temporal lobe, and cerebellar activity with sequence learning, correlations were examined within these regions. We also examined the ventral portion of the premotor cortex that was included in our FOV. The human prefrontal cortex is not bound by sulcal landmarks. To isolate BA 9/46 in our analyses, we defined the DLPFC as the area outlined by Rajkowska and Goldman-Rakic (1995): [BA 9: Anterior/Posterior (A/P) +53 to +26, Dorsal/Ventral (D/V) +50 (if FOV allowed) to + 25; BA 46: A/P +50 to +29, D/V +36 to +14]. An activation cluster was included in the DLPFC if its maximum voxel or a majority of its voxels were located within these criteria. The premotor cortex included areas anterior to the precentral sulcus ranging from approximately A/P +4 to −10 and D/V +50 (capped by FOV) to +6. Basal ganglia structures included the caudate nucleus, putamen, globus pallidus, subthalamic nuclei, and substantia nigra. Analysis of the medial temporal lobe included parahippocampal regions and the hippocampus body. We examined all areas of the cerebellum available within the FOV, which included all but the most inferior aspect of the cerebellum. Measures of learning were based on the RT difference between the mean of random RT minus the mean of sequence RT within blocks for Blocks 2, 5, and 8, which corresponded to EARLY, MIDDLE, and LATE phases of sequence exposure, respectively. The mean RT for Block 11 (SWITCH) minus the mean RT for sequence trials in Block 10 represented the RT disruption following the switch from sequence to random stimulus exposure. The more positive the trial type difference, the greater the magnitude of sequence learning.
Results
Behavioral Data
Implicit Sequence Learning Test
Mean RTs for correct responses were calculated for each block for every participant. RTs were derived separately for each trial type (sequence and random) within the S blocks. We examined the first 80 trials of each block because these trials occurred during the time interval of tracer uptake within the brain. In addition to the statistics described below, analysis of variance (ANOVA) tables that include effect sizes for the major comparisons are provided in Tables 1–4 in the supplemental materials.
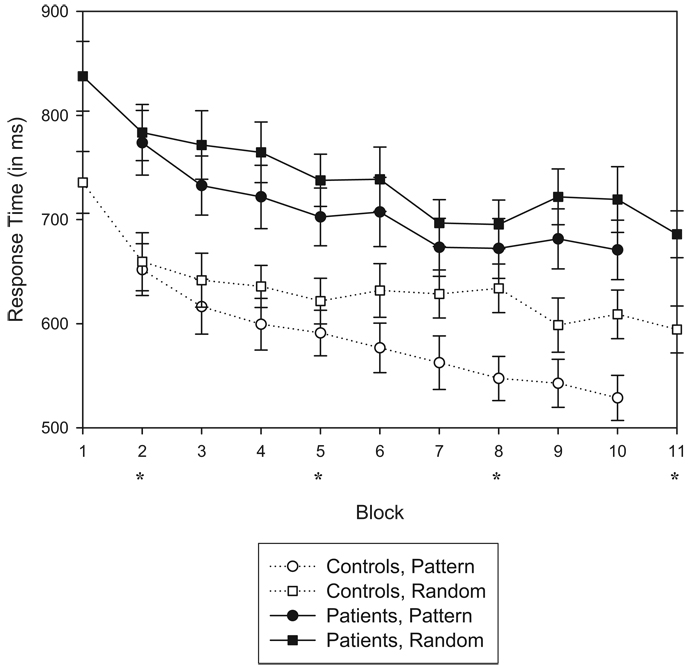
Mean RTs for accurate sequence and random trials in each block are plotted in Figure 2. A 2 (Group: controls vs. patients) × 2 (Trial Type: sequence vs. random) × 9 (Blocks: 2–10) ANOVA on RT scores for the S blocks yielded four main findings. First, there was a main effect of block, F(8, 400) = 21.92, p < .001, indicating that RT improved with practice. Second, there was a main effect of trial type, F(1, 50) = 64.183, p < .001, indicating that participants became sensitive to the sequence over time. Third, sensitivity to the sequence increased across blocks, shown by a significant interaction of Trial Type × Block, F(8, 400) = 3.01, p < .01. Finally, and importantly, increased sensitivity to the sequence differed between the two groups, resulting in a three-way interaction of Group × Trial Type × Block, F(8, 400) = 2.04, p < .05. Controls showed a greater increase in learning across blocks than did the patients. When patients with a prior history of substance dependence (n = 7) were removed from the overall analyses, the three-way interaction of Group × Trial Type × Block remained, F(8, 344) = 2.46, p < .05. Patients were significantly slower than controls, F(1, 50) = 11.169, p < .01. It should be noted, however, that the degree of improvement (e.g., general motor learning) was not different between groups (i.e., no Group × Block interaction), F(1, 50) = 0.156, p = .70. An ANOVA conducted on the patient group only yielded a main effect of trial type, F(1, 26) = 24.84, p < .001, confirming that patients developed sensitivity to the sequence. They also showed a main effect of block, F(8, 208) = 9.40, p < .001. Patient performance, however, did not yield an interaction of Trial Type × Block, F(8, 208) = 0.72, p = .67, indicating that the trial type difference remained constant throughout testing.
Figure 2.
Mean response times for correct responses as a function of group, block, and trial type for all 27 patients and 25 controls for the first 80 trials within each block. Error bars of 1 SE are plotted for each data point. Blocks 1 and 11 were comprised of 100% random trials. Blocks 2–10 consisted of sequence trials embedded with random trials (17%) and were performed under implicit conditions. Asterisks denote blocks that were performed in conjunction with positron emission tomography for 12 patients and 11 controls.
Mean accuracy scores for the first 80 trials of each block were calculated for sequence and random trial types within Blocks 2–10 for every participant. A 2 (Group) × 2 (Trial Type) × 9 (Block) ANOVA on accuracy means yielded a main effect of trial type, indicating that participants committed more errors on random trials relative to sequence trials, F(1, 50) = 12.19, p < .01. Although accuracy was very high for both groups (ranging from 92% to 98%), overall performance was significantly lower for patients, F(1, 50) = 5.26, p < .05. There were no additional main effects or interactions of group, trial type, or block for this measure.
