Abstract
Purpose
To determine the predictors of distress in older patients with cancer.
Patients and Methods
Patients age ≥ 65 years with a solid tumor or lymphoma completed a questionnaire that addressed these geriatric assessment domains: functional status, comorbidity, psychological state, nutritional status, and social support. Patients self-rated their level of distress on a scale of zero to 10 using a validated screening tool called the Distress Thermometer. The relationship between distress and geriatric assessment scores was examined.
Results
The geriatric assessment questionnaire was completed by 245 patients (mean age, 76 years; standard deviation [SD], 7 years; range, 65 to 95 years) with cancer (36% stage IV; 71% female). Of these, 87% also completed the Distress Thermometer, with 41% (n = 87) reporting a distress score of ≥ 4 on a scale of zero to 10 (mean score, 3; SD, 3; range, zero to 10). Bivariate analyses demonstrated an association between higher distress (≥ 4) and poorer physical function, increased comorbid medical conditions, poor eyesight, inability to complete the questionnaire alone, and requiring more time to complete the questionnaire. In a multivariate regression model based on the significant bivariate findings, poorer physical function (increased need for assistance with instrumental activities of daily living [P = .015] and lower physical function score on the Medical Outcomes Survey [P = .018]) correlated significantly with a higher distress score.
Conclusion
Significant distress was identified in 41% of older patients with cancer. Poorer physical function was the best predictor of distress. Further studies are needed to determine whether interventions that improve or assist with physical functioning can help to decrease distress in older adults with cancer.
INTRODUCTION
Psychological distress is common among patients with cancer; however, it often goes unrecognized.1 The National Comprehensive Cancer Network defines distress as a “multifactorial, unpleasant emotional experience of a psychological (cognitive, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”2 Despite the fact that approximately 60% of cancer diagnoses and 70% of cancer mortality occur in patients age ≥ 65 years,3 few studies have specifically focused on the prevalence or causes of distress in older adults with this disease.
Studies have reported that 27% to 48% of older adults who live in the community experience psychological distress.4–6 Sociodemographic, clinical, and psychosocial variables have been identified as risk factors for distress, including sex (females more likely than males), age (younger and older more likely than middle age), lower education, lower perceived social support, functional dependence, comorbid conditions, and use of escape/avoidance coping strategies.4,7,8 On the basis of these prior studies, there are several potential reasons why older adults with cancer are at risk for psychological distress. First, they are more likely to require assistance with their daily functions than are older adults without cancer.9 Furthermore, this increased need for assistance persists in cancer survivors.10 Second, changes in the social support structure that often accompany aging, such as the death of a spouse or friends or the loss of a job, can lead to social isolation in older adults, isolation that can be exacerbated by a serious illness. Third, treatment-related short- and long-term toxicity is common in older adults because of age-related declines in physiologic function.11 Fourth, comorbid medical conditions increase with age, may have an impact on tolerance to cancer therapy, or may be acquired as adverse effects to therapy.12–14 All of these factors can contribute to psychological distress in older adults with cancer.
The National Comprehensive Cancer Network (NCCN) recommends that all patients with cancer should be evaluated regularly for psychosocial distress as a part of routine care.2 The NCCN guidelines for distress management recommend using the Distress Thermometer as a screening tool.2 This is a single-item, zero- to 10-point scale with a threshold score of 4 indicating significant distress that warrants further evaluation. The Distress Thermometer has been suggested as a quick and valid alternative to other psychometric instruments for use in busy outpatient cancer clinics. However, once a distressed older adult is identified, there is little research to guide oncologists in determining which of the risk factors is most likely to cause distress. An understanding of the predictors of distress would help streamline the evaluation and guide interventions for decreasing the level of distress.
The goal of this study was to determine the prevalence of distress in a cohort of older adults with cancer by using the Distress Thermometer. In addition, we sought to determine whether predictors of distress could be identified using a brief, comprehensive geriatric assessment that captured information regarding the individual's functional status, comorbid medical conditions, psychological state, social support, and nutritional status.
