Abstract
Rationale
The levels of a small heat shock protein 20 (Hsp20) and its phosphorylation are increased upon ischemic insults, and overexpression of Hsp20 protects the heart against ischemia/reperfusion injury. However, the mechanism underlying cardioprotection of Hsp20 and especially the role of its phosphorylation in regulating ischemia/reperfusion-induced autophagy, apoptosis and necrosis remain to be clarified.
Objective
Herein we generated a cardiac-specific overexpression model, carrying non-phosphorylatable Hsp20, where serine 16 was substituted with alanine (Hsp20S16A). By subjecting this model to ischemia/reperfusion, we addressed whether: 1) the cardioprotective effects of Hsp20 are associated with serine 16 phosphorylation; 2) blockade of Hsp20 phosphorylation influences the balance between autophagy and cell death; and 3) the aggregation pattern of Hsp20 is altered by its phosphorylation.
Methods and Results
Our results demonstrated that Hsp20S16A hearts were more sensitive to ischemia/reperfusion injury, evidenced by lower recovery of contractile function and increased necrosis and apoptosis, compared with non-transgenic (TG) hearts. Interestingly, autophagy was activated in non-TG hearts, but significantly inhibited in Hsp20S16A hearts following ischemia/reperfusion. Accordingly, pre-treatment of Hsp20S16A hearts with rapamycin, an activator of autophagy, resulted in improvement of functional recovery, compared with saline-treated Hsp20S16A hearts. Furthermore, upon ischemia/reperfusion, the oligomerization pattern of Hsp20 appeared to shift to higher aggregates in Hsp20S16A hearts.
Conclusion
Collectively, these data indicate that blockade of Ser16-Hsp20 phosphorylation attenuates the cardioprotective effects of Hsp20 against ischemia/reperfusion injury, which may be due to suppressed autophagy and increased cell death. Therefore, phosphorylation of Hsp20 at serine 16 may represent a potential therapeutic target in ischemic heart disease.
Keywords: Apoptosis, Myocardial infarction, Autophagy, Heat-shock protein
Introduction
Coronary reperfusion is the primary therapeutic strategy in ischemic heart disease, nevertheless, it may at first exacerbate cellular damage sustained during the ischemic period (ischemia/reperfusion injury).1 As a consequence of such injury, adaptive stress-responses occur immediately, some of which are involved with upregulation of heat shock proteins (Hsps).2 In fact, Hsps synthesis arises transiently under a wide spectrum of stressful stimuli as a protective mechanism.3 Within the superfamily of Hsps, the small Hsps with molecular weights ranging from 12 ~ 43 kD have received particular attention.4–6 Recently, several members of small Hsps have been identified as protective mediators during myocardial ischemia, including αB-crystallin, Hsp27 and Hsp20.4–6
Hsp20, sharing a conserved domain with αB-crystallin and Hsp27,7 is the only member within the sHsps family that contains a consensus peptide motif (RRAS) for protein kinase A (PKA)/protein kinase G (PKG)-dependent phosphorylation at Ser16.8 We and others have demonstrated that the levels of cardiac Hsp20 and its phosphorylation were significantly increased, compared with Hsp27 and αB-crystallin, in animal hearts upon ischemic conditions, exercise training, rapid right ventricular pacing, and pharmacological treatment by doxorubicin and chronic β-adrenergic stimulation.6, 9–11 More recently, we have identified a P20L substitution in human Hsp20, which was associated with diminished phosphorylation at Ser16 and complete abrogation of the protective effects of Hsp20, suggesting an instrumental role of phosphorylated Hsp20 in cardioprotection.12 Indeed, the constitutively phosphorylated mutant of Hsp20 ( Hsp20S16D ) conferred protection against β-agonist-induced apoptosis in cultured myocytes;13 conversely, the constitutively dephosphorylated mutant, namely Hsp20S16A, displayed no anti-apoptotic properties,13 implying a mechanistic link between phosphorylated Hsp20 and its protection.
Notably, studies have shown that ischemia/reperfusion-induced cardiomyocyte necrosis14 and apoptosis15 contribute to ventricular dysfunction and end-stage failure. However, there is an increasing awareness that necrosis and apoptosis are not the only mechanisms for cell death. Macroautophagy (commonly referred to as autophagy), which involves the bulk degradation of cytoplasmic contents, may provide alternative mechanisms that determine cell fate in ischemia/reperfusion.16,17 In the present study, we sought to address the effects of blocking Hsp20 phosphorylation at Ser16 on ischemia/reperfusion-induced cell injury. Our findings herein demonstrate a detrimental role of non-phosphorylated Hsp20S16A in ischemia/reperfusion injury through suppression of autophagy and increased cell death, which further implicate phosphorylation of Hsp20 as a potential therapeutic strategy for ischemic heart disease.
Methods and Materials
Acquisition of Failing and Donor Human Heart Samples
Human tissues from the anterior wall of 4 non-failing (donor) and 5 failing left ventricles were obtained from Dr. Roger Hajjar’s Laboratory (Mount Sinai School of Medicine). The failing hearts were obtained at the time of cardiac transplantation. Information about each patient was collected and linked to the subject number (but not the patient name) and documented in a spreadsheet. Once in the laboratory, the tissue was cut into pieces, frozen in liquid nitrogen, and stored at −80°C. All investigation conforms with the principles outlined in the Declaration of Helsinki.18
Generation of Transgenic Mice
We generated TG mice in the FVB/N background that carry the mouse cardiac Hsp20S16A cDNA under the control of the α-MHC mouse promoter, as described.6 A 0.5kb mutant mouse Hsp20S16A cDNA was ligated with the murine cardiac α-myosin heavy chain gene promoter (5.5-kb). In all studies, male Hsp20S16A transgenic and nontransgenic control littermate mice, between 12 to 16 weeks of age, were used. The care of all animals used in the present study was in accordance with the University of Cincinnati animal care guidelines.
Results
Increased Level of Phosphorylated Ser16-Hsp20 in Ischemia/Reperfused and Failing Hearts
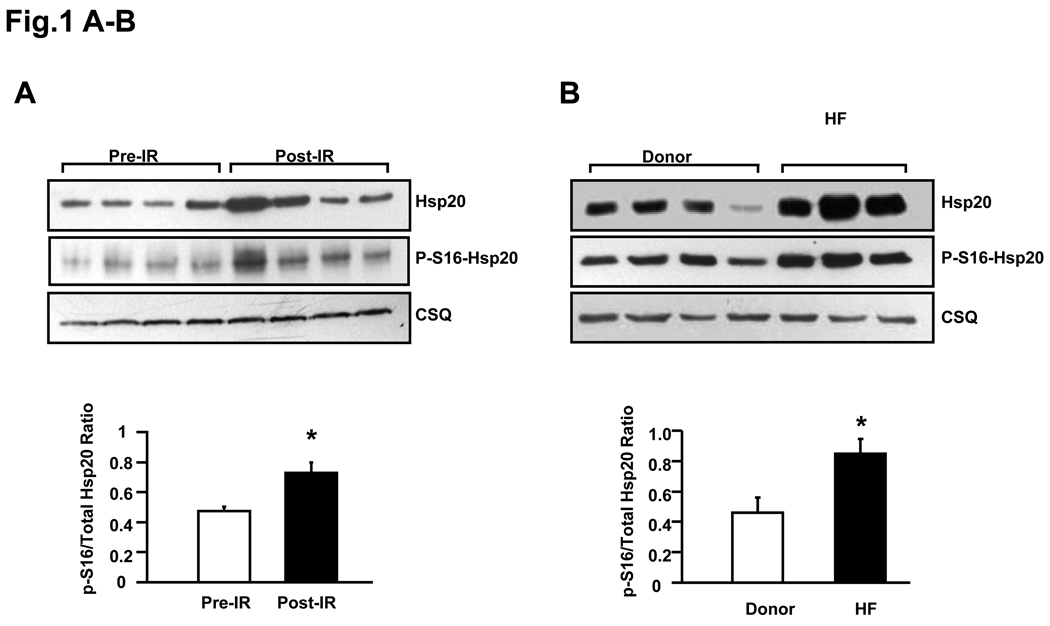
It has been shown that the levels of sHsps and their phsophorylation were increased in response to stress signals.3 Thus, in initial studies, we assessed the levels of total Hsp20 and phospho-Ser16-Hsp20, by quantitation of the ratio of phosphorylated Ser16 versus total Hsp20 in wild type mouse hearts (n=6) after ex vivo ischemia (45min) and reperfusion (2h), compared with that in pre-ischemic wild type hearts (n=6). As expected, expression of total Hsp20 was upregulated by ischemic/reperfusion insults (Figure 1A). The specific anti-phospho-Ser16-Hsp20 antibody was used and the ratio of phospho-Ser16/total Hsp20 was increased by 40%, compared with its pre-ischemic level (Figure 1A). Correspondingly, an increase in total Hsp20 and the ratio of phospho-Ser16/total Hsp20 was observed in failing human hearts , compared with donors (Figure 1B), albeit variations that existed in protein levels from individual hearts, similar to previous studies.19–21 These results indicate that Hsp20 and its phosphorylation at Ser16 may function as an innate protector during cardiac ischemia/reperfusion and heart failure.