Tests for Explicit Knowledge of the Sequence
Verbal report
When participants were asked whether they noticed any regularities in the way the stimuli were changing on the screen, none acknowledged rules of any kind that governed stimulus locations. Almost all participants, however, reported seeing back and forth repetitions (e.g., moving back and forth between Quadrants 2 and 3) or “triangles” (e.g., Quadrants 4–2–1–4–2–1–4–2–1, etc.). We wondered whether the identification of triangles stemmed from repeated instances in which random trials were consistent with the rule set (i.e., random trials that followed the rule by chance several times in a row). The chance of this occurring on consecutive random trials was very low (1/3 × 1/3 × 1/3 …), but triangles formed by these occurrences may have been particularly salient. Participants were asked to specify the quadrants that comprised the repeating triangles. Eight controls and 3 patients articulated a specific frequently occurring triangular sequence that was, in fact, consistent with their given rule set. When we eliminated these 11 participants from the RT data set, the relevant three-way interaction of sequence learning remained: Group × Trial Type × Block interaction, F(8, 213) = 2.188, p < .03.
Generation
For 12 trials, participants were shown two consecutive stimulus locations and asked to verbally indicate the quadrant in which the stimulus was most likely to appear next. Because participants never saw the stimulus appear in the same quadrant twice in a row, we assumed that participants would select one of the remaining three quadrants. Thus, chance performance was determined to be 33% accuracy, or 4 of 12 correct trials. The mean performance for controls was 4.64 (SD = 1.73), which did not significantly differ from chance, t(24) = 1.85, p = .08. The mean performance for patients was 6.19 (SD = 1.59), which was significantly higher than chance, t(26) = 7.12, p < .001, and was significantly higher than controls, t(50) = –3.35, p < .01. This finding raised the possibility that the patients relied more on explicit learning systems than did the controls. To investigate, we correlated the full patient and control groups’ Generation scores to implicit learning measures for Blocks 2–10. For the patients, positive correlations were observed for Blocks 2, 5, and 7 (r = .46, p < .02; r = .43, p < .03; r = .56, p < .01; respectively). However, Generation scores did not correlate to any blocks for the controls (all ps > .10).
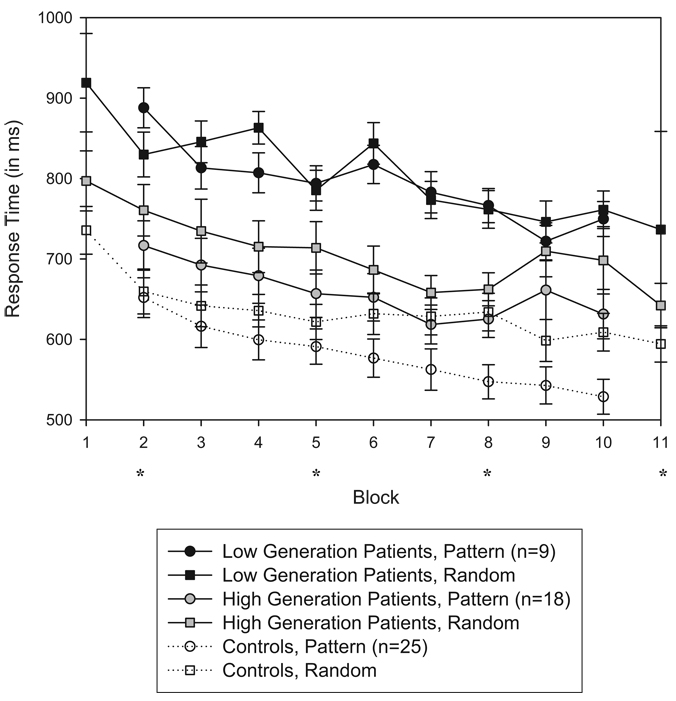
To further examine the relation between Generation scores and implicit sequence learning, we split the patients into high-generation (≥6; n = 18) and low-generation (<6; n = 9) subgroups. As can be seen in Figure 3, direct comparison of the two patient subgroups using a 2 (Subgroup) × 9 (Block) × 2 (Trial Type) repeated-measures ANOVA of the implicit sequence learning test yielded a main effect of subgroup, F(1, 25) = 5.038, p < .04; block, F(8, 200) = 9.574, p < .001; and trial type, F(1, 25) = 19.274, p < .001. This analysis also yielded an interaction of Subgroup × Block, F(8, 200) = 2.168, p < .04, and Subgroup × Trial Type, F(1, 25) = 9.682, p < .01. There were no Block × Trial Type, or Subgroup × Block × Trial Type interactions (all ps > .10). Thus, the high-generation patients exhibited overall faster RTs and a greater magnitude of implicit learning than did the low-generation patients. An interesting finding is that the high-generation patients slowed their RTs during the final blocks of testing, which appears to be the source of the Subgroup × Block interaction, rather than general subgroup differences in motor learning. A post hoc analysis of each subgroup revealed that the low-generation patient group did not reach a significant trial type learning effect, F(1, 8) = 0.425, p = .533, whereas the high-generation patient group did, F(1, 17) = 53.297, p < .001.
Figure 3.
Mean response times for correct responses as a function of performance on the Generation test (high-and low-generation patients vs. controls), block, and trial type for the first 80 trials within each block. Error bars represent 1 SE. Blocks 1 and 11 were comprised of 100% random trials. Blocks 2–10 consisted of sequence trials embedded with random trials (17%) and were performed under implicit conditions. Asterisks denote blocks that were performed in conjunction with positron emission tomography for 12 patients and 11 controls.
We also compared implicit sequence learning measures of each subgroup with that of controls (shown in Figure 3). A repeated-measures ANOVA between the low-generation patients versus controls yielded a main effect of group, F(1, 32) = 16.988, p < .001; block, F(8, 256) = 20.086, p < .001; and trial type, F(1, 32) = 14.387, p < .01. There were significant interactions of Group × Trial Type, F(1, 32) = 7.678, p < .01; Block × Trial Type, F(8, 256) = 3.481, p < .01; and Group × Block × Trial Type, F(8, 256) = 2.324, p < .03. Thus, controls showed a greater increase in learning (trial type differences) across blocks than did low-generation patients. The patients’ rate of motor learning was normal, however, as there was no Group × Block interaction, F(8, 256) = 1.777, p = .08. A comparison between the high-generation patients versus controls also yielded main effects of group, F(1, 41) = 4.745, p < .04; block, F(8, 328) = 19.935, p < .001; and trial type, F(1, 41) = 77.221, p < .001. There were interactions of Group × Block, F(8, 328) = 3.018, p < .01; and Block × Trial Type, F(8, 328) = 2.458, p < .02; as well as a Group × Block × Trial Type, F(8, 328) = 2.098, p < .04. The interesting finding of Group × Block appears to be driven by the unexpected RT slowing by this subgroup of patients toward the end of the test. However, there was no Group × Trial Type effect, F(1, 41) = 0.158, p = .69, indicating that trial type differences between these two groups were generally equivalent. Visual inspection of Figure 3 suggests that controls’ RT differences started small but grew large, whereas patients’ RT differences held constant across blocks. This interpretation was supported by a Block × Trial Type interaction in the control group, F(8, 192) = 5.578, p < .001, that was absent in the high-generation group, F(8, 136) = 0.458, p = .88. It is this group difference in the temporal pattern of sequence sensitivity that resulted in the three-way interaction.