PATIENTS AND METHODS
Patients age ≥ 65 years were recruited from Memorial Sloan-Kettering Cancer Center's (New York, NY) main campus and satellite community clinics situated in the New York City area. Patients received the geriatric assessment questionnaire either by mail or when they checked in for their oncology appointments.
Domains evaluated in the geriatric assessment include functional status, comorbid medical conditions, psychological state, social support, and nutritional status (Table 1). These measures were chosen on the basis of their ability to predict morbidity and/or mortality in geriatric patients as well as on their reliability, validity, and brevity. Most of these measures had been included in a prior study aimed at developing a cancer-specific geriatric assessment.24 The feasibility of someone completing this mailed geriatric assessment has been previously reported.25 The questionnaire was designed to be self-administered; however, if the patient required assistance, a family member or staff member could help. After the patient completed the questionnaire, a member of the health care team scored the assessment and reviewed the results with the treating physician. A medical record review was performed to record whether patients received chemotherapy, endocrine therapy, immunotherapy, or radiation; however, the timing between receipt of cancer therapy and completion of this questionnaire was not captured.
Table 1.
Domains and Measures in the Geriatric Assessment Questionnaire
| Domain | Measure | Description |
|---|---|---|
| Functional status | Activities of Daily Living (subscale of MOS Physical Health)15 | Ten items measuring the limitations in a wide range of physical function (from bathing/dressing to vigorous activities such as running) |
| Instrumental Activities of Daily Living (subscale of the OARS)16 | Seven items measuring the ability to complete activities required to maintain independence in the community (such as shopping, meal preparation, making telephone calls, money management) | |
| Karnofsky self-reported performance rating scale17 | One item global indicator of patient function determined by patient self-report ranging from “normal” to “severely disabled” | |
| Number of falls in the last 6 months18 | One item indicating number of times fallen in the last 6 months | |
| Comorbidity | Physical Health Section (subscale of the OARS)16 | List of 13 comorbid illnesses and the degree to which they impair daily activities, as well as a rating of eyesight and hearing |
| Psychological | Distress Thermometer19 | One item measuring level of distress on a scale of 0 to 10 |
| Social support | MOS Social Support Survey: Emotional/Information and Tangible Subscales20 | Twelve items measuring the perceived availability of social support |
| Nutrition | Body mass index21 | One item: weight/(height)2 |
| Percent unintentional weight loss in last 6 months22,23 | One item: (unintentional weight lost in last 6 months/baseline body weight) × 100 |
Abbreviations: MOS, Medical Outcomes Study; OARS, Older American Resources and Services.
Patient distress was measured by the Distress Thermometer, which is a self-report questionnaire consisting of one item. On a scale of zero to 10, patients were asked to circle the number that best described how distressed they had been in the past week, with zero indicating “no distress” and 10 indicating “extreme distress.” Prior studies have reported on the efficacy of the Distress Thermometer as a screening aid.26,27 Roth et al19 performed a study of patients with prostate cancer and found a high concordance between scores on the Distress Thermometer and the Hospital Anxiety and Depression Scale. Ransom et al28 evaluated 491 patients scheduled to undergo a bone marrow transplantation and found that a cutoff score of 4 had the greatest sensitivity and specificity when compared with the Center for Epidemiological Studies Depression scale. Jacobsen et al29 studied 380 patients with cancer and reported that patients who scored ≥ 4 on the Distress Thermometer were more likely to also report physical, emotional, practical, and family problems. On the basis of this review of the literature, a cutoff score of ≥ 4 on the Distress Thermometer was used to analyze these data.
Raw scores from the Distress Thermometer were divided into two categories, < 4 and ≥ 4, and were analyzed as a bivariate variable. The associations between the bivariate distress score and important patient characteristics, as well as other measures from the geriatric assessment questionnaire, were assessed using a two-sample t test or χ2 test. On the basis of significant findings, a multivariate logistic regression was fit to the data to identify which geriatric assessment or patient characteristic variables were independent predictors of distress. Institutional review board approval was obtained to review and report on these data.