Figure 1.
Phosphorylation of Hsp20 in ex vivo ischemia/reperfusion injured wild-type mouse hearts and failing human hearts. The ratio of phospho-Ser16-Hsp20/total Hsp20 was increased in post-ischemia/reperfusion myocardium (Figure 1A, n=6, *: P<0.01, Post vs. Pre) and in failing human hearts (Figure 1B, n=5 for HF, n=4 for Donor, *: P<0.01, HF vs. Donor,). CSQ: calsequestrin (loading control). HF: heart failure.
Transgenic Mice with Cardiac-Overexpression of Hsp20S16A
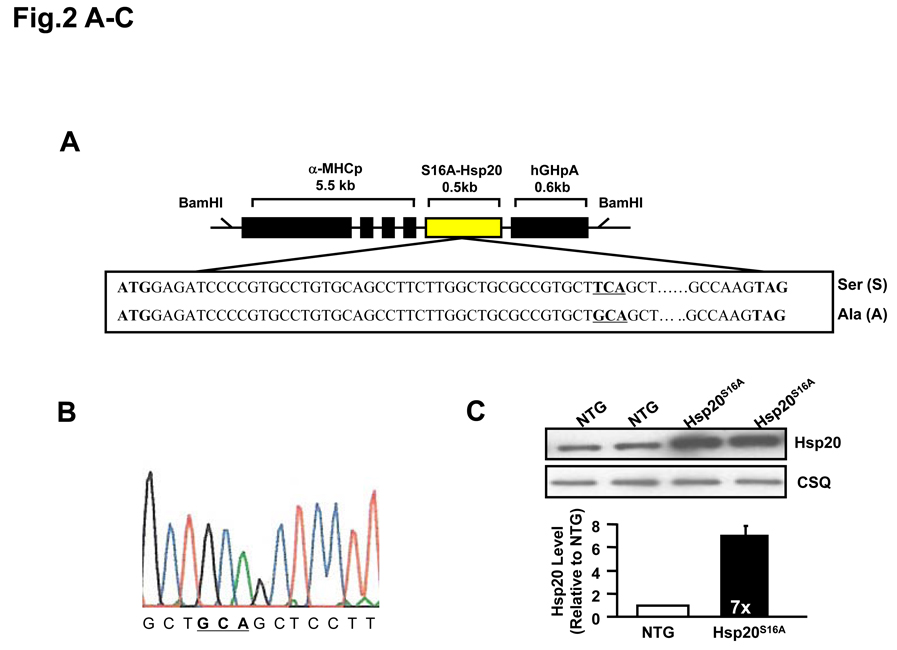
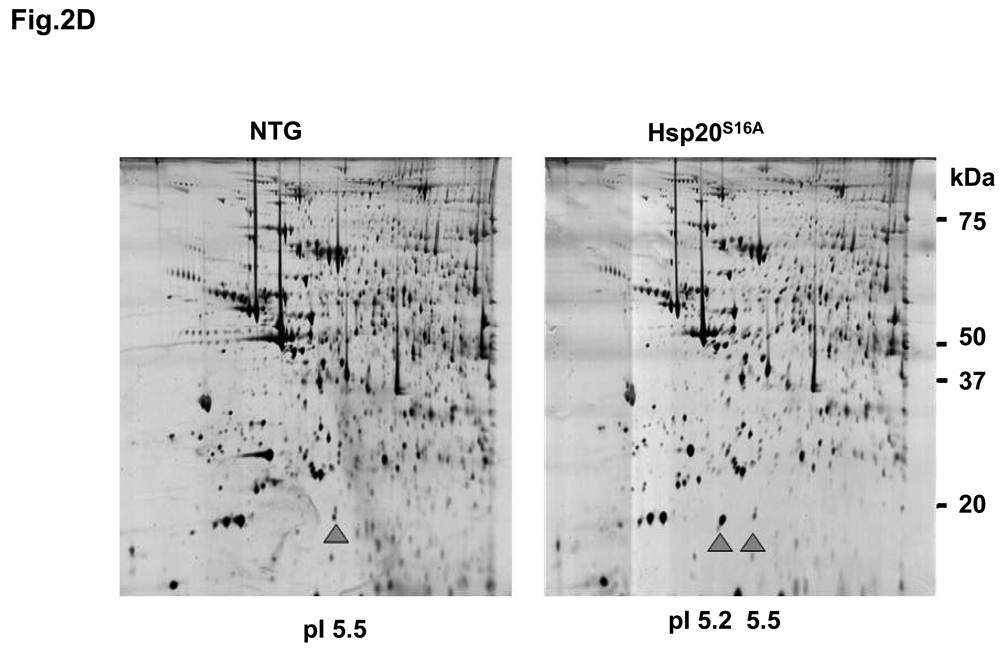
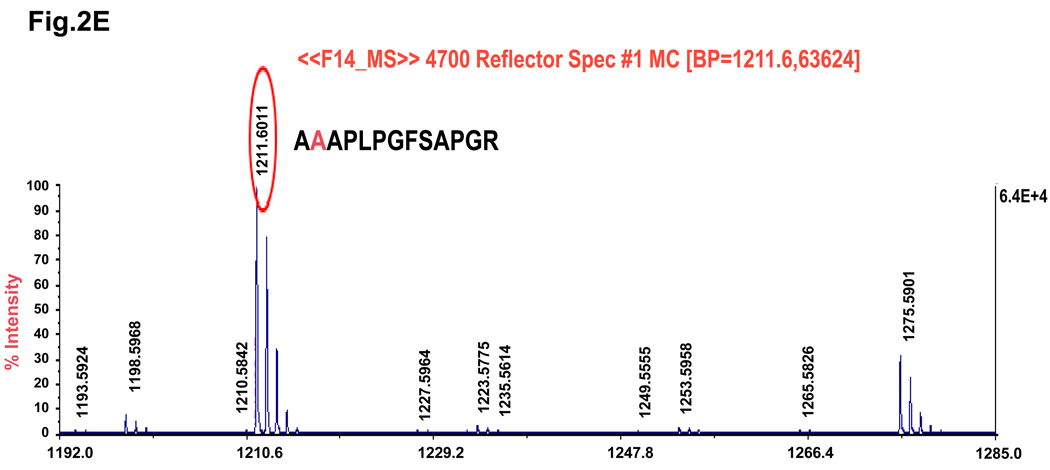
Sympathetic overactivity is closely connected with cell injury and contractile dysfunction during myocardial ischemia/reperfusion.22, 23 Thus, regulation of the β-adrenergic receptor-activated cAMP-PKA signaling pathway and its downstream targets might be important to the improvement of cardiac performance after reperfusion. Our findings above and previous studies6 have suggested a potential role of cAMP-dependent phosphorylation of Hsp20 under ischemic conditions. To examine whether blocking Hsp20 phosphorylation at Ser16 confers any in vivo effects on ischemia/reperfusion injury, we generated transgenic mice that carried the mouse cardiac mutant Hsp20 cDNA, in which TCA encoding for Ser16 were substituted with GCA for alanine (Figure 2A). To verify whether the Hsp20S16A cDNA was present in the mouse genome, we performed PCR amplification of genomic DNA followed by DNA sequencing. This confirmed that the TCA codon, encoding Ser16, was mutated to GCA, which encrypted the alanine residue (Figure 2B). Western-blotting analysis revealed two transgenic lines of Hsp20S16A with overexpression levels of 5-fold and 7-fold, relative to non-TG controls (Figure 2C). The 5-fold and 7-fold lines shared similar basal cardiac contractile functional phenotypes, which were not different from wild type hearts (Online Figure I). Subsequently, the 7-fold overexpression line of Hsp20S16A was used. The expression of non-phosphorylatable Hsp20S16A was further confirmed by proteomics.8 As shown in Figure 2D, only one spot of Hsp20 protein with PI 5.5 was detected by 2-D electrophoresis in non-TG hearts, and two protein spots of Hsp20 (PI 5.5 and 5.2) were observed in Hsp20S16A hearts (Figure 2D). Mass spectrometry revealed that the spot with PI value of 5.2 in TG hearts contained the mutant Hsp20S16A peptide (Figure 2E). It is of note that Hsp20S16A hearts contained endogenous WT-Hsp20 (spot of PI 5.5). However the phosphorylated Hsp20 levels in these hearts were significantly lower than non-TG hearts, either at basal conditions or after ex vivo ischemia/reperfusion injury (Online Figure II), suggesting that overexpression of mutant Hsp20 prevented the phosphorylation of endogenous WT-Hsp20.
