Abstract
Purpose
To investigate the long-term impact of pathologic characteristics and an extra boost dose of 16 Gy on local relapse, for stage I and II invasive breast cancer patients treated with breast conserving therapy (BCT).
Patients and Methods
In the European Organisation for Research and Treatment of Cancer boost versus no boost trial, after whole breast irradiation, patients with microscopically complete excision of invasive tumor, were randomly assigned to receive or not an extra boost dose of 16 Gy. For a subset of 1,616 patients central pathology review was performed.
Results
The 10-year cumulative risk of local breast cancer relapse as a first event was not significantly influenced if the margin was scored negative, close or positive for invasive tumor or ductal carcinoma in situ according to central pathology review (log-rank P = .45 and P = .57, respectively). In multivariate analysis, high-grade invasive ductal carcinoma was associated with an increased risk of local relapse (P = .026; hazard ratio [HR], 1.67), as was age younger than 50 years (P < .0001; HR, 2.38). The boost dose of 16 Gy significantly reduced the local relapse rate (P = .0006; HR, 0.47). For patients younger than 50 years old and in patients with high grade invasive ductal carcinoma, the boost dose reduced the local relapse from 19.4% to 11.4% (P = .0046; HR, 0.51) and from 18.9% to 8.6% (P = .01; HR, 0.42), respectively.
Conclusion
Young age and high-grade invasive ductal cancer were the most important risk factors for local relapse, while margin status had no significant influence. A boost dose of 16 Gy significantly reduced the negative effects of both young age and high-grade invasive cancer.
INTRODUCTION
Breast conserving therapy (BCT) is now a standard therapeutic option for the treatment of many women with early-stage invasive breast cancer and is a preferred treatment to mastectomy because of the cosmetic benefit of breast preservation.1–5 Four percent to 15% of patients with early-stage breast carcinoma treated with BCT will have a local relapse within 10 years.1–10 We and Romestaing et al11–13 demonstrated that a higher radiation dose to the tumor bed significantly reduced the local relapse rate. The Early Breast Cancer Trialists Collaborative Group meta-analysis demonstrated that preventing breast recurrences improves survival.14 Thus, minimizing the risk of local relapse in patients choosing BCT remains an important clinical objective.
There is increasing recognition of the complex role prognostic factors other than those included in the conventional staging system have in determining the risk of local relapse after BCT. Integrating and interpreting these relationships between prognostic factors such as margin status, age, histologic grade, size of primary tumor, number of positive nodes, HER2 status, peritumoral vascular invasion, and other such clinicopathological features will provide a useful platform for interpreting the risk of local recurrence and additionally provide guidance as to the role of adjuvant therapy and the intensity of such adjuvant therapy. Several publications indicate that the final margin status ranks among the pathologic risk factors most strongly associated with local relapse.9–23 This association has also been reported in three prospective randomized trials that compared BCT with mastectomy in early-stage breast cancer patients.24–25 Several factors have been associated with a reduced risk for local relapse in patients with positive margins such as focally positive margins compared to more extensively involved margins.18,19,21 These patients have been considered candidates for BCT, particularly in the absence of an associated extensive intraductal component (EIC).26 A higher total dose of radiation to the tumor bed has been found to decrease the risk of local relapse associated with positive margins in some series,27–33 but not in others.18,20,34 To address these complex issues, a subset analysis of patients with completely excised tumors entered in the first years of the European Organisation for Research and Treatment of Cancer (EORTC) trial 22881/10882 was performed. Pathologic factors including the margin status were assessed by central pathology review. The purpose of the study was to examine each of these pathologic features as prognostic factors on the subsequent risk of local relapse after BCT with or without a16-Gy boost.
PATIENTS AND METHODS
The EORTC 22881/10882 boost versus no boost trial accrued 5,569 patients from 1989 to 1996. The main objective of the trial was to assess the effect of the boost dose in early -stage breast cancer patients treated with BCT. All patients underwent lumpectomy and axillary dissection followed by whole breast irradiation (WBI; total dose of 50 Gy in 5 weeks with a dose per fraction of 2 Gy). After informed consent, patients with microscopically complete excision (negative margin for the invasive component) were randomly assigned to receive WBI with either no boost or a boost of 16 Gy on the tumor bed. Patients with microscopically incomplete excision (positive margins for the invasive component) received WBI of 50 Gy to the breast and were randomly assigned to receive an extra boost dose of 10 or 26 Gy to the tumor bed. Details of this trial have been published previously.11–12,34 Data with a median follow-up time of 10 years were used for this analysis.