RESULTS
The geriatric assessment questionnaire was given to 250 patients age ≥ 65 years. Of these, five patients (2%) did not complete the questionnaire, leaving 245 evaluable patients (mean age, 76 years; standard deviation [SD], 7 years; range, 65 to 95 years). Of these, 214 patients (87%) also completed the Distress Thermometer. The patient characteristics are summarized in Table 2. Patients with all stages of cancer were included: stage I (31%), stage II (22%), stage III (10%), and stage IV (36%). The most common tumor types were breast (41%), GI (19%), and gynecologic or genitourinary (17%). Seventy-one percent of participants were female, 95% were white, 52% were married, 9% were working full-time, and 46% had completed a college degree or higher education.
Table 2.
Patient Characteristics (N = 245)
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| 65-75 | 115 | 47 |
| 76-85 | 110 | 45 |
| 86-95 | 20 | 8 |
| Sex | ||
| Female | 175 | 71 |
| Male | 70 | 29 |
| Cancer type | ||
| Breast | 100 | 41 |
| Lymphoma | 21 | 9 |
| Gynecologic or genitourinary | 42 | 17 |
| GI | 46 | 19 |
| Other | 36 | 14 |
| Cancer stage | ||
| Localized | 157 | 64 |
| Metastatic | 88 | 36 |
| Educational level | ||
| < High school | 21 | 8 |
| High school graduate | 112 | 46 |
| Bachelor's degree | 52 | 21 |
| Advanced degree | 51 | 21 |
| Other | 9 | 4 |
| Marital status | ||
| Married | 127 | 52 |
| Widowed | 77 | 31 |
| Single | 24 | 10 |
| Separated, divorced, other | 17 | 7 |
| Employment status | ||
| Full or part time | 22 | 9 |
| Retired, homemaker, unemployed | 220 | 90 |
| Other | 3 | 1 |
| Household composition | ||
| Lives alone | 81 | 33 |
| Lives with spouse, partner, or child | 164 | 67 |
| Ethnicity | ||
| White | 232 | 95 |
| Black, Asian | 13 | 5 |
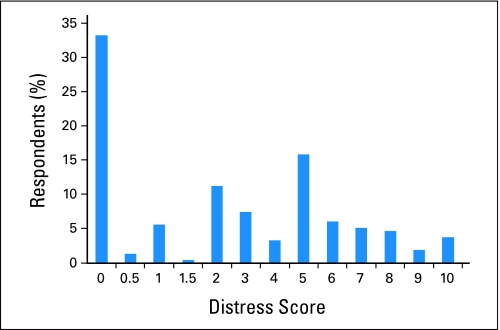
Figure 1 summarizes the distribution of Distress Thermometer scores. The mean score was 3 (SD, 3) and the median score was 2, with a range of scores from zero to 10. Sixty-seven percent of the patients reported a distress score of ≥ 1, whereas 41% scored ≥ 4 on the Distress Thermometer. Participants were asked to circle the number that best described the distress they experienced in the last week. Four patients (1.9%) indicated that their level of distress was between two numbers on the scale (three patients specified a distress level of 0.5 and one patient specified a distress level of 1.5), and their responses were included in the analysis.
Fig 1.
Distribution of Distress Thermometer responses (N = 214).
The association between patient characteristics and distress levels is shown in Table 3. The following categoric variables were significantly associated with increased distress scores: requiring assistance with instrumental activities of daily living (P < .0001), a Karnofsky performance score of < 70 (P = .001), having three or more comorbid medical conditions (P = .047), poor eyesight (P = .002), requiring services at home (P = .052), and needing assistance to complete the geriatric questionnaire (P = .003). The following continuous variables were associated with increased distress scores: requiring more time to complete the questionnaire (P = .01), requiring increased assistance with instrumental activities of daily living (P < .0001), and a lower Medical Outcomes Survey Physical Function score (P < .0001). A multivariate regression model based on the significant bivariate findings was then applied, and it revealed that requiring assistance with instrumental activities of daily living (P = .015) and a lower score on the Medical Outcomes Survey Physical Function scale (P = .018) remained significantly correlated with distress. Patients who required assistance with instrumental activities of daily living had a 2.7 times increased odds of having a distress score of ≥ 4 compared with those who did not require assistance. In addition, each 10-point decrease in the Medical Outcomes Survey Physical Function score (on a scale of zero to 100) was associated with a 1.2 times increased odds of having a distress score ≥ 4. There was no association between the type of therapy received (chemotherapy, endocrine therapy, immunotherapy, or radiation) and a distress score of more than 4.