Figure 2.
Generation of Hsp20S16A transgenic mouse models. (A) Diagram of Hsp20S16A TG constructs. The mutant mouse Hsp20 cDNA, in which Serine 16 encoded by codon TCA was mutated into GCA (encoding alanine), was driven by the α-myosin heavy chain promoter (a -MHCp). (B) DNA sequencing of PCR products from Hsp20S16A mice genome confirmed the TCA to GCA mutation. (C) Quantitative immunoblotting analysis showed that in Hsp20S16A TG hearts there was a 7-fold increase in total Hsp20 levels relative to non-TG hearts (NTG). (D) 2-D gel electrophoresis identified one non-modified Hsp20 spot (PI=5.5) in WT hearts, while there was another S16A modified spot in Hsp20S16A hearts (PI=5.2). (E) The amino acid sequence of S16A-modified Hsp20 spot was indentified by Mass Spectrometry.
Characterization of non-TG and Hsp20S16A mice showed no alterations in body weight (BW: 28.9±0.74g vs. 28.2±0.56g, n=10, P>0.05) or tibia length (TL: 1.79±0.09 cm vs. 1.80±0.10 cm). Furthermore, there were no differences in heart weight/body weight and heart weight/tibia length ratio (Online Figure IIIA) between these two groups. In addition, histological analysis revealed no signs of fibrosis, inflammation, cardiomyocyte hypertrophy or dystrophy in Hsp20S16A hearts, compared with non-TG controls (Online Figure IIIB). Of importance, 7-fold overexpression of Hsp20S16A did not alter the expression of other small heat shock proteins, such as Hsp25 or αB-crystallin in the heart (Online Figure IIIC).
Impaired Functional Recovery in Hsp20S16A Hearts during Ischemia/Reperfusion Injury
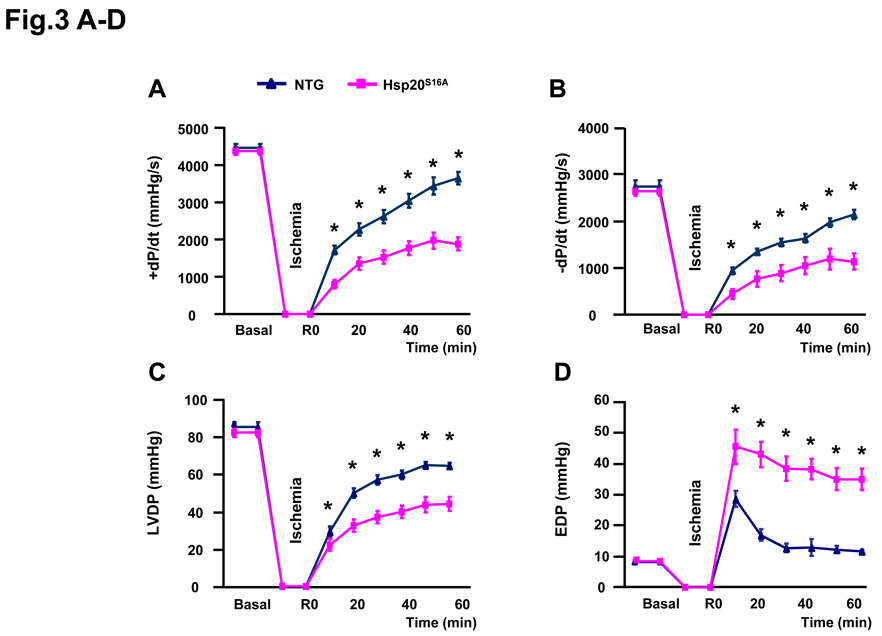
As shown in Figure 1, the levels of phosphorylated Hsp20 were increased in the heart upon ischemia/reperfusion injury. However, it is unclear whether this phosphorylation is essential to the cardioprotective effects of Hsp20 against ischemic stress. Thus, we subjected the Hsp20S16A hearts to ex vivo 45 minutes of no-flow global ischemia, followed by 2h of reperfusion. Non-TG hearts were used as controls. There were no differences in ±dP/dt, left ventricular developed pressure (LVDP) and end diastolic pressure (EDP) between the two groups under basal conditions (Figure 3A–3D). However, under ischemia/reperfusion, functional recovery of Hsp20S16A hearts was significantly depressed, as determined by the parameters of +dP/dt (43.1±4.7% versus non-TG control: 81.6±5.0%; Figure 3A), −dP/dt (42.8± 4.4% versus 78.2± 3.1%; Figure 3B) and LVDP (53.7± 4.3% versus 75.5± 2.2%; Figure 3C) (P<0.01). In addition, EDP was significantly greater in Hsp20S16A hearts after global, no-flow ischemia/reperfusion, compared with non-TG controls (Figure 3D, P<0.01). Taken together, these data suggest that blockade of Hsp20 phosphorylation in Hsp20S16A hearts is associated with impaired functional recovery upon ischemia/reperfusion.
Figure 3.
Overexpression of Hsp20S16A increased susceptibility to ex vivo ischemia/reperfusion injury. During reperfusion, (A, B) recovery of ±dP/dt was significantly lower in Hsp20S16A hearts compared with non-TGs, (C) LVDP recovery was lower in Hsp20S16A TG hearts compared with non-TGs, (D) the increase of EDP in Hsp20S16A hearts was higher than non-TGs, (non-TGs: n=10, Hsp20S16A: n=8; * : P<0.01, Hsp20S16A vs. non-TG).