To characterize the development of learning across blocks, we performed post hoc t tests within the control group and patient subgroups on the sequence and random trial types for each block. The time point of successful sequence learning was defined as the block in which a trial type difference was observed and maintained from that point forward for the duration of the test. Controls maintained a significant trial type difference in Blocks 6–10 (all ps < .001). High-generation patients maintained a significant trial type difference in Blocks 2–10 (all ps < .05) except Block 4, which was marginal, t(17) = 3.53, p = .07. Low-generation patients showed a trial type difference only during Block 2, t(8) = − 2.76, p < .03, which was never regained in other blocks. We also compared trial type differences between Block 11 and Block 10 (sequence trials only) for all three groups. This trial type comparison represented the standard SRT learning measure in which performance on a block of random trials is compared with performance on the preceding block of sequence trials. Using this measure of learning, the trial type difference was significant for controls, t(24) = 3.90, p < .01; but not for high-generation patients, t(17) = 0.59, p = .57; or low-generation patients, t(8) = −0.60, p = .56.
Taken together, these Generation analyses indicate that the high-generation patients were able to demonstrate sensitivity to the sequence, whereas the low-generation patients were not. Furthermore, the magnitude of learning based on trial type differences appears to be equivalent between the high-generation patients and controls. The characterization of learning differed between these two groups, however. The high-generation patients demonstrated a trial type difference as early as Block 2 that remained constant. Controls revealed trial type differences in Block 6 that continued to grow larger across blocks. This group difference in the time course and pattern of learning suggests that high-generation patients relied on an alternative sequence learning strategy that possibly involved explicit learning systems.
Recognition
Participants were shown two three-element stimulus locations side by side and asked to indicate which set of stimuli (the one on the left or the one on the right) may have appeared more often during the test. Chance was determined to be 50%, or 6 of 12 trials correct. The mean performance for controls was 6.16 (SD = 1.18) and for patients was 6.30 (SD = 1.64). The two groups’ means were similar, and neither mean significantly differed from chance (all ps > .10).
Sequence Learning and Clinical Variables
Clinical variables were compared with the learning measure in Block 10 (random RT minus sequence RT). There were no significant correlations between positive, negative, or disorganized symptom severity and implicit sequence learning on the basis of scores derived from the CASH. There were significant age differences between PET groups but not between groups overall, and age did not correlate to learning for patients or controls. The effects of medication on learning were examined in two ways. First, medications were converted to chlorpromazine (CPZ) equivalents (CPZ = estimate of patients’ daily dose of neuroleptic medications), which was then compared with the learning measure in Block 10. Second, duration of illness, which provided a rough estimate of a patient’s length of medication exposure, was compared with the learning measure. However, neither medication variable correlated to learning (all ps > .10).
We also compared the two subgroups of patients who performed differentially on the Generation test to see whether demographic variables could help explain their differences in implicit sequence learning performance. We found that the patient subgroups were equated on positive, negative, and disorganized symptom severity, age, CPZ, and duration of illness (all ps > .10). Of the 7 total patients with a history of drug or alcohol dependence, 2 were included in the low-generation group, and 5 were included in the high-generation group.
Imaging Data
Within-group differences were computed by comparing all conditions with BASELINE. A detailed listing of these activations is included in Tables 5 and 6 in the supplemental materials. Controls showed a consistent rCBF increase in cerebellar lobes V/VI on the right side during every condition that required a motor response, clearly indicating that this area was related to button pressing with the right hand. Right cerebellar lobes VIIIA/B were activated during EARLY, MIDDLE, and LATE (and subthreshold during SWITCH), suggesting that this area’s role was also motor-related. Posterior visual areas BA 17/18 were activated during SWITCH and FIXATION. A more anterior region of BA 17/18 showed greater activation during BASELINE relative to FIXATION, suggesting that this area was related to tracking spatial stimuli.
There were 4 low-generation patients and 8 high-generation patients who underwent PET imaging. When we excluded the 4 low-generation patients from analyses, results for the remaining 8 high-generation patients were similar to the overall group, but activations were often smaller, less intense, and in some cases below threshold. In other words, the lower N increased false negatives but not false positives (Andreasen et al., 1996). This indicated that the high-generation group was not driving the overall patient group findings for the subtraction analyses. Therefore, data for the low- and high-generation patients were collapsed in this comparison. Similar to controls, schizophrenia patients showed increased right-sided rCBF in cerebellar lobes V/VI on every condition that required a motor response. Right cerebellar lobes VIIIA/B were activated during EARLY and LATE only (and subthreshold during MIDDLE). Visual areas BA 17/18 were activated during LATE, SWITCH, and FIXATION. As with the controls, a more anterior region of BA 17/18 showed greater activation during BASELINE relative to FIXATION. There was an area of activation in the left parahippocampus that showed greater activation during BASELINE relative to conditions that required a motor response, suggesting that this area’s activation was related to passive viewing in the patients.
Correlational analyses also were used to examine the relation between normalized rCBF in a priori regions of interest and behavioral measures of implicit sequence learning. Here, we collapsed data across high- and low-generation subgroups because generation performance was factored into SRT learning (i.e., the high-generation group showed superior learning). As listed in Table 3, correlations in controls were primarily motor-related regions, such as the cerebellum VIIIA, premotor cortex, and substantia nigra, all of which were negatively correlated to learning. Positive correlations included cerebellar Crus I, an area that has been implicated in cognitive support (Chen & Desmond, 2005), and the putamen/globus pallidus, which has been demonstrated to increase activity with learning (Grafton et al., 1995; Rauch et al., 1997). An area of the left parahippocampus revealed a negative correlation with sequence learning during the LATE condition and a positive correlation during SWITCH. The DLPFC, however, showed no correlation with implicit sequence learning in controls.
Table 3.