Table 3.
Predictors of Distress
| Variable | No. of Patients | Distress Score ≥ 4 |
P | |
|---|---|---|---|---|
| No. | % | |||
| Sex | ||||
| Female | 155 | 67 | 43 | .215 |
| Male | 59 | 20 | 34 | |
| Age, years | ||||
| 65-80 | 157 | 65 | 41 | .712 |
| ≥ 81 | 57 | 22 | 39 | |
| Cancer stage | ||||
| Localized | 135 | 55 | 41 | .968 |
| Metastatic | 78 | 32 | 41 | |
| Education | ||||
| ≤ High school | 121 | 54 | 45 | .242 |
| Postsecondary school | 85 | 31 | 36 | |
| Marital status | ||||
| Not married | 101 | 45 | 45 | .296 |
| Married | 112 | 42 | 38 | |
| Living companion | ||||
| Lives alone | 144 | 62 | 43 | .305 |
| Lives with spouse, partner, or child | 70 | 25 | 36 | |
| Employment | ||||
| Employed | 19 | 4 | 21 | .063 |
| Not employed | 193 | 83 | 43 | |
| Use of services | ||||
| Used no services | 182 | 69 | 37 | .052 |
| Used services | 32 | 18 | 56 | |
| Eyesight | ||||
| Excellent, good, or fair | 206 | 80 | 39 | .002 |
| Poor | 7 | 7 | 100 | |
| Hearing health | ||||
| Excellent, good, or fair | 192 | 77 | 40 | .414 |
| Poor | 18 | 9 | 50 | |
| OARS IADL score | ||||
| < 14 | 98 | 59 | 60 | < .0001 |
| 14 | 108 | 25 | 23 | |
| Falls in previous 6 months | ||||
| No falls | 168 | 64 | 38 | .120 |
| Experienced falls | 43 | 22 | 51 | |
| Ability to complete questionnaire alone | ||||
| No | 47 | 28 | 60 | |
| Yes | 163 | 58 | 36 | .003 |
| No. of comorbid illnesses | ||||
| ≤ 2 | 105 | 35 | 33 | .047 |
| ≥ 3 | 107 | 50 | 47 | |
| Unintentional weight loss in previous 6 months | ||||
| None | 132 | 49 | 38 | .097 |
| Experienced weight loss | 69 | 34 | 49 | |
| KPS | ||||
| 40, 50, 60 | 37 | 24 | 65 | .001 |
| 70, 80, 90, 100 | 176 | 62 | 35 | |
| Continuous Variable | No. of Patients | Mean Values for Distress Scores 0-4 | Mean Values for Distress Score ≥ 4 | P |
|---|---|---|---|---|
| Age, years | 214 | 75.83 | 76.12 | .75 |
| Body mass index | 210 | 26.67 | 27.51 | .30 |
| OARS IADL (scale 0-14) | 206 | 13.20 | 11.35 | < .0001 |
| MOS Physical (scale 0-100) | 214 | 73.30 | 49.91 | < .0001 |
| MOS Tangible (scale 0-100) | 212 | 70.58 | 72.80 | .61 |
| MOS Emotional (scale 0-100) | 211 | 80.80 | 78.45 | .55 |
| MOS Social Support (scale 0-100) | 211 | 77.39 | 76.55 | .82 |
| Time to complete questionnaire (minutes) | 197 | 13.31 | 17.56 | .01 |
| No. of medications | 210 | 4.77 | 5.28 | .33 |
NOTE. Only patients with a reported distress score were included in this analysis.
Abbreviations: OARS, Older American Resources and Services; IADL, Instrumental Activities of Daily Living; KPS, Karnofsky performance score; MOS, Medical Outcomes Study.