Pathology Review
From the first years of the accrual period (1989 to 1996), pathology slides from 1,616 patients (which represent 30% of the whole population) with clinical stage I or II breast cancer were centrally collected. The pathologic characteristics and the status of the surgical margins were determined by one pathologist (H.L.P.). Tumors were scored according to their proximity to the inked surgical margin. Thus, margins status was defined as followed: a positive surgical margin as tumor (invasive or ductal carcinoma in situ [DCIS]) seen immediately at the inked edge of the resection, a close margin as tumor (invasive or DCIS) seen at 2 mm or less from the inked resection edge, and a negative margin as greater than 2 mm of tumor free margin from the inked resection edge or no residual tumor on re-excision. The extent of invasive tumor positivity at the margin was not recorded. The extent of DCIS was estimated by counting the number of ducts involved with DCIS in the breast tissue adjacent to the primary tumor. The DCIS component was considered as minimal if three or fewer ducts were involved, as moderate if four to nine ducts were involved, and as extensive if 10 or more ducts were involved. DCIS tumors with focal areas of invasion were classified as invasive carcinomas with an EIC. For 1,494 patients, the margin status could be scored for involvement of invasive cancer. In 805 patients with invasive tumor and a DCIS component, the margin for DCIS could be scored. The histologic grade of all 1,616 invasive tumors was defined according to the Elston/Ellis modification of the Bloom-Richardson system35 and the histologic grade of the DCIS component was classified as low, intermediate, or high.36 Mitotic activity index was also scored and represented the number of mitoses in 10 consecutive high power fields of 0.045 mm2. Vascular invasion was considered to be present when distinct tumor emboli were seen in at least three endothelium-lined (blood or lymphatic) vessels in breast tissue surrounding the tumor.
Statistical Analysis
Differences in patient, treatment, and tumor characteristics (ie, menopausal status, size of biopsy specimen, nodal status, estrogen and progesterone receptor status, DCIS, systemic treatment [chemo- or hormone therapy], and histologic grade) between margin status categories were investigated with Kruskal-Wallis test for continuous and the χ2 test for categoric variables.
To investigate the association between margin status and local failure, univariate and multivariate survival analysis was performed with Cox proportional hazard models, which takes censoring into account. Time was calculated from the random assignment date. An event was defined as first recurrence in the ipsilateral breast. All other patients were censored at the time of another breast cancer event, death from any cause, or at last follow-up. Age was calculated from date of birth until date of random assignment. Patients were excluded from a given analysis, if the data for the required factor was missing. Local failure was defined as disease recurrence in the treated breast. Hazard ratio (HR) and 95% CI are presented. At the time of this analysis, 126 local failures were observed in this patient subset of completely excised tumors according to the local pathologist, 81 in the no boost, and 45 in the 16-Gy boost, respectively. Because of the exploratory nature and the limited statistical power, no formal correction for multiple comparisons was applied. Thus, P values less than .01 were considered statistically significant. All analyses were performed using SAS version 9.1 (SAS Inc, Bethesda, MD).
RESULTS
In the 1,616 patients randomly assigned after complete resection according to local pathology, the central pathology review revealed that the final resection margins for invasive tumors were negative in 1,137 patients (76%), close in 306 patients (20%), and positive in 51 patients (3.4%; Table 1). The final resection margins were negative for DCIS in 478 (59%), close in 216 patients (27%), and positive in 111 patients (14%; Table 2). Compared to patients with negative margins, the patients with close margins were younger and had different histologic tumor features, such as lobular cancer, slightly more DCIS component, and had a higher risk of regional nodal involvement. A minority of the patients received adjuvant hormone therapy (22.2%) or adjuvant chemotherapy (15.7%); however, patients with invasive tumor involving the margins or close to the margins were more likely to receive chemotherapy (P = .004). There were a few patients in any subgroup with EIC-positive tumor, which was associated with DCIS involved margins (Table 2). High-grade invasive tumors were more common in patients who were younger, had a larger tumor, those with positive lymph nodes, and in those who received systemic treatment (Table 3). A total of 126 recurrences was found in the breast as a first event. Two of these local failures were diagnosed concurrently with distant metastases. These local failures were also scored as primary event.
Table 1.