Increased Necrosis and Apoptosis in Hsp20S16A Hearts upon Ex Vivo Ischemia/Reperfusion
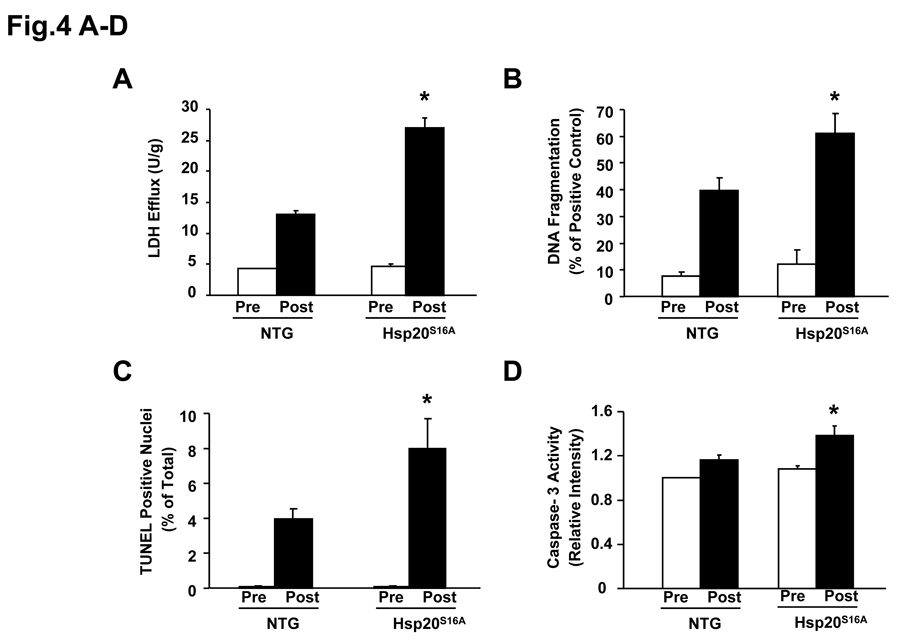
It is recognized that maintaining adequate numbers of myocyte is critical to the overall preservation of structural integrity and cardiac function following ischemia/reperfusion.1 Thus, strategies to maximize post-ischemic salvage have aimed at preventing two forms of cell death, necrosis1, 14 and apoptosis.1, 15 To delineate the detrimental effects conferred by Hsp20S16A in post-ischemic cellular damage, the extent of necrotic and apoptotic cell death was examined after ex vivo 45min ischemia followed by 2h reperfusion. Under basal conditions, LDH release, a biochemical marker of necrotic cell death, did not differ between the Hsp20S16A and non-transgenic hearts (Figure 4A). However, upon ischemia/reperfusion, LDH release was significantly increased by 2-fold in Hsp20S16A hearts, compared to non-TG hearts (Figure 4A, P<0.05). These results indicate that overexpression of Hsp20S16A promote ischemia/reperfusion-initiated cellular disruption in the myocardium.
Figure 4.
Hsp20S16A overexpression increased ischemia/reperfusion-induced necrosis and apoptosis. At basal level, there is no significant differences in LDH release (A), DNA fragmentation (B), TUNEL positive nuclei (C) and caspase-3 activity (D), between Hsp20S16A and non-transgenic hearts (non-TGs: n=6, Hsp20S16A: n=6, P>0.05). Hsp20S16A hearts, subjected to no-flow ischemia followed by reperfusion, exhibited significantly increased total LDH release (A), DNA fragmentation (B), TUNEL-positive nuclei (C) and caspase-3 activity (D), compared to non-TGs (non-TGs: n=6, Hsp20S16A: n=6, *: P <0.01 vs. non-TG).
Furthermore, we examined whether the functional deterioration of the Hsp20S16A TG hearts was related to increased apoptosis. Heart lysates from a subset of experimental animals were assayed for DNA fragmentation by a quantitative nucleosome assay. Hsp20S16A hearts exhibited a 1.5-fold increase over non-TGs (61.1±7.5% versus 39.8±4.8%; n=6; P<0.05, Figure 4B). Additionally, TUNEL-positive nuclei reached 3.97±0.6% in non-TGs versus 7.95±1.68% in Hsp20S16A hearts (P<0.01, Figure 4C). The apparent discrepancy between the percentage of DNA fragmentation and TUNEL-positive nuclei was due to the technique differences between these two methods, which have also been observed in our previous studies.11, 12 Moreover, the levels of active caspase-3 were significantly elevated in Hsp20S16A TG hearts, relative to non-TGs (Figure 4D; P<0.01, n=6). Therefore, all three assays (DNA fragmentation, TUNEL assay and caspase-3 activity) demonstrate significant increases in apoptosis in Hsp20S16A hearts.
Increased Necrosis and Apoptosis in Hsp20S16A Hearts upon In vivo Ischemia/Reperfusion
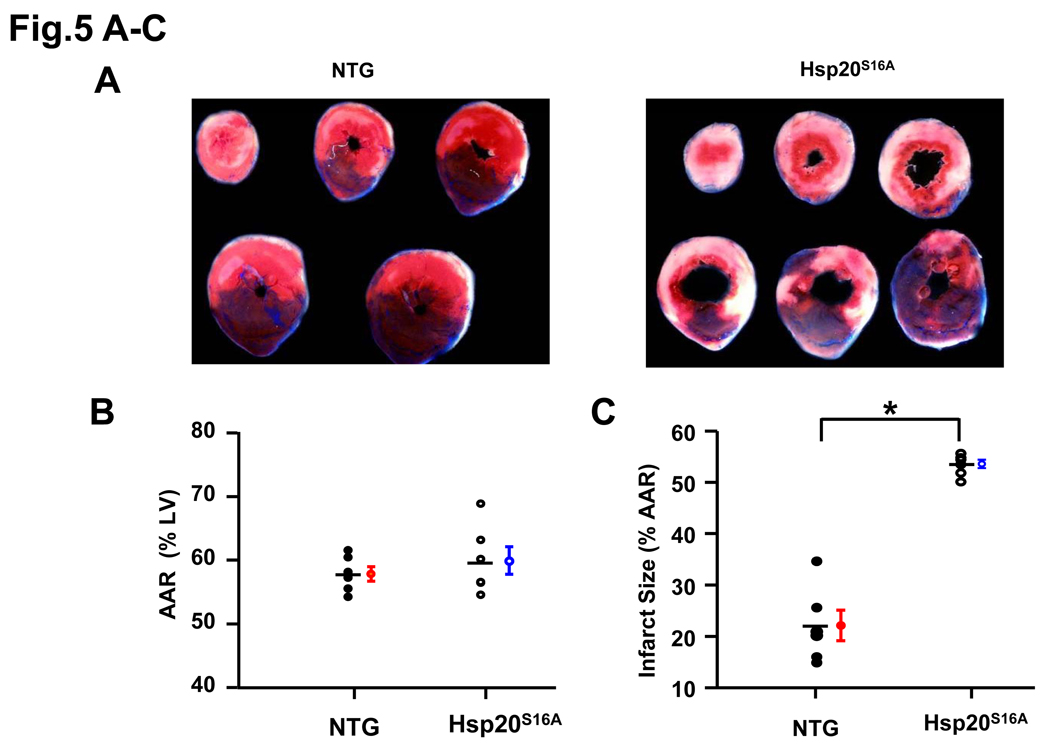
To further examine whether overexpression of Hsp20S16A may be detrimental in ischemic/reperfused hearts in vivo, the Hsp20S16A and non-TG animals were subjected to 30 min myocardial ischemia, via LAD occlusion, followed by 24 h reperfusion.24 The area at risk (AAR), determined by negative staining after reperfusion with phthalo blue dye and expressed as percent of left ventricle, was not significantly different between Hsp20S16A and non-TG hearts (Figure 5B: Hsp20S16A, 57.8±1.1%; non-TG, 59.9±2.2%; P>0.05, n=6), indicating that a comparable degree of ischemic jeopardy existed between these two groups after occlusion of the LAD. The infarct-to-risk region ratio was 22.0±3.0% in non-TG hearts post-I/R, similar to previous reports,25, 26 whereas it was significantly increased (53.5±0.8%, P<0.005) in Hsp20S16A hearts (Figure 5C, n=6). Furthermore, apoptotic TUNEL-positive nuclei27 were also increased in Hsp20S16A hearts after 24h reperfusion (Online Figure IV). Consistent with the ex vivo finding, overexpression of Hsp20 S16A aggravates in vivo ischemia/reperfusion injury.
Figure 5.
Infarct areas were increased in Hsp20S16A TG hearts after prolonged ischemia/reperfusion injury. (A) In vivo infarction was induced by 30 minutes occlusion of the left anterior descending artery. The mice were euthanized after 24 hours of reperfusion and infarct size was determined as described previously24. (B) There were no significant differences in size of the risk region in transgenic vs. non-TG groups. (C) Infarct size, expressed as a percentage of the region at risk, was 2-fold larger in Hsp20S16A hearts, compared to non-TGs (Hsp20S16A: 53.5±0.8%; non-TG: 22.0±3.0%; n=6, P<0.005).