DISCUSSION
Across the age spectrum, various factors contribute to distress. However, few studies have specifically evaluated the factors contributing to distress in older adults with cancer. In our study cohort of older adults with cancer, significant distress (score ≥ 4 on the Distress Thermometer) was identified in 41% of patients. By using a brief, comprehensive, self-administered geriatric assessment questionnaire, we were able to explore the relationship between distress and several variables that independently predict morbidity and mortality in older adults, such as functional status, comorbid medical condition, nutritional state, and social support. The following variables were significantly associated with increased distress scores by bivariate analysis: poor physical functioning, having more than three comorbid medical conditions, poor eyesight, requiring services at home, and needing more time or requiring assistance to complete the geriatric questionnaire. A multivariate regression model based on the significant bivariate findings revealed that poor physical function correlated significantly with distress.
Previous studies that used the distress thermometer reported distress scores of ≥ 4 in 42.5% to 66.6% of patients with cancer.26,28–33 These studies were performed in patients of all ages. Risk factors for distress included younger age33; female sex29,31,34; poor performance status27,29; and physical,28,29,32,33 emotional,27–29,32–34 family,28,29 and cognitive33 problems. Our finding of distress in 41% of older adults with cancer is slightly lower than the prevalence reported in other studies, reinforcing the findings in previous reports that demonstrate that younger age is a risk for distress and therefore older age may be protective.
The study results also help to pinpoint the unique causes of distress that face older adults with cancer. In particular, loss of independence is a key risk factor contributing to distress. This finding is consistent with the risk factors for distress reported in the geriatric literature for patients without cancer4,6–8 but are particularly relevant for patients with cancer, given that the burden of cancer or cancer therapy can further jeopardize an older adult's physical independence. The impact of therapy on a patient's ability to maintain independence plays a key role in the decision about whether a patient will proceed with treatment. This was illustrated in a survey of older adults in which the majority reported that they would refuse potentially life sustaining therapy if that therapy would cause functional or cognitive decline.35 Functional decline is often associated with feeling that one is a burden to others.36 This is especially true among patients who are terminally ill.36,37 In a report of 43 patients (median age, 70 years) who requested physician-assisted suicide in Oregon, the most frequent underlying illness was cancer (72%), and the most common end-of-life concerns were loss of autonomy (79%) and the inability to participate in activities that make life enjoyable (77%).38
In daily oncology practice, functional status is reported as a Karnofsky performance score39 or Eastern Cooperative Oncology Group40 performance status. These brief scales of functional status predict overall survival and treatment morbidity, but they do not evaluate a patient's ability to complete specific daily activities, nor do they provide information about the impact of functional decline on someone's mental health. This study highlights the importance of asking these questions in daily practice. Studies of interventions aimed at improving or assisting with physical function in order to decrease distress in older patients with cancer need to be conducted. In addition, research into the longitudinal impact of cancer and cancer therapy on an older patient's physical function is warranted for patients and physicians to weigh the risks and benefits of cancer therapies.
We recognize the limitations of this study. First, the study consisted of a convenience sample of patients seen in an outpatient oncology practice and the majority of study participants were female (71%), white (95%), diagnosed with breast cancer (41%), and treated at a tertiary care cancer center. This may limit the ability to generalize these results to all adults with cancer. Second, the time from initial diagnosis and the timing since initiation of specific cancer therapies were not captured. The current or recent receipt of chemotherapy may influence the distress level. Third, these data were obtained by self-report, and it is possible that patients may have over- or underestimated their own abilities on certain self-report measures. In addition, there may be domains other than those evaluated in the geriatric assessment questionnaire that are predictors of distress. We did not capture a problem list, which is often used in association with the Distress Thermometer, and this might have provided further insight into causes of patient distress. Finally, we do not know whether distress or declines in physical function are directly due to cancer or another specific comorbid medical condition.
Despite these limitations, this study has important strengths. Few studies have specifically evaluated predictors of distress in older adults with cancer, a population that is expected to grow rapidly in the next 25 years. Our study demonstrates that a significant proportion of patients (41%) score above the threshold for psychological distress and that poorer physical function is associated with increased distress levels. From these results, we conclude that screening tools to evaluate an individual's distress level and physical function should be incorporated into daily oncology practice, and interventions should be put in place to maintain or assist with physical functioning. Ultimately, further research is needed to determine whether these interventions would decrease the distress and suffering that accompanies a cancer diagnosis in older adults.