Population Characteristics by Margins for Invasive Cancer According to Central Pathology Review
| Parameter | Margin for Invasive Tumor |
P | |||||
|---|---|---|---|---|---|---|---|
| Positive |
Close |
Free |
|||||
| No. | % | No. | % | No. | % | ||
| No. of patients | 51 | 306 | 1,137 | ||||
| Complete WBI, 50 Gy | |||||||
| No boost | 27 | 53 | 160 | 52 | 549 | 48 | .4003 |
| Boost 16 Gy | 24 | 47 | 146 | 48 | 588 | 52 | |
| Volume of excision biopsy specimen, cm3 | |||||||
| Median | 104.8 | 108.0 | 120.0 | .04056 | |||
| Range | 7.9-540.0 | 4.5-1,800.0 | 1.3-1,680.0 | ||||
| Largest diameter, mm | |||||||
| Median | 15.0 | 17.0 | 15.0 | .00163 | |||
| Range | 5.0-35.0 | 4.0-50.0 | 2.0-50.0 | ||||
| Age at random assignment, years | |||||||
| Median | 54 | 51.5 | 55 | .01788 | |||
| Range | 29-69 | 27-71 | 28-76 | ||||
| Younger than 50 | 19 | 37 | 132 | 43 | 375 | 33 | .0088 |
| 50-60 | 17 | 33 | 106 | 35 | 411 | 36 | |
| Older than 60 | 15 | 29 | 68 | 22 | 351 | 31 | |
| N+ according to local pathology | 12 | 24 | 79 | 26 | 242 | 21 | .2167 |
| Postmenopausal | 33 | 65 | 164 | 54 | 718 | 63 | .0084 |
| Receptor status | |||||||
| Estrogen+ | 22 | 71 | 145 | 67 | 621 | 73 | .1628 |
| Progesterone+ | 25 | 83 | 128 | 67 | 472 | 62 | .0330 |
| Systemic treatment | 21 | 41 | 120 | 39 | 379 | 33 | .0992 |
| Mitotic activity index | |||||||
| < 10 | 40 | 80 | 203 | 67 | 831 | 74 | .0967 |
| 10-19 | 4 | 8 | 51 | 17 | 142 | 13 | |
| ≥ 20 | 6 | 12 | 51 | 17 | 156 | 14 | |
| Histology | |||||||
| Ductal | 28 | 56 | 207 | 68 | 839 | 74 | .0006 |
| Lobular | 9 | 18 | 20 | 7 | 55 | 5 | |
| Mixed pattern | 11 | 22 | 58 | 19 | 162 | 14 | |
| Other | 2 | 4 | 20 | 7 | 74 | 7 | |
| Differentation of the invasive tumor | |||||||
| Low | 28 | 60 | 143 | 48 | 561 | 51 | .2737 |
| Intermediate | 9 | 19 | 73 | 24 | 292 | 26 | |
| High | 10 | 21 | 83 | 28 | 250 | 23 | |
| Extensive intraductal component | 8 | 16 | 39 | 13 | 111 | 10 | .1552 |
| Differentiation of DCIS | |||||||
| Low | 10 | 20 | 64 | 21 | 173 | 15 | .0788 |
| Intermediate | 7 | 14 | 78 | 25 | 277 | 24 | |
| High | 7 | 14 | 42 | 14 | 145 | 13 | |
| No DCIS | 27 | 53 | 122 | 40 | 540 | 47 | |
| Vascular invasion | |||||||
| None | 37 | 74 | 220 | 72 | 866 | 77 | .2843 |
| Present | 10 | 20 | 50 | 16 | 152 | 14 | |
| Doubtful | 3 | 6 | 35 | 11 | 107 | 10 | |
NOTE. Patients were randomly assigned according to local pathology.
Abbreviations: WBI, whole breast irradiation; DCIS, ductal carcinoma in situ.
Table 2.