Areas of Normalized Regional Cerebral Blood Flow Correlated to Implicit Sequence Learning (p < .005)
| Condition | Brain region or Brodmann’s area (BA) |
Talairach coordinates X, Y, Z |
Voxels | r |
|---|---|---|---|---|
| Controls | ||||
| EARLY: Block 2 | R precentral gyrus BA 4/6 | 55, 0, 25 | 64 | −.78 |
| R cerebellar Lobe VIIIA | 35, −40, −48 | 1,208 | −.93 | |
| L cerebellar Vermis VIIIA/B (pyramid) | −10, −62, −37 | 80 | .83 | |
| L cerebellar Crus I | −28, −68, −37 | 64 | .80 | |
| MIDDLE: Block 5 | ||||
| LATE: Block 8 | R precentral gyrus BA 6 | 54, −6, 24 | 80 | −.83 |
| L parahippocampus | −21, −16, −20 | 152 | −.82 | |
| Red nucleus/substantia nigra | 0, −20, −4 | 72 | −.80 | |
| SWITCH: Block 11 | L globus pallidus/putamen | −20, 2, 1 | 96 | .86 |
| L parahippocampus | −17, −8, −20 | 832 | .90 | |
| Cerebellar Lobe IX | 7, −48, −41 | 128 | −.83 | |
| Patients | ||||
| EARLY: Block 2 | R globus pallidus | 12, −6, −1 | 56 | −.82 |
| MIDDLE: Block 5 | R middle frontal gyrus BA 9/46 | 34, 38, 27 | 120 | .80 |
| L precentral gyrus BA 6 | −55, 0, 8 | 232 | .84 | |
| L cerebellar Crus II | −15, −82, −39 | 664 | −.90 | |
| LATE: Block 8 | L globus pallidus/putamen | −18, 2, 1 | 128 | −.84 |
| L precentral gyrus BA 6 | −57, −2, 12 | 72 | .80 | |
| L precentral gyrus BA 6 | −55, −6, 32 | 440 | .85 | |
| R cerebellar Lobes V/VI | 20, −58, −17 | 408 | .83 | |
| SWITCH: Block 11 | R middle frontal gyrus BA 9/46 | 36, 36, 24 | 320 | .89 |
| R precentral gyrus BA 6 | 57, 1, 16 | 392 | .89 | |
| L parahippocampus | −26, −35, −12 | 752 | −.91 | |
| L cerebellar Lobes V/VI | −27, −50, −22 | 184 | −.90 | |
| L cerebellar dentate | −15, −50, −39 | 1,264 | −.80 | |
| R cerebellar dentate | 15, −52, −39 | 136 | −.76 |
Note. The measure of sequence learning was the trial type difference within Blocks 2, 5, and 8. For Block 11, the learning measure was the response time (RT) for Block 11 minus the RT for sequence trials in Block 10. Learning measures were correlated to normalized positron emission tomography activity per block, respectively. Talaraich coordinates represent the point of highest correlation. R = right; L = left.
For patients, correlational analyses revealed positive correlations between learning and DLPFC activity during MIDDLE and SWITCH conditions. In each case, a positive DLPFC correlation was accompanied by a negative correlation in the contralateral cerebellum. Because the cerebellum is interconnected to the DLPFC via the thalamus (Dum & Strick, 2003; Kelly & Strick, 2003), the two brain areas may be influencing each other during implicit sequence learning. Correlations within the premotor cortex were revealed during MIDDLE, LATE, and SWITCH conditions. Unlike in the controls, these correlations were positive and consistent throughout testing, suggesting that patients used an alternative strategy that benefited from utilization of the premotor region. (See the supplemental materials for further discussion of motor-related activations.) Finally, activity in the putamen/globus pallidus negatively correlated to learning during EARLY and LATE conditions. Because increased activity would be expected to positively correlate with sequence learning, this inverse correlation may represent the patient’s inability to rely on the basal ganglia normally and instead adopt alternative strategies of learning.
We performed a second correlational analysis to examine whether Generation scores correlated with patterns of rCBF. In this comparison, Generation scores of the patient group were correlated to normalized rCBF during each of the sequence learning conditions. We had no a priori hypotheses about brain regions that might be important for the patients’ alternative sequence learning strategies, so we observed correlations in the whole brain with the criteria that correlations must be at least .75 and cluster size at least 50 voxels. We did not perform this exploratory analysis on the control group’s whole-brain PET data because their Generation scores were at chance levels and showed no relation to implicit sequence learning. There were several consistent positive correlations observed across conditions, which are listed in Table 4. Notably, activity in the right parahippocampus correlated to Generation performance across all conditions: EARLY, MIDDLE, LATE, and SWITCH (in the supplemental materials, see Figure 1 for PET images and Figure 2 for scatterplots). Lateral and ventral portions of the precentral gyrus (i.e., the premotor cortex) correlated to Generation during the MIDDLE, LATE, and SWITCH conditions. This finding was comparable with the previous correlations observed between the premotor cortex and sequence learning. Finally, sensorimotor (BA1/2) and visual areas (BA 19) were correlated to Generation scores during the EARLY and SWITCH conditions. This suggests that sensory and visual areas were recruited for novel stimulus processing early in testing and again with the introduction of random stimuli.
Table 4.
Patients: Areas of Normalized rCBF Correlated to Generation Scores (p < 005)
| Condition | Brain region or Brodmann’s area (BA) |
Talairach coordinates X, Y, Z |
Voxels | r |
|---|---|---|---|---|
| EARLY: Block 2 | L postcentral gyrus BA 1/2 | −49, −12, 24 | 112 | .81 |
| R parahippocampus | 19, −45, −4 | 328 | .91 | |
| L cerebellar Lobes 3/4 | −10, −44, −26 | 104 | −.81 | |
| Midline posterior cingulate BA 29/30 | 0, −45, 12 | 608 | .83 | |
| L fusiform gyrus BA 19 | −35, −70, −16 | 96 | .80 | |
| MIDDLE: Block 5 | R inferior frontal gyrus BA 47 | 33, 19, −4 | 72 | .84 |
| L precentral gyrus BA 4/6 | −52, 2, 8 | 144 | .82 | |
| L hippocampus/parahippocampus | −32, −27, −8 | 704 | .90 | |
| R parahippocampus | 19, −40, −1 | 1,136 | .91 | |
| LATE: Block 8 | L precentral gyrus BA 4/6 | −50, 8, 8 | 72 | .77 |
| R superior temporal gyrus BA 22 | 47, −8, 1 | 208 | .85 | |
| L middle temporal gyrus BA 21 | −53, −26, −4 | 96 | −.83 | |
| L parahippocampus | −25, −38, −4 | 120 | .81 | |
| L posterior cingulate BA 29 | −4, −44, 10 | 112 | .78 | |
| R parahippocampus | 13, −55, −4 | 1,232 | .95 | |
| R middle occipital gyrus BA 19 | 41, −75, 18 | 96 | −.91 | |
| SWITCH: Block 11 | R middle frontal gyrus BA 9/46 | 28, 38, 28 | 80 | −.81 |
| L precentral gyrus BA 4/6 | −48, 2, 8 | 136 | .81 | |
| R superior temporal gyrus BA 22 | 50, −16, 0 | 272 | .85 | |
| R parahippocampus | 30, −17, −20 | 80 | .81 | |
| L postcentral gyrus BA 1/2 | −51, −20, 24 | 288 | .87 | |
| R parahippocampus | 13, −46, −4 | 2,592 | .94 | |
| L fusiform gyrus BA 19 | −35, −60, −10 | 368 | .88 |
Note. Scores on the Generation test were correlated to normalized regional cerebral blood flow (rCBF) throughout the whole brain for each of the implicit sequence learning conditions. Talaraich coordinates represent the point of highest correlation. R = right; L = left.