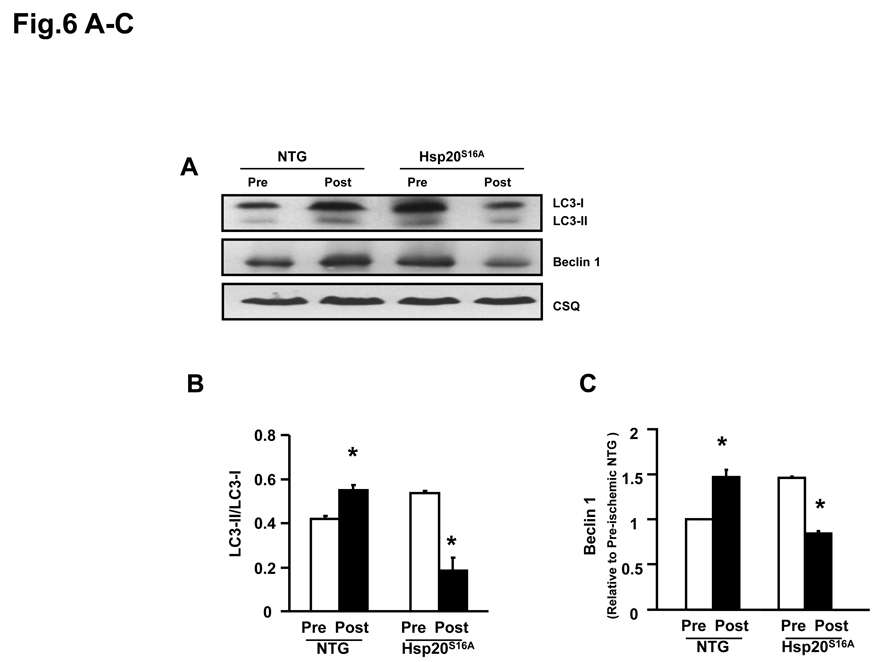
Inactivation of Autophagy in Hsp20S16A Hearts upon Ex Vivo Ischemia/Reperfusion
Autophagy is an intracellular bulk degradation process, whereby cytosolic, long-lived proteins and organelles are degraded and recycled.16 Recent studies have shown that autophagy plays an important role in ischemia/reperfusion injury,17 and its activity levels determine its benifical or detrimental effects in such injury.17 Thus, we assessed the autophagy activity in Hsp20S16A hearts by measurement of the microtubule-associated protein light chain 3 (LC3) and specifically the ratio of LC3-II/LC3-I, as well as Beclin 1 protein level,28 in comparison with non-TG hearts. Conversion of cytosolic LC3-I to membrane-conjugated LC3-II is correlated with the number of autophagosomes, indicative of autophagic activity.28,29 Furthermore, Beclin 1, an autophagy related protein, is a critical player in the formation of autophagosome.30 Western blotting analysis showed that the ratio of LC3-II/LC3-I was increased in non-TG hearts after ischemia/reperfusion (Figure 6A and B), suggesting that ischemia/reperfusion initiated autophagy. Interestingly, the ratio of LC3-II/LC3-I was elevated in Hsp20S16A hearts under basal conditions, but was significantly decreased following ischemia/reperfusion (Figure 6A and B). Alterations of another autophagy-related protein, Beclin1 (Figure 6A and C), were parallel to the changes of the LC3-II/LC3-1 ratio in Hsp20S16A and non-TG hearts. Taken together, these data indicate that suppression of ischemia/reperfusion-induced autophagy may contribute to the depressed cardiac functional recovery in Hsp20S16A hearts.
Figure 6.
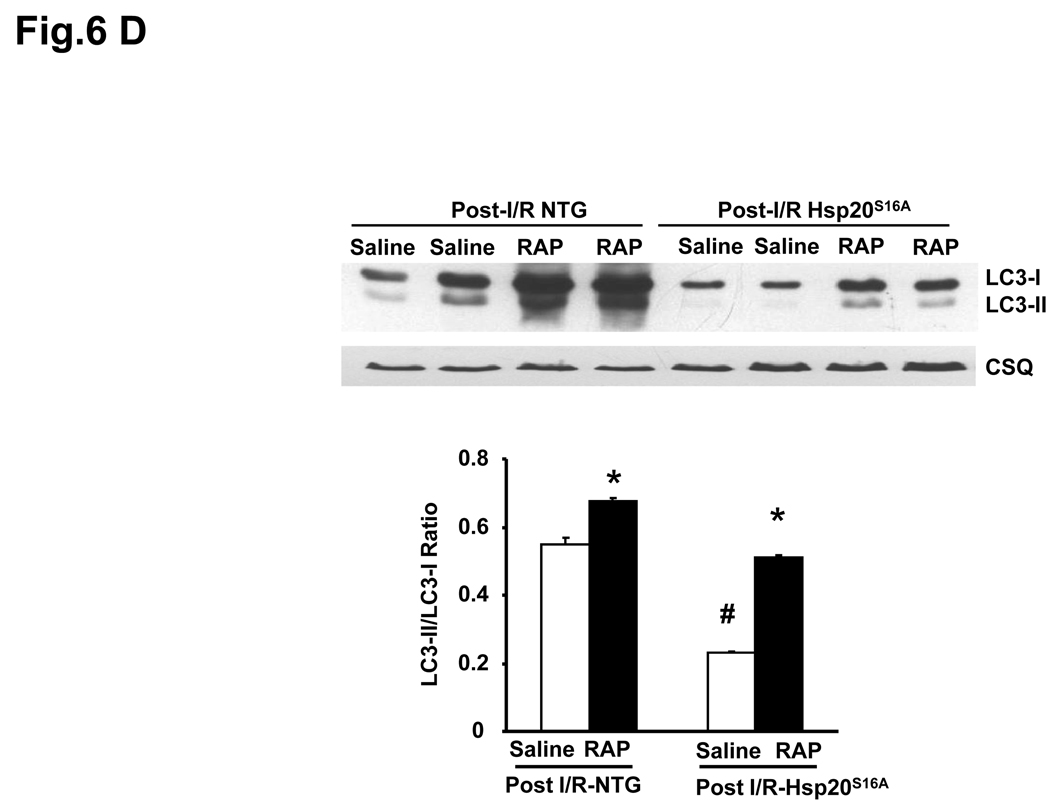
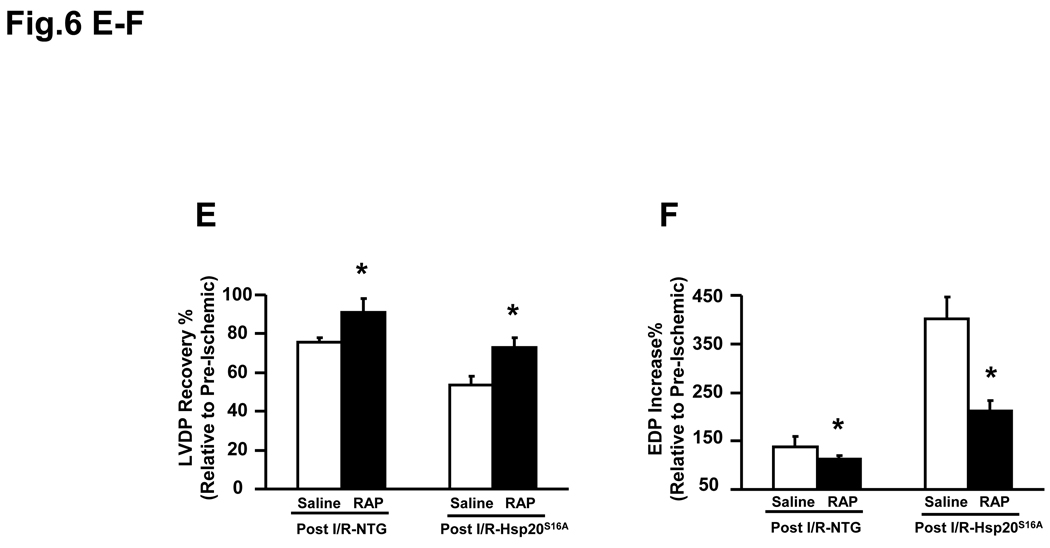
Autophagy was inhibited in Hsp20S16A hearts in response to ischemia/reperfusion injury. Cardiac homogenates from Hsp20S16A and non-TG mice were subjected to immunoblot analysis for detection of LC3-II/LC3-I and Beclin1 (A). Quantitative analysis results of LC3-II/-LC3-I ratio (B) and Beclin 1 level (C) are from 3 different preparations (six hearts for each group,* : P<0.05, Post- vs. Pre-ischemic group). Pretreatment with the autophagy inducer rapamycin in Hsp20S16A hearts increased the LC3-II/LC3-1 ratio (D) and improved recovery of left ventricular developed pressure (E) and decreased EDP (F) following ischemia/reperfusion, compared with saline-treated controls. (non-TG: n=6, Hsp20S16A: n=6, *: P<0.01, rapamycin vs. saline group).