Population Characteristics by Margins for DCIS According to Central Pathology Review
| Parameter | Margin at DCIS |
P | |||||
|---|---|---|---|---|---|---|---|
| Positive |
Close |
Negative |
|||||
| No. | % | No. | % | No. | % | ||
| No. of patients | 111 | 216 | 478 | ||||
| Volume of excision biopsy specimen, cm3 | |||||||
| Median | 110.0 | 120.0 | 120.0 | .81720 | |||
| Range | 4.5-567.0 | 5.6-1,800.0 | 1.3-1,584.0 | ||||
| Largest diameter, mm | |||||||
| Median | 15.0 | 15.0 | 15.0 | .13729 | |||
| Range | 4.0-40.0 | 4.0-40.0 | 4.0-40.0 | ||||
| Age at random assignment, years | |||||||
| Median | 51 | 52 | 54 | .00997 | |||
| Range | 28-70 | 27-69 | 28-76 | ||||
| Younger than 50 | 52 | 47 | 84 | 39 | 154 | 32 | .0122 |
| 50-60 | 42 | 38 | 78 | 36 | 186 | 39 | |
| Older than 60 | 17 | 15 | 54 | 25 | 138 | 29 | |
| N+ according to local pathology | 30 | 27 | 55 | 26 | 116 | 25 | .8314 |
| Postmenopausal | 57 | 51 | 125 | 58 | 292 | 61 | .1611 |
| Receptor status | |||||||
| Estrogen+ | 54 | 68 | 103 | 64 | 277 | 77 | .0029 |
| Progesterone+ | 44 | 61 | 92 | 63 | 219 | 68 | .3858 |
| Systemic treatment | 46 | 41 | 88 | 41 | 162 | 34 | .1218 |
| Mitotic activity index | |||||||
| < 10 | 75 | 68 | 133 | 62 | 377 | 79 | < .0001 |
| 10-19 | 16 | 14 | 50 | 23 | 60 | 13 | |
| ≥ 20 | 20 | 18 | 32 | 15 | 39 | 8 | |
| Histology | |||||||
| Ductal | 88 | 79 | 177 | 82 | 391 | 82 | .8108 |
| Mixed pattern | 23 | 21 | 39 | 18 | 87 | 18 | |
| Differentation of the invasive tumor | |||||||
| Low | 50 | 46 | 85 | 41 | 247 | 53 | < .0001 |
| Intermediate | 21 | 19 | 66 | 32 | 136 | 29 | |
| High | 38 | 35 | 58 | 28 | 80 | 17 | |
| Extensive intraductal component | 59 | 53 | 46 | 21 | 44 | 9 | < .0001 |
| Differentiation of DCIS | |||||||
| Low | 52 | 47 | 73 | 34 | 122 | 26 | < .0001 |
| Intermediate | 27 | 24 | 91 | 42 | 244 | 51 | |
| High | 32 | 29 | 52 | 24 | 110 | 23 | |
| Vascular invasion | |||||||
| None | 80 | 72 | 142 | 66 | 353 | 74 | .125 |
| Present | 23 | 21 | 45 | 21 | 75 | 16 | |
| Doubtful | 8 | 7 | 29 | 13 | 48 | 10 | |
Abbreviation: DCIS, ductal carcinoma in situ.
Table 3.
Population Characteristics for Histologic Grade According to Central Pathology Review
| Parameter | Histologic Grade of Invasive Carcinoma |
P | |||||
|---|---|---|---|---|---|---|---|
| Low |
Intermediate |
High |
|||||
| No. | % | No. | % | No. | % | ||
| No. of patients | 782 | 395 | 362 | ||||
| Volume of excision biopsy specimen, cm3 | |||||||
| Median | 105.0 | 122.5 | 140.0 | < .00001 | |||
| Range | 4.5-1,584.0 | 1.3-1,616.0 | 9.0-1,800.0 | ||||
| Largest diameter, mm | |||||||
| Median | 15.0 | 15.0 | 20.0 | < .00001 | |||
| Range | 2.0-40.0 | 2.0-45.0 | 5.0-50.0 | ||||
| Age at random assignment, years | |||||||
| Median | 55.0 | 54.0 | 50.0 | < .00001 | |||
| Range | 27.0-76.0 | 28.0-75.0 | 28.0-73.0 | ||||
| Younger than 50 | 231 | 30 | 130 | 33 | 179 | 49 | < .0001 |
| 50-60 | 289 | 37 | 155 | 39 | 107 | 30 | |
| Older than 60 | 262 | 34 | 110 | 28 | 76 | 21 | |
| N+ according to local pathology | 153 | 20 | 101 | 26 | 92 | 25 | .0275 |
| Postmenopausal | 509 | 65 | 241 | 61 | 187 | 52 | .0001 |
| Receptor status | |||||||
| Estrogen+ | 462 | 84 | 228 | 75 | 119 | 42 | < .0001 |
| Progesterone+ | 386 | 79 | 189 | 67 | 81 | 33 | < .0001 |
| Systemic treatment | 243 | 31 | 158 | 40 | 145 | 40 | .0012 |
| Mitotic activity index | |||||||
| < 10 | 778 | 99 | 273 | 69 | 56 | 15 | < .0001 |
| 10-19 | 3 | 0.4 | 119 | 30 | 88 | 24 | |
| ≥ 20 | 1 | 0.1 | 3 | 0.8 | 218 | 60 | |
| Histology | |||||||
| Invasive ductal carcinoma | 422 | 54 | 376 | 95 | 323 | 89 | < .0001 |
| Invasive lobular carcinoma | 90 | 12 | 1 | 0.3 | |||
| Mixed invasive pattern | 211 | 27 | 16 | 4 | 3 | 0.8 | |
| Other | 59 | 8 | 2 | 0.5 | 36 | 10 | |
| Extensive intraductal component | 58 | 7 | 47 | 12 | 53 | 15 | .0004 |
| Differentiation of DCIS | |||||||
| Low | 13 | 2 | 63 | 16 | 161 | 44 | ≤ .0001 |
| Intermediate | 207 | 26 | 143 | 36 | 12 | 3 | |
| High | 168 | 21 | 18 | 5 | 3 | 0.8 | |
| No DCIS | 394 | 50 | 171 | 43 | 186 | 51 | |
| Vascular invasion | |||||||
| None | 665 | 86 | 231 | 59 | 255 | 71 | < .0001 |
| Present | 63 | 8 | 89 | 23 | 69 | 19 | |
| Doubtful | 49 | 6 | 74 | 19 | 33 | 9 | |
Abbreviation: DCIS, ductal carcinoma in situ.