Our correlational analyses revealed some interesting activation patterns that were difficult to interpret. For example, correlations were found in some, but not all, conditions, and correlations differed across conditions by group. We charted the blood flow dynamics throughout sequence learning of six regions of interest that were particularly relevant to this study. These analyses may be reviewed in Figure 3 in the supplemental materials. Notably, these analyses revealed that patients and controls did not differ in their DLPFC activity, but that patients showed higher dentate activity.
To determine whether clinical symptoms predicted functional imaging measures, we correlated CASH scores for positive, negative, and disorganized symptoms to normalized rCBF during the LATE condition. Correlational criteria included a Pierson r value of at least .75 and a cluster size of at least 50 voxels. Several brain regions showed a correlation between symptoms and blood flow in the LATE condition (see Table 7 in the supplemental materials). Upon closer examination, however, we found that these same regions also showed correlations between symptoms and blood flow in the FIXATION condition. This finding suggested that clinical symptoms did not predict aspects of learning; rather, blood flow reflected symptom state at the time of test.
Discussion
This study examined the neural correlates associated with implicit sequence learning impairments in schizophrenia. Both groups demonstrated learning, as evidenced by a divergence in RT performance between sequence and random trials. A subset of schizophrenia patients failed to show sequence learning even though general motor learning was intact. These patients were identified by their relatively poor performance on the postexperimental Generation test. In contrast, the high-generation patients exhibited an overall normal degree of sequence learning. Their pattern of performance, however, differed from that of controls, which suggested a reliance on adaptive cognitive strategies to process the sequential stimuli.
Our documentation of sequence sensitivity in schizophrenia patients is contrary to the findings of Kumari et al. (2002) and researchers who used nonprobabilistic SRT paradigms (Exner, Boucsein, et al., 2006; Green et al., 1997) in which no significant sequence learning was observed by the patients. Adjustments to the current task from that used by Kumari et al., such as equating the number of trials per block between groups and measuring trial type differences within blocks, may have led to differences in results between the two studies. Our findings agreed with two other studies that used probabilistic SRT paradigms in schizophrenia (Marvel et al., 2005; Schwartz et al., 2003). Measuring trial type differences throughout testing may be a more sensitive assessment of learning than the more traditional approach of comparing trial types at the end of the test. We did not observe a significant trial type difference between Block 11 (random trials) and Block 10 (sequence trials only) in our high-generation patient group, yet learning was represented by trial type differences within Blocks 2–10.
This study also reported the pattern of neural activity associated with SRT learning. On the basis of our subtraction analyses, patients showed generally normal rCBF activity changes in motor areas of the cerebellum (Lobes V/VI and VII/VIII) and in visual processing areas (BA 17/18). This suggests that the patients’ fundamental perceptual processing was intact. Instead, it is likely that adaptive learning strategies were driven by disruptions in higher order processes.
We focused our rCBF correlational analysis on key areas of implicit sequence learning that have been demonstrated in SRT-type tasks; specifically, the DLPFC, premotor cortex, basal ganglia, medial temporal lobe, and cerebellum. These brain regions have also been implicated in the pathophysiology of schizophrenia, which made this investigation particularly important for gaining insight into the underlying mechanisms of this population’s implicit sequence learning strategies. Our correlational analyses showed that SRT learning-related patterns of brain activity differed between the groups. Controls demonstrated that greater learning correlated to decreased activity in motor-related areas, such as cerebellar Lobe VIIIA and the premotor cortex. Areas that are currently thought to function on the interface between motor and cognition, such as the cerebellar Crus I and the putamen/globus pallidus, correlated positively with sequence learning. However, a correlation with learning was not observed in the DLPFC.
Patients demonstrated that the right DLPFC correlated to learning the MIDDLE condition and again when random stimuli were presented during the SWITCH condition. When the direction of the correlation with implicit sequence learning in patients was positive for the DLPFC, it was negative for the cerebellum. These two brain areas have known interconnections via the thalamus (Dum & Strick, 2003; Kelly & Strick, 2003; Middleton & Strick, 2001), providing a means for the cerebellum to influence (or perhaps facilitate) prefrontal lobe functions. A correlation in opposite directions in our study may represent a functional disruption in which these brain areas could not work together in a normal way to complete the task. However, it is unclear whether the patients’ learning deficits were directly related to a breakdown in this pathway or whether attempts were made to use this (faulty) pathway secondarily after upstream processes failed. (For further discussion of the role of the DLPFC and cerebellum in SRT learning in this study, see the supplemental materials.)
When we examined the relation between performance on the Generation test and brain activation in patients, we found that the posterior parahippocampus, especially on the right side, was positively correlated to the patients’ Generation scores. The right parahippocampus has been associated with spatial processing (Burgess, Maguire, & O’Keefe, 2002), particularly with the encoding of spatial scenes (Epstein, Harris, Stanley, & Kanwisher, 1999). It is possible that patients relied on spatial encoding strategies to process the moving stimuli. Persistent activation of the parahippocampus by patients who performed well on the Generation test may represent an ongoing effort to process spatial relations among stimuli at face value (e.g., Quadrant 2 follows Quadrant 4). This strategy may reflect a stimulus-driven approach that should have segued into sensitivity to abstract relations (e.g., horizontal often follows diagonal). Patients who performed well on the Generation test also showed the most learning on the implicit sequence learning test. However, an inability to become sensitive to the abstract quality of the spatial relations may explain why these high-generation patients failed to show a continued increase in trial type differences across blocks, as controls did. Using a similar probabilistic sequence learning paradigm, Marvel et al. (2005) also found that trial type differences in the schizophrenia group leveled off early in testing and did not change across sessions. Taken together, these studies indicate that patients may have a limit, not on how quickly they learn sequential regularities but rather on the level of sensitivity they develop with extended exposure (i.e., their asymptote). The patients’ overreliance on external stimuli may have facilitated their ability to recall short spans of sequences but hampered their ability to acquire sensitivity to the overarching abstract spatial pattern.