Footnotes
Supported by a Paul Beeson Career Development Award in Aging Research (K23 AG026749-01; A.H.) and the American Society of Clinical Oncology Association of Specialty Professors Junior Development Award in Geriatric Oncology.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Arti Hurria, Enid Zuckerman, Mark Lachs
Financial support: Arti Hurria
Administrative support: Arti Hurria, Mark Lachs
Provision of study materials or patients: Arti Hurria, Stuart M. Lichtman, Paul Hamlin, William P. Tew, Enid Zuckerman
Collection and assembly of data: Arti Hurria, Daneng Li, Kurt Hansen, Ravi Gupta, Stuart M. Lichtman, William P. Tew, Enid Zuckerman, Jonathan Gardes, Sewanti Limaye, Eva Kelly
Data analysis and interpretation: Arti Hurria, Daneng Li, Sujata Patil, Ravi Gupta, Christian Nelson, Jonathan Gardes
Manuscript writing: Arti Hurria, Daneng Li, Sujata Patil, William P. Tew
Final approval of manuscript: Arti Hurria, Daneng Li, Kurt Hansen, Sujata Patil, Christian Nelson, Stuart M. Lichtman, William P. Tew, Paul Hamlin, Enid Zuckerman, Jonathan Gardes, Mark Lachs
REFERENCES
- 1.Söllner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counselling? Br J Cancer. 2001;84:179–185. doi: 10.1054/bjoc.2000.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland JC, Bultz BD. The NCCN guideline for distress management: A case for making distress the sixth vital sign. J Natl Compr Cancer Netw. 2007;5:3–7. [PubMed] [Google Scholar]
- 3.Ries LAG, Harkins D, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2006. SEER Cancer Statistics Review, 1975-2003. [Google Scholar]
- 4.Couture M, Larivière N, Lefrancois R. Psychological distress in older adults with low functional independence: A multidimensionalperspective. Arch Gerontol Geriatr. 2005;41:101–111. doi: 10.1016/j.archger.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Blazer D, Hughes DC, George LK. The epidemiology of depression in an elderly community population. Gerontologist. 1987;27:281–287. doi: 10.1093/geront/27.3.281. [DOI] [PubMed] [Google Scholar]
- 6.Preville M, Hebert R, Bravo G, et al. Predisposing and facilitating factors of severe psychological distress among frail elderly adults. Can J Aging. 2002;21:195–204. [Google Scholar]
- 7.Reich JW, Zautra AJ, Guarnaccia CA. Effects of disability and bereavement on the mental health and recovery of older adults. Psychol Aging. 1989;4:57–65. doi: 10.1037//0882-7974.4.1.57. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW, Beekman AT, Ormel J, et al. Psychological status among elderly people with chronic diseases: Does type of disease play a part? J Psychosom Res. 1996;40:521–534. doi: 10.1016/0022-3999(95)00620-6. [DOI] [PubMed] [Google Scholar]
- 9.Stafford RS, Cyr PL. The impact of cancer on the physical function of the elderly and their utilization of health care. Cancer. 1997;80:1973–1980. doi: 10.1002/(sici)1097-0142(19971115)80:10<1973::aid-cncr15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, Norredam M, Landrum MB, et al. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005;53:2145–2152. doi: 10.1111/j.1532-5415.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 11.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 12.Yancik R, Ries LA. Aging and cancer in America: Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. 2000;14:17–23. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 13.Frasci G, Lorusso V, Panza N, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18:2529–2536. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- 14.Welch HG, Albertsen PC, Nease RF, et al. Estimating treatment benefits for the elderly: The effect of competing risks. Ann Intern Med. 1996;124:577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 15.Stewart AL, Ware JE. Durham, NC: Duke University Press; 1992. Measuring functioning and well-being: The Medical Outcomes Study approach. [Google Scholar]
- 16.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 17.Loprinzi CL, Laurie JA, Wieand HS, et al. Prospective evaluation of prognostic variables from patient-completed questionnaires: North Central Cancer Treatment Group. J Clin Oncol. 1994;12:601–607. doi: 10.1200/JCO.1994.12.3.601. [DOI] [PubMed] [Google Scholar]