Pretreatment of Hsp20S16A Hearts with Rapamycin Improved Functional Recovery in Response to Ischemia/Reperfusion
To further examine whether activation of autophagy in Hsp20S16A TG hearts would rescue its post-ischemic function, we administered rapamycin to Hsp20S16A mice, which induces autophagy by inhibiting the mammalian target of rapamycin (mTOR).31 Pretreatment with rapamycin did not alter the pre-ischemic cardiac function (Online Figure V). As shown in Figure 6D, treatment with rapamycin significantly increased the LC3-II/LC3-I in both non-TG and Hsp20S16A hearts. Consequently, functional recovery was significantly improved in Hsp20S16A hearts following 2h reperfusion, compared with saline-treated controls (Figure 6E–6F). These findings suggest that enhancement of autophagy in post- ischemia/reperfusion Hsp20S16A hearts may ameliorate tissue injury.
Effects of Mutant S16A on Hsp20 Oligomerization Patterns Following Ischemia/Reperfusion
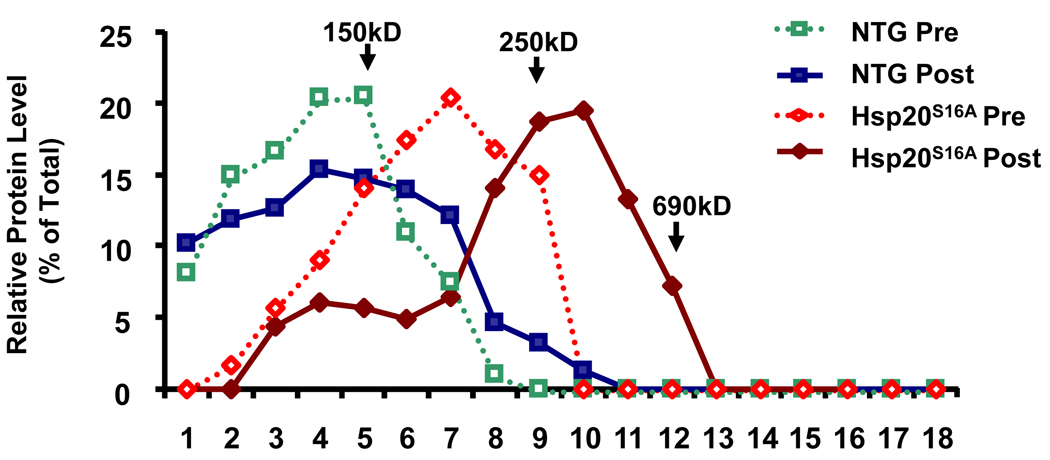
The phosphorylation status of Hsp20 has been suggested to have a role in its structural organization, manifesting in the aggregation patterns of the protein, which might be associated with its function.32, 33 As a result, we examined the oligomerization pattern of Hsp20 in non-transgenic and Hsp20S16A transgenic hearts before and after global I/R by sucrose gradient ultracentrifugation. This method revealed that non-TG hearts displayed Hsp20 oligomers up to 150kD before I/R, and this pattern slightly shifted to the right (larger complex) after I/R. In contrast, at basal levels, Hsp20S16A hearts displayed Hsp20 aggregate patterns composed primarily of oligomers between 150kD to 250kD. After I/R, Hsp20S16A hearts displayed Hsp20 oligomers that were shifted to a larger complex profile (>250kD, Figure 7), suggesting that phosphorylation of Ser16 may alter the ability of Hsp20 to aggregate.
Figure 7.
Sucrose-gradient centrifugation analysis of Hsp20 proteins. Cardiac lysates (100µg total protein) from pre- and post-ischemic/reperfused Hsp20S16A and non-TG hearts were layered on the top of 5%-40% sucrose gradients. After centrifugation, as described in Methods, fractions of the gradients (labeled 1–18 from top to bottom) were collected, and resolved by electrophoresis. A blot of the gel was probed with anti-Hsp20 antibody. Relative protein levels in each fraction were calculated by densitometric scans of each immunoreactive band/total Hsp20. Mutant Hsp20S16A promoted a shift of Hsp20 oligomers to a larger complex profile after ischemia/reperfusion, compared with non-TG controls. Arrows indicate standards: alcohol dehydrogenase (ADH) (150 kDa), β-amylase (250 kDa), and thyroglobulin (690 kDa). Other abbreviations are as defined in the text.
Discussion
The findings herein indicate that phosphorylation of Hsp20 at Ser16 is essential for protecting the myocardium from ischemia/reperfusion damage. Blockade of this phosphorylation site in a model with cardiac overexpression of Hsp20S16A resulted in loss of cardioprotective effects exhibited by overexpression of wild type Hsp20,6 and fostered worse functional recovery from ischemia/reperfusion. More interestingly, this detrimental effect of Hsp20S16A was associated with increased necrosis and apoptosis, but decreased autophagy. To our knowledge, this is the first study showing that blockade of Hsp20 phosphorylation reduces ischemia/reperfusion-induced autophagy and compromises its cardioprotection.
It is noteworthy that in vivo or ex vivo myocardial ischemia involves a large and progressive release of catecholamines from adrenergic nerve terminals, and excessive stimulation of myocardial β-adrenergic receptors by catecholamines may further accelerate the ischemia-induced cell damage.22, 23 Indeed, we observed an increased ratio of phospho-Ser16/total Hsp20 in ischemia/reperfusion injured wild type mouse hearts, as well as in failing human hearts. This could be interpreted as a compensatory protective response to the accumulated catecholamines in the ischemic myocardial tissue. In fact, our previous study has shown that the protective effect of Hsp20 against ischemia/reperfusion was associated with increased phosphorylation of Hsp20.6, 12 Our data presented here further support the in vivo significance of Hsp20 phosphorylation in its cardioprotective effects against ischemia/reperfusion injury.
The mechanisms underlying the detrimental effects of Hsp20S16A on ischemia/reperfusion may involve several pathways. Firstly, the increase of apoptosis is likely an important factor responsible for severe injuries in post-ischemic Hsp20S16A hearts. It is well accepted that cardiomyocyte apoptosis could be a fundamental part of the myocardial process, that initiates or aggravates cardiac injury.1, 34–36 For example, conditional overexpression of active caspase-8 demonstrated that very low levels of myocyte apoptosis were sufficient to cause lethal, dilated cardiomyopathy.37 Accordingly, our laboratory also observed that reduced cardiac apoptosis significantly contributed to better functional recovery in Hsp20-overexpressing hearts upon ischemia/reperfusion.6 Hence, blockade of Hsp20 phosphorylation at Ser16 is associated with increased cardiomyocyte apoptosis triggered by ischemia/reperfusion, leading to lower functional recovery.