Positive correlations between the patients’ Generation performance and rCBF also were observed in the left premotor cortex for MIDDLE, LATE, AND SWITCH conditions. In particular, we observed this correlation in a ventral portion of the premotor cortex that has been linked to so-called mirror neurons (Rizzolatti & Arbib, 1998). Such neurons respond to specific actions as well as to the observation of those same actions by others. For example, single-cell recordings in nonhuman primates have demonstrated that neurons in the ventral premotor cortex respond to grasping an object and to observing that object grasped by an experimenter but are unresponsive when observing the same object grasped with a pair of pliers (Rizzolatti & Arbib, 1998). Moreover, the premotor cortex has been associated with externally guided movements, such as those cued by spatial events (Hazeltine et al., 1997; Hazeltine & Ivry, 2002; Mushiake et al., 1991). In our study, activation of the ventral premotor cortex in association with higher Generation scores suggests that patients relied on visual cues to guide their responses throughout the test and further supports our interpretation of the patients’ stimulus-driven approach to SRT learning.
An overreliance on external cues to guide key pressing may explain why patients performed particularly well on the Generation test, yet were unable to spontaneously recall the nature of the sequence upon questioning or to recognize parts of the sequence in the Recognition test. Of the three postexperimental probes for explicit knowledge, the Generation test was the one that most closely resembled the way patients processed the sequential information. Unlike the other two probes, the Generation test prompted a (motor) response based on visually guided cues. Participants were permitted to practice key pressing on the response box to help them generate the third item of the sequence. We did not collect data on whether patients or controls actually used finger movements before deciding on a response, but we can infer from these data that such a strategy would have aided the patients’ performance. Marvel et al. (2005) noted a similar phenomenon in a similar probabilistic paradigm of implicit sequence learning in which the patient group appeared to have greater explicit knowledge of the learned sequence relative to controls. Because this trend has held up across two studies, it suggests that stimulus-driven strategies are commonly used by schizophrenia patients to acquire information about the occurrence of consecutive events.
One point of note is that the visual display and response buttons used in the task were not related to each other via a natural spatial mapping. That is, the visual display of quadrants was numbered in a vertical fashion, whereas the response buttons were positioned left to right in a horizontal fashion. Learning the spatial mapping between visual display and response buttons may have been part of the learning process in this task. A study by Grafton, Salidis, and Willingham (2001) tested several experimental groups who learned sequences within the context of incompatible and compatible visuomotor maps. Grafton et al. determined that when learning incompatible visuomotor maps, reliance on the visual presentations of the target (i.e., perceptual representation) was more beneficial than reliance on the motor sequence of the key presses. Grafton et al. also examined the neural correlates of incompatible visuomotor mapping. They found learning related changes in dorsal areas of the frontal lobe, especially in the precentral gyrus (BA 4/6) bilaterally. These regions were substantially more anterior and superior to the activations we saw in our analyses. The regions reported by Grafton et al. were within our FOV, and we should have been able to observe activations occurring there. Therefore, it is unlikely that our participants engaged these regions in the same way that participants did in Grafton et al.’s study. Nevertheless, we cannot overlook the potential influence that incompatible visuomotor mapping may have had on implicit sequence learning or the degree to which each study group’s learning was affected by it.
Another aspect of learning in this task was the ability to develop sensitivity to a sequence that was embedded in a steady stream of random noise. The process of filtering relevant information from random noise relies heavily on the DA system. DA regulates the excitability of neurons and refines the signal-to-noise ratio (Durstewitz, Seamans, & Sejnowski, 2000). High signal-to-noise ratio leads to the development of context. As such, DA may provide a means for participants to learn a context (i.e., the sequence) and respond appropriately (Braver et al., 1999). DA receptors in the basal ganglia are the primary site of action for most antipsychotic medications, and the degree of antipsychotic efficacy is correlated with the degree of DA blockade (Patterson & Kotrla, 2002). If DA systems were disrupted in the basal ganglia and its projections to the prefrontal cortex in the patients of our study, then the ability to learn the sequence could have been compromised. Subsequently, the ability to develop and maintain contextual information also could have been compromised (Braver et al., 1999; Winterer & Weinberger, 2004). To compensate, patients may have relied on salient stimulus features to guide them through testing. It is not possible to distinguish whether DA-related disturbances were driven by schizophrenia proper or by the medication used to treat it given the current study design. It should be noted, however, that no relation was found between medications or duration of illness and test performance. Future studies that include neuroleptic-naïve patients would help tease apart the basis of cognitive difficulties related to dopaminergic disruption in schizophrenia.
The finding that schizophrenia patients have implicit sequence learning difficulties offers insight into the deficits that often accompany this disorder. In everyday experience, complex structural relations are presented within the context of random and meaningless information. If these relationships are not learned, the ability to draw on reliable information to prepare appropriate responses is impaired. Thus, if patients fail to learn structural relations between events (i.e., context), or to overemphasize the importance of rare co-occurrences, then abnormal relationships between events may form, leading to an inability to respond appropriately (Manschreck et al., 2000). Consequently, self-initiated behavior becomes unreliable, and the patients’ actions, instead, tend to be carried out directly in response to environmental stimuli (Frith, 1992). This specific type of learning failure may contribute to the cognitive and social dysfunction often observed in schizophrenia (American Psychiatric Association, 2000).
In summary, this research extends what is known about the cognitive and neural strategies used during implicit sequence learning in schizophrenia. The patients’ learning strategies were associated with abnormal activation in several brain regions, including the DLPFC, premotor cortex, basal ganglia, medial temporal lobe, and cerebellum. Disturbances in any one of these areas may have contributed to an unusual pattern of sequence learning. Our results suggest that, without the full benefit of implicit processing to develop context within their environment, individuals with schizophrenia may be forced to adopt a more stimulus-driven strategy. This could lead to a number of cognitive and behavioral difficulties that have been associated with this clinical disorder.
Supplementary Material
Acknowledgments
This research was funded by a Nellie Ball Research Trust, T32 MH19113, T32 CA82106, and R01 MH60990. We are grateful to David Elleson, Frank Fleming, Suzanne Monkman, Melanie Sievers-Rient, and Alane Tranel for assisting with patient recruitment, symptom ratings, and PET administration, as well as Gene Zeien for processing the PET and magnetic resonance imaging data. Grant Golds-berry and Travis Jewitt assisted with pilot data collection. Deborah Ellis assisted with article preparation.