- 18.Naeim A, Reuben D. Geriatric syndromes and assessment in older cancer patients. Oncology (Williston Park) 2001;15:1567–1577. 1580. discussion 1581, 1586, 1591. [PubMed] [Google Scholar]
- 19.Roth AJ, Kornblith AB, Batel-Copel L, et al. Rapid screening for psychologic distress in men with prostate carcinoma: A pilot study. Cancer. 1998;82:1904–1908. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 21.Landi F, Onder G, Gambassi G, et al. Body mass index and mortality among hospitalized patients. Arch Intern Med. 2000;160:2641–2644. doi: 10.1001/archinte.160.17.2641. [DOI] [PubMed] [Google Scholar]
- 22.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients: Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Yanez D, Harris T, et al. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 24.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: A feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 25.Hurria A, Lichtman SM, Gardes J, et al. Identifying vulnerable older adults with cancer: Integrating geriatric assessment into oncology practice. J Am Geriatr Soc. 2007;55:1604–1608. doi: 10.1111/j.1532-5415.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman BM, Zevon MA, D'Arrigo MC, et al. Screening for distress in cancer patients: The NCCN rapid-screening measure. Psychooncology. 2004;13:792–799. doi: 10.1002/pon.796. [DOI] [PubMed] [Google Scholar]
- 27.Akizuki N, Akechi T, Nakanishi T, et al. Development of a brief screening interview for adjustment disorders and major depression in patients with cancer. Cancer. 2003;97:2605–2613. doi: 10.1002/cncr.11358. [DOI] [PubMed] [Google Scholar]
- 28.Ransom S, Jacobsen PB, Booth-Jones M. Validation of the Distress Thermometer with bone marrow transplant patients. Psychooncology. 2006;15:604–612. doi: 10.1002/pon.993. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494–1502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- 30.Bulli F, Miccinesi G, Maruelli A, et al. The measure of psychological distress in cancer patients: The use of Distress Thermometer in the Oncological Rehabilitation Center of Florence. Support Care Cancer. doi: 10.1007/s00520-008-0543-9. epub ahead of print on December 3, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Bauwens S, Baillon C, Distelmans W, et al. The ‘Distress Barometer’: Validation of method of combining the Distress Thermometer with a rated complaint scale. Psychooncology. 2009;18:534–542. doi: 10.1002/pon.1425. [DOI] [PubMed] [Google Scholar]
- 32.Tuinman MA, Gazendam-Donofrio SM, Hoekstra-Weebers JE. Screening and referral for psychosocial distress in oncologic practice: Use of the Distress Thermometer. Cancer. 2008;113:870–878. doi: 10.1002/cncr.23622. [DOI] [PubMed] [Google Scholar]
- 33.Graves KD, Arnold SM, Love CL, et al. Distress screening in a multidisciplinary lung cancer clinic: Prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55:215–224. doi: 10.1016/j.lungcan.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keir ST, Calhoun-Eagan RD, Swartz JJ, et al. Screening for distress in patients with brain cancer using the NCCN′s rapid screening measure. Psychooncology. 2008;17:621–625. doi: 10.1002/pon.1271. [DOI] [PubMed] [Google Scholar]
- 35.Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 36.McPherson CJ, Wilson KG, Murray MA. Feeling like a burden to others: A systematic review focusing on the end of life. Palliat Med. 2007;21:115–128. doi: 10.1177/0269216307076345. [DOI] [PubMed] [Google Scholar]
- 37.Wilson KG, Curran D, McPherson CJ. A burden to others: A common source of distress for the terminally ill. Cogn Behav Ther. 2005;34:115–123. doi: 10.1080/16506070510008461. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan AD, Hedberg K, Fleming DW. Legalized physician-assisted suicide in Oregon—the second year. N Engl J Med. 2000;342:598–604. doi: 10.1056/NEJM200002243420822. [DOI] [PubMed] [Google Scholar]
- 39.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 40.Zubrod C, Schneiderman M, Frei E. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11:7–33. [Google Scholar]