Secondly, there is an increasing awareness that autophagy plays a critical role in ischemia/reperfusion injury,16,17 which is an important addition to the well-known necrosis14 and apoptosis processes.15 Under basal conditions, low level of autophagy is necessary for the turnover of long-lived proteins and cytoplasmic organelles in the heart.38 In response to ischemia, the extent of autophagy depends on the severity and duration of ischemic insults. For example, modest levels of autophagy, induced by mild to moderate hypoxia/ischemia, appear to be protective by degrading and removing damaged mitochondria, therefore preventing activation of apoptosis.16, 39 On the other hand, high levels of autophagy triggered by severe hypoxia or ischemia/reperfusion may cause self-digestion and eventual cell death.40 Accordingly, Decker and Wildenthal16 reported that as early as 40min of ischemia led to upregulation of autophagy, and that subsequent reperfusion of 1h induced a drastic enhancement of autophagy in Langendorff perfused rabbit hearts. In their model, induction of modest levels of autophagy was correlated with functional recovery of rabbit hearts after ischemia/reperfusion.16 Therefore, the level of autophagy may determine whether it is protective or detrimental to the heart in response to ischemia/reperfusion. The benifical increase of autophagy may be responsible for elimination of damaged, presumably nonfunctional organelles, including mitochondria, along with the restoration of normal cardiac structure and function.17, 40 Furthermore, autophagy can spill over into regulation of apoptotic and necrotic cell death, specifically, through autophagic proteases.41 And the key machinery of autophagy, autophagasome itself, when fused with the lysosome, can also regulate necrotic cell death.42
Consistently, we found that a brief period (20 minutes) of ischemia followed by 2h reperfusion induced reversible cardiac injury (complete contractile recovery) in wild type murine hearts. By contrast, prolonged ischemia (60 minutes) and 2h reperfusion resulted in a large infarct along with minimal recovery of contractility (~10%) (Online Figure VI). The degree of injury inflicted during 45 minutes of ischemia followed by 2h reperfusion was intermediate, and correlated with a modest activation of autophagy (1.3-fold increase of the LC3-II/LC3-I ratio). Thus, we selected a 45min/2h of ischemia-reperfusion protocol for our present study. As expected, autophagy was increased in our non-TG hearts upon ischemia/reperfusion, which corresponded to ~75% of functional recovery. However, Hsp20S16A hearts displayed inhibition of ischemia/reperfusion-activated autophagy, and this may contribute to deterioration of energy homeostasis, leading to impaired functional recovery (~54%). Accordingly, activation of the autophagy process in Hsp20S16A hearts by pretreatment with rapamycin restored their functional recovery upon ischemia/reperfusion. Furthermore, we have noticed a modest (1.27 fold) increase of autophagy in Hsp20S16A hearts under basal conditions, which did not affect its basal myocardial function, compared with non-TG controls. We also found that pretreatment of Hsp20S16A hearts with rapamycin did not alter basal contractile function (Online Figure V). These observations suggest that a modest increase of autophagy has no effects on basal cardiac function. However, it should be admitted that rapamycin might have some other protective effects beyond activating autophagy, such as inducing potent preconditioning-like effects against myocardial infarction through opening of mitochondrial KATP channels.43
Finally, non-phosphorylatable Hsp20 may affect its oligomerization, which offsets the protective effects of Hsp20. It is well-known that the phosphorylative capacity and the resulting aggregation patterns of small heat shock proteins may influence their cytoprotective ability during cellular stress.44–46 For example, the phosphorylated form of Hsp27 was concentrated in small and medium-sized oligomers, whereas its non-phosphorylated form was present in larger oligomers.44, 45 Unexpectedly, non-phosphorylatable mutants of Hsp25, the rodent form of Hsp27, conferred better protection than wild-type Hsp25 in L929 cells subjected to tumor necrosis factor (TNF)-α and H2O2-induced cytotoxicity.46 Furthermore, overexpression of wild-type Hsp27 or a non-phosphorylatable Hsp27 mutant was equally capable of protecting from an ischemic insult in adult rat myocytes44 and transgenic mouse models.47 These conflicting results may be partially due to the use of different models and variability in Hsp27 protein levels between studies. Previous in vitro studies have shown that phosphorylation at Ser16 regulates the aggregation pattern of Hsp20, 33 which is consistent with the observation that the N-terminal residues of sHsps are necessary for complex formation.48 Interestingly, our pull-down assay indicated that phospho-Ser16-Hsp20 is associated with autophagy-related Beclin-1 (Online Figure VII), suggesting that Beclin-1 may be a potential target of phosphorylated Hsp20 in regulating autophagy. The blockade of Hsp20 phosphorylation at Ser16 promoted formation of large aggregates and possibly disassociated Beclin-1, which may promote the detrimental effects under stress conditions.
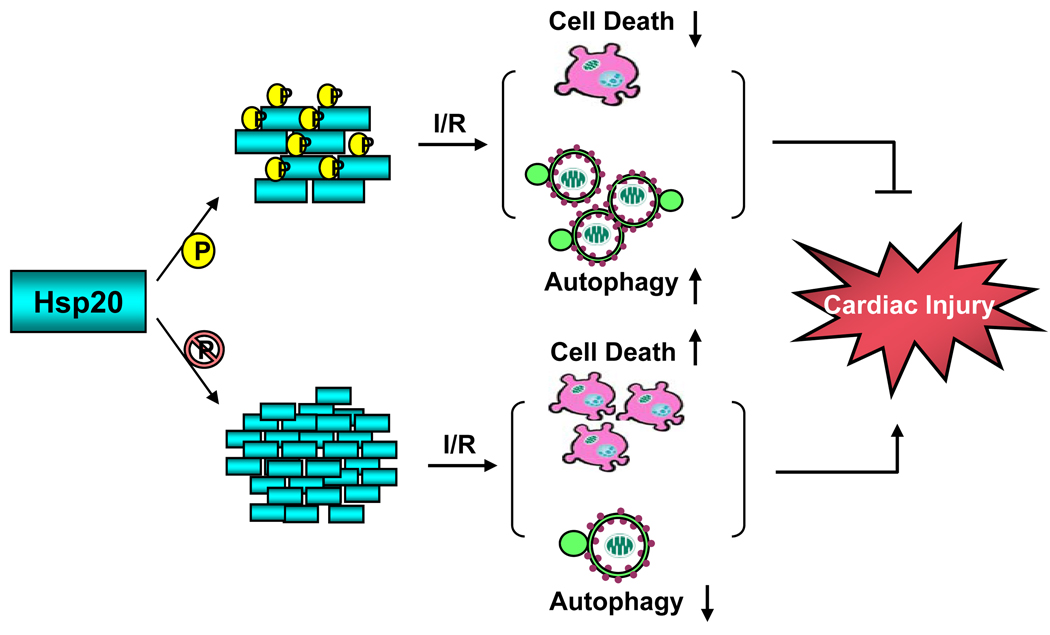
In conclusion, the present findings indicate that blockade of Hsp20 phosphorylation at Ser16 is associated with increased cardiac ischemia/reperfusion injury, partially through reduced activation of autophagy and increased apoptosis as well as necrosis (Figure 8). Thus, Hsp20 and its phosphorylation may constitute an important modality for cardioprotection against myocardial infarction.
Figure 8.
Proposed scheme for phosphorylation of Ser16-Hsp20 protecting against cardiac ischemia/reperfusion injury. Upon phosphorylation, Hsp20 tends to form small oligomers which increase autophagy activity and decrease cell death, therefore preventing cardiac injury during ischemia/reperfusion. When phosphorylation of the Ser16 site is blocked (Hsp20S16A hearts), during ischemia/reperfusion injury, Hsp20 forms large oligomers, suppresses autophagy activity and increases cell death, which leads to cardiac injury.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Roger Hajjar for providing the human samples.
Source of Funding
This study was supported by NIH grant HL-087861 (Dr. G-C Fan), and by NIH grants HL26057, HL64018, HL 77101 and the Leducq Foundation (Dr. E.G. Kranias).
Non-standard Abbreviations and Acronyms
- 2-D
2 dimensional
- AAR
Area at risk
- EDP
End-diastolic pressure
- Hsp20
Heat shock protein 20
- Hsps
Heat shock proteins
- LAD
Left anterior descending coronary artery
- LC3
Microtubule-associated protein light chain 3
- LDH
Lactate dehydrogenase
- LVDP
Left ventricular developed pressure
- mTOR
Mammalian target of rapamycin
- PKA
Protein kinase A
- PKG
Protein kinase G
- TTC
2,3,5-Triphenyltetrazolium chloride
- TUNEL
Terminal dUTP nick end-labeling
- WGA
Wheat germ agglutinin
Footnotes
Disclosures
Dr. E.G. Kranias is a scientific founder of Nanocor.