Footnotes
Portions of these data were presented at the 10th International Congress on Schizophrenia Research, Savannah, Georgia, April 2005, and the 11th Annual Meeting of the Organization for Human Brain Mapping, Toronto, Ontario, Canada, June 2005.
Supplemental materials: http://dx.doi.org/10.1037/0894-4105.21.6.761.supp
Contributor Information
Cherie L. Marvel, Department of Psychiatry, Roy J. and Lucille A. Carver College of Medicine, University of Iowa
Beth M. Turner, Department of Psychiatry, Roy J. and Lucille A. Carver College of Medicine, University of Iowa
Daniel S. O’Leary, Department of Psychiatry, Roy J. and Lucille A. Carver College of Medicine, University of Iowa
Hans J. Johnson, Department of Psychiatry, Roy J. and Lucille A. Carver College of Medicine, University of Iowa
Ronald K. Pierson, Department of Psychiatry, Roy J. and Lucille A. Carver College of Medicine, University of Iowa
Laura L. Boles Ponto, Department of Radiology/Positron Emission Tomography (PET) Center, Roy J. and Lucille A. Carver College of Medicine, University of Iowa
Nancy C. Andreasen, Department of Psychiatry, Roy J. and Lucille A. Carver College of Medicine, University of Iowa
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC: Author; 2000. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1984a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984b. [Google Scholar]
- Andreasen NC. Comprehensive Assessment of Symptoms and History (CASH) Iowa City: University of Iowa; 1985. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Cizadlo T, O’Leary DS, Watkins GL, Ponto LL, et al. Sample size and statistical power in [15O]H2O studies of human cognition. Journal of Cerebral Blood Flow and Metabolism. 1996;16:804–816. doi: 10.1097/00004647-199609000-00005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, et al. Short-term and long-term verbal memory: A positron emission tomography study. Proceedings of the National Academy of Sciences, USA. 1995;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, et al. Hypofrontality in schizophrenia: Distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997 June 14;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical–subcortical–cerebellar circuitry? Schizophrenia Bulletin. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Berman KF, Torrey EF, Daniel DG, Weinberger DR. Regional cerebral blood flow in monozygotic twins discordant and concordant for schizophrenia. Archives of General Psychiatry. 1992;49:927–934. doi: 10.1001/archpsyc.1992.01820120015004. [DOI] [PubMed] [Google Scholar]
- Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness. Science. 1997 May 23;276:1272–1275. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: A computational model of dopamine and prefrontal function. Biological Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia: A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Archives of General Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Calarge C, Andreasen NC, O’Leary DS. Visualizing how one brain understands another: A PET study of theory of mind. American Journal of Psychiatry. 2003;160:1954–1964. doi: 10.1176/appi.ajp.160.11.1954. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. American Journal of Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Temporal dynamics of cerebro–cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: Typical versus atypical neuroleptics. American Journal of Psychiatry. 1999;156:1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- Curran T. Implicit sequence learning from a cognitive neuroscience perspective: What, how, and where? In: Stadler MA, Frensch PA, editors. Handbook of implicit learning. Thousand Oaks, CA: Sage; 1998. pp. 365–400. [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. European Journal of Neuroscience. 1996;8:637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. Journal of Neurophysiology. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nature Neuroscience Supplement. 2000;3:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: Recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Exner C, Boucsein K, Degner D, Irle E. State-dependent implicit learning deficit in schizophrenia: Evidence from 20-month follow-up. Psychiatry Research. 2006;142:39–52. doi: 10.1016/j.psychres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Exner C, Weniger G, Schmidt-Samoa C, Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophrenia Research. 2006;84:386–396. doi: 10.1016/j.schres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Feeney JJ, Howard JH, Jr, Howard DV. Implicit learning of higher order sequences in middle age. Psychology and Aging. 2002;17:351–355. [PubMed] [Google Scholar]
- Frensch PA, Miner CS. Effects of presentation rate and individual differences in short-term memory capacity on an indirect measure of serial learning. Memory & Cognition. 1994;22:95–110. doi: 10.3758/bf03202765. [DOI] [PubMed] [Google Scholar]
- Frith CD. The cognitive neuropsychology of schizophrenia. Hove, United Kingdom: Erlbaum; 1992. [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Archives of General Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Gomez-Beldarrain M, Garcia-Monco JC, Rubio B, Pascual-Leone A. Effect of focal cerebellar lesions on procedural learning in the serial reaction time task. Experimental Brain Research. 1998;120:25–30. doi: 10.1007/s002210050374. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. Journal of Cognitive Neuroscience. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Salidis J, Willingham DB. Motor learning of compatible and incompatible visuomotor maps. Journal of Cognitive Neuroscience. 2001;13:217–231. doi: 10.1162/089892901564270. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: Learning new tricks and loving it. Current Opinion in Neurobiology. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Green M, Kern R, Williams O, McGurk S, Kee K. Procedural learning in schizophrenia: Evidence from serial reaction time. Cognitive Neuropsychiatry. 1997;2:123–134. doi: 10.1080/135468097396360. [DOI] [PubMed] [Google Scholar]
- Hallett M, Grafman J. Executive function and motor skill learning. In: Schmahmann JD, editor. The cerebellum and cognition. San Diego, CA: Academic Press; 1997. pp. 297–319. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding: A PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Ivry RB. Neural structures that support implicit sequence learning. In: Jimenez L, editor. Attention and implicit learning. Amsterdam: John Benjamins; 2002. pp. 71–107. [Google Scholar]
- Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: Clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biological Psychiatry. 2004;55:1146–1153. doi: 10.1016/j.biopsych.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Waldram K, Kesner RP. Sequences assessed by declarative and procedural tests of memory in amnesic patients with hippocampal damage. Neuropsychologia. 2004;42:1877–1886. doi: 10.1016/j.neuropsychologia.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Jr, Japikse KC, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: Effects of level of structure, adult age, and extended practice. Psychology and Aging. 2004;19:79–92. doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV. Age differences in implicit learning of higher order dependencies in serial patterns. Psychology and Aging. 1997;12:634–656. doi: 10.1037//0882-7974.12.4.634. [DOI] [PubMed] [Google Scholar]
- Japikse KC, Negash S, Howard JH, Jr, Howard DV. Intermanual transfer of procedural learning after extended practice of probabilistic sequences. Experimental Brain Research. 2003;148:38–49. doi: 10.1007/s00221-002-1264-9. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning: I. Frontal cortex and attention to action. Journal of Neurophysiology. 1997;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, McGrath J, Saunders JB, Dore G, Clark D. Substance misuse in patients with schizophrenia: Epidemiology and management. Drugs. 2002;62:743–755. doi: 10.2165/00003495-200262050-00003. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. Journal of Neuroscience. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D, Nissen MJ. Procedural learning is impaired in Huntington’s disease: Evidence from the serial reaction time task. Neuropsychologia. 1991;29:245–254. doi: 10.1016/0028-3932(91)90085-m. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SC, et al. Procedural learning in schizophrenia: A functional magnetic resonance imaging investigation. Schizophrenia Research. 2002;57:97–107. doi: 10.1016/s0920-9964(01)00270-5. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Intuition: A social cognitive neuroscience approach. Psychological Bulletin. 2000;126:109–137. doi: 10.1037/0033-2909.126.1.109. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biological Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Candela SF, Redmond D, Yurgelun-Todd D, Tsuang M. Impaired verbal memory is associated with impaired motor performance in schizophrenia: Relationship to brain structure. Schizophrenia Research. 2000;43:21–32. doi: 10.1016/s0920-9964(99)00179-6. [DOI] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Rucklos ME, Vereen DR. Disturbed voluntary motor activity in schizophrenic disorder. Psychological Medicine. 1982;12:73–84. doi: 10.1017/s0033291700043300. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Schwartz BL, Howard DV, Howard JH., Jr Implicit learning of non-spatial sequences in schizophrenia. Journal of the International Neuropsychological Society. 2005;11:659–667. doi: 10.1017/S1355617705050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Schwartz BL, Rosse RB. A quantitative measure of postural sway deficits in schizophrenia. Schizophrenia Research. 2004;68:363–372. doi: 10.1016/j.schres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, et al. Auditory p300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Archives of General Psychiatry. 1993;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Glover GH, Pfefferbaum A. Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. American Journal of Psychiatry. 2001;158:646–649. doi: 10.1176/appi.ajp.158.4.646. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. Journal of Neurophysiology. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. Abnormal Involuntary Movement Scale. Rockville, MD: U.S. Department of Health, Education, and Welfare; 1976. [Google Scholar]
- Negash S, Howard DV, Japikse KC, Howard JH., Jr Age-related differences in implicit learning of non-spatial sequential patterns. Aging, Neuropsychology and Cognition. 2003;10:108–121. [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Nissen MJ, Willingham D, Hartman M. Explicit and implicit remembering: When is learning preserved in amnesia? Neuropsychologia. 1989;27:341–352. doi: 10.1016/0028-3932(89)90023-7. [DOI] [PubMed] [Google Scholar]
- Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC. An MRI study of cerebellar vermis morphology in patients with schizophrenia: Evidence in support of the cognitive dysmetria concept. Biological Psychiatry. 1999;46:703–711. doi: 10.1016/s0006-3223(99)00093-1. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Sedvall G, Nordstrom M, Andreasen N, Pierson R, Magnotta V, et al. Selective reduction of the posterior superior vermis in men with chronic schizophrenia. Schizophrenia Research. 2002;55:61–67. doi: 10.1016/s0920-9964(01)00248-1. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barnes TR, Nelson HE, Tanner S, Weatherley L, Owen AM, et al. Frontal–striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997;120:1823–1843. doi: 10.1093/brain/120.10.1823. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, et al. Procedural learning in Parkinson’s disease and cerebellar degeneration. Annals of Neurology. 1993;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994 March 4;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- Patterson JC, Jr, Kotrla KJ. Functional imaging in psychiatry. In: Yudofsky SC, Hales RE, editors. The American Psychiatric Publishing textbook of neuropsychiatry and clinical neurosciences. 4th ed. Washington, DC: American Psychiatric Publishing; 2002. pp. 285–322. [Google Scholar]
- Psychology Software Tools. MEL professional (Version 2.01) [Computer software] Pittsburgh, PA: Author; 1995. [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of Areas 9 and 46 and relationship to the Talairach coordinate system. Cerebral Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD, et al. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Human Brain Mapping. 1997;5:124–132. [PubMed] [Google Scholar]
- Reber AS. Implicit learning and tacit knowledge. Journal of Experimental Psychology: General. 1989;118:219–235. [Google Scholar]
- Reber PJ, Squire LR. Parallel brain systems for learning with and without awareness. Learning & Memory. 1994;1:217–229. [PubMed] [Google Scholar]
- Reiss JP, Campbell DW, Leslie WD, Paulus MP, Ryner LN, Polimeni JO, et al. Deficit in schizophrenia to recruit the striatum in implicit learning: A functional magnetic resonance imaging investigation. Schizophrenia Research. 2006;87:127–137. doi: 10.1016/j.schres.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends in Neurosciences. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Tormos JM, Maeda F, Pascual-Leone A. The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information. Cerebral Cortex. 2001;11:628–635. doi: 10.1093/cercor/11.7.628. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Schultz SK, O’Leary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to mid-life adults. NeuroReport. 1999;10:2493–2496. doi: 10.1097/00001756-199908200-00011. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Howard DV, Howard JH, Jr, Hovaguimian A, Deutsch SI. Implicit learning of visuospatial sequences in schizophrenia. Neuropsychology. 2003;17:517–533. doi: 10.1037/0894-4105.17.3.517. [DOI] [PubMed] [Google Scholar]
- Seger CA. Implicit learning. Psychological Bulletin. 1994;115:163–196. doi: 10.1037/0033-2909.115.2.163. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA. The role of dopamine in cognitive sequence learning: Evidence from Parkinson’s disease. Behavioural Brain Research. 2005;156:191–199. doi: 10.1016/j.bbr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson’s disease? A meta-analysis. Neuropsychology. 2006;20:490–495. doi: 10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Shear PK, Cahn-Weiner DA, Stein M, Zipursky RB, et al. Motor sequencing deficits in schizophrenia: A comparison with Parkinson’s disease. Neuropsychology. 2001;15:342–350. doi: 10.1037//0894-4105.15.3.342. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. In: Co-planar stereotaxic atlas of the human brain 3-D proportional system, an approach to cerebral imaging. Rayport M, translator. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Interference of left and right cerebellar rTMS with procedural learning. Journal of Cognitive Neuroscience. 2004;16:1605–1611. doi: 10.1162/0898929042568488. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Archives of General Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scandinavian Journal of Psychology. 2001;42:239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—III (WAIS–III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in Neurosciences. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziota JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Heinrichs RW. Schizophrenia and the frontal brain: A quantitative review. Journal of the International Neuropsychological Society. 1999;5:556–566. doi: 10.1017/s1355617799566101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.