References
- 1.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001;51:637–646. doi: 10.1016/s0008-6363(01)00354-6. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin IJ, McMillan D. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Vander Heide RS. Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2002;282:H935–H941. doi: 10.1152/ajpheart.00660.2001. [DOI] [PubMed] [Google Scholar]
- 5.Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for alphaB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol. 2004;286:H847–H855. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- 6.Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 7.Beall A, Bagwell D, Woodrum D, Stoming TA, Kato K, Suzuki A, Rasmussen H, Brophy CM. The small heat shock-related protein, HSP20, is phosphorylated on serine 16 during cyclic nucleotide-dependent relaxation. J Biol Chem. 1999;274:11344–11351. doi: 10.1074/jbc.274.16.11344. [DOI] [PubMed] [Google Scholar]
- 8.Chu G, Egnaczyk GF, Zhao W, Jo SH, Fan GC, Maggio JE, Xiao RP, Kranias EG. Phosphoproteome analysis of cardiomyocytes subjected to beta-adrenergic stimulation: identification and characterization of a cardiac heat shock protein p20. Circ Res. 2004;94:184–193. doi: 10.1161/01.RES.0000107198.90218.21. [DOI] [PubMed] [Google Scholar]
- 9.Dohke T, Wada A, Isono T, Fujii M, Yamamoto T, Tsutamoto T, Horie M. Proteomic analysis reveals significant alternations of cardiac small heat shock protein expression in congestive heart failure. J Card Fail. 2006;12:77–84. doi: 10.1016/j.cardfail.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Burniston J. Adaptation of the rat cardiac proteome in response to intensity-controlled endurance exercise. Proteomics. 2009;9:106–115. doi: 10.1002/pmic.200800268. [DOI] [PubMed] [Google Scholar]
- 11.Fan GC, Zhou X, Wang X, Song G, Qian J, Nicolaou P, Chen G, Ren X, Kranias EG. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolaou P, Knöll R, Haghighi K, Fan GC, Dorn GW, 2nd, Hasenfub G, Kranias EG. Human mutation in the anti-apoptotic heat shock protein 20 abrogates its cardioprotective effects. J Biol Chem. 2008;283:33465–33471. doi: 10.1074/jbc.M802307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan GC, Chu G, Mitton B, Song Q, Yuan Q, Kranias EG. Small heat-shock protein Hsp20 phosphorylation inhibits beta-agonist-induced cardiac apoptosis. Circ Res. 2004;94:1474–1482. doi: 10.1161/01.RES.0000129179.66631.00. [DOI] [PubMed] [Google Scholar]
- 14.Tavernarakis N. Cardiomyocyte necrosis: alternative mechanisms, effective interventions. Biochim Biophys Acta. 2007;1773:480–482. doi: 10.1016/j.bbamcr.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Masri C, Chandrashekhar Y. Apoptosis: a potentially reversible, meta-stable state of the heart. Heart Fail Rev. 2008;13:175–179. doi: 10.1007/s10741-007-9069-3. [DOI] [PubMed] [Google Scholar]
- 16.Decker RS, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol. 1980;98:425–444. [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res. 1997;35:2–3. [PubMed] [Google Scholar]
- 19.Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG. Regulation of myocardial function by histidine-rich, calcium-binding protein. Am J Physiol Heart Circ Physiol. 2004;287:H1705–H1711. doi: 10.1152/ajpheart.01211.2003. [DOI] [PubMed] [Google Scholar]
- 20.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Bovill E, Westaby S, Crisp A, Jacobs S, Shaw T. Reduction of four-and-a-half LIM-protein 2 expression occurs in human left ventricular failure and leads to altered localization and reduced activity of metabolic enzymes. J Thorac Cardiovasc Surg. 2009;137:853–861. doi: 10.1016/j.jtcvs.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Schömig A, Dart AM, Dietz R, Mayer E, Kübler W. Release of endogenous catecholamines in the ischemic myocardium of the rat. Part A: Locally mediated release. Circ Res. 1984;55:689–701. doi: 10.1161/01.res.55.5.689. [DOI] [PubMed] [Google Scholar]
- 23.Lameris TW, de Zeeuw S, Alberts G, Boomsma F, Duncker DJ, Verdouw PD, Veld AJ, van Den Meiracker AH. Time course and mechanism of myocardial catecholamine release during transient ischemia in vivo. Circulation. 2000;101:2645–2650. doi: 10.1161/01.cir.101.22.2645. [DOI] [PubMed] [Google Scholar]
- 24.Ren X, Wang Y, Jones WK. TNF-alpha is required for late ischemic preconditioning but not for remote preconditioning of trauma. J Surg Res. 2004;121:120–129. doi: 10.1016/j.jss.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, Mitton B, Pathak A, Robbins J, Hajjar R, Jones K, Kranias E. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren X, Wu J, Wang X, Sartor M, Qian J, Jones K, Nicolaou P, Pritchard T, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jugdutt BI, Idikio HA. Apoptosis and oncosis in acute coronary syndromes:assessment and implications. Mol Cell Biochem. 2005;270:177–200. doi: 10.1007/s11010-005-4507-9. [DOI] [PubMed] [Google Scholar]
- 28.Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–387. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 32.van de Klundert FA, Smulders RH, Gijsen ML, Lindner RA, Jaenicke R, Carver JA, de Jong WW. The mammalian small heat-shock protein Hsp20 forms dimers and is a poor chaperone. Eur J Biochem. 1998;258:1014–1021. doi: 10.1046/j.1432-1327.1998.2581014.x. [DOI] [PubMed] [Google Scholar]
- 33.Chernik IS, Seit-Nebi AS, Marston SB, Gusev NB. Small heat shock protein Hsp20 (HspB6) as a partner of 14-3-3gamma. Mol Cell Biochem. 2007;295:9–17. doi: 10.1007/s11010-006-9266-8. [DOI] [PubMed] [Google Scholar]
- 34.Bialik S, Geenen DL, Sasson IE, Cheng R, Horner JW, Evans SM, Lord EM, Koch CJ, Kitsis RN. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest. 1997;100:1363–1372. doi: 10.1172/JCI119656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colucci WS. Apoptosis in the heart. N Engl J Med. 1996;335:1224–1226. doi: 10.1056/NEJM199610173351610. [DOI] [PubMed] [Google Scholar]
- 36.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 39.Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. 2006;20:445–462. doi: 10.1007/s10557-006-0583-7. [DOI] [PubMed] [Google Scholar]
- 40.Gustafsson AB, Gottlieb RA. Eat your heart out: Role of autophagy in myocardial ischemia/reperfusion. Autophagy. 2008;4:416–421. doi: 10.4161/auto.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fortunato F, Bürgers H, Bergmann F, Rieger P, Büchler MW, Kroemer G, Werner J. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. 2009;137:350–360. doi: 10.1053/j.gastro.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Fortunato F, Kroemer G. Impaired autophagosome-lysosome fusion in the pathogenesis of pancreatitis. Autophagy. 2009;5:850–853. doi: 10.4161/auto.8839. [DOI] [PubMed] [Google Scholar]
- 43.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Martin JL, Hickey E, Weber LA, Dillmann WH, Mestril R. Influence of phosphorylation and oligomerization on the protective role of the small heat shock protein 27 in rat adult cardiomyocytes. Gene Expr. 1999;7:349–355. [PMC free article] [PubMed] [Google Scholar]
- 45.Mehlen P, Arrigo AP. The serum-induced phosphorylation of mammalian hsp27 correlates with changes in its intracellular localization and levels of oligomerization. Eur J Biochem. 1994;221:327–334. doi: 10.1111/j.1432-1033.1994.tb18744.x. [DOI] [PubMed] [Google Scholar]
- 46.Préville X, Schultz H, Knauf U, Gaestel M, Arrigo AP. Analysis of the role of Hsp25 phosphorylation reveals the importance of the oligomerization state of this small heat shock protein in its protective function against TNFalpha- and hydrogen peroxide-induced cell death. J Cell Biochem. 1998;69:436–452. [PubMed] [Google Scholar]
- 47.Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- 48.Leroux MR, Ma BJ, Batelier G, Melki R, Candido EP. Unique structural features of a novel class of small heat shock proteins. J Biol Chem. 1997;272:12847–12853. doi: 10.1074/jbc.272.19.12847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.