Univariate and Multivariate Analysis of Prognostic Factors for Local Relapse
In the univariate analysis (Table 4), high tumor grade of invasive tumor and young age (younger than 50 years) were predictive for an increased risk of local relapse, whereas adjuvant hormone or chemotherapy and an additional boost on the tumor bed were associated with a decreased risk of local relapse. In the multivariate analysis, the boost dose significantly reduced the local relapse rate (P = .0006; HR, 0.47). The presence of high grade invasive ductal carcinoma was associated with an increased risk of local relapse (P = .026; HR, 1.67), as was age younger 50 years (P < .0001; HR, 2.38). Margin involvement or differentiation grade of DCIS had no significant impact on the risk of local relapse (Table 5).
Table 4.
Univariate Analysis for Local Relapse in Patients With 0 or 16 Gy Boost
| Contrast by Factor | P | Hazard Ratio | 95% CI |
|---|---|---|---|
| Age > 50, yes v no | < .0001 | 0.40 | 0.28 to 0.57 |
| N+ according to local pathology, N+ v N− | .2585 | 0.77 | 0.48 to 1.22 |
| Systemic treatment (chemotherapy or tamoxifen), yes v no | .0088 | 0.57 | 0.38 to 0.87 |
| Volume of excision biopsy specimen, cm3 | .1068 | 1.00 | 1.00 to 1.00 |
| Treatment, no boost v boost 16 Gy | .0008 | 1.86 | 1.29 to 2.68 |
| Vascular invasion, yes v no | .9715 | 0.99 | 0.65 to 1.50 |
| Extensive intraductal component, yes v no | .8975 | 1.04 | 0.57 to 1.90 |
| Histology | |||
| Lobular v ductal | .4451 | 0.72 | 0.32 to 1.66 |
| Mixed pattern v ductal | .3184 | 0.72 | 0.37 to 1.38 |
| Other v ductal | .6439 | 0.88 | 0.50 to 1.54 |
| Histologic grade | |||
| Intermediate v high | .0320 | 0.60 | 0.37 to 0.96 |
| Low v high | .0003 | 0.46 | 0.31 to 0.70 |
| Ductal component involved, yes v no | .5254 | 1.17 | 0.72 to 1.89 |
| Lobular component involved, yes v no | .2482 | 0.73 | 0.43 to 1.24 |
| Mitotic activity index, < 10 v > 10 | .0267 | 0.66 | 0.45 to 0.95 |
| Margin involved of DCIS, not involved v involved | .3710 | 0.82 | 0.53 to 1.27 |
| Margin involved of DCIS/invasive tumor, not involved v involved | .6779 | 0.92 | 0.64 to 1.34 |
| Margin involved of invasive tumor, not involved v involved | .4127 | 1.20 | 0.78 to 1.84 |
| Margin of DCIS | |||
| No DCIS v positive | .4862 | 0.78 | 0.38 to 1.58 |
| Close v positive | .9276 | 0.97 | 0.46 to 2.03 |
| Negative v positive | .8362 | 0.93 | 0.48 to 1.81 |
| Grade of DCIS | |||
| No DCIS v high grade | .9640 | 1.02 | 0.51 to 2.03 |
| Low v high | .1826 | 1.57 | 0.81 to 3.06 |
| Moderate v high | .7437 | 1.12 | 0.58 to 2.16 |
| Margin of invasive tumor | |||
| Negative v close | .6589 | 1.11 | 0.71 to 1.73 |
| Incomplete v close | .3191 | 0.48 | 0.11 to 2.03 |
| Estrogen positive, yes v no | .0330 | 0.63 | 0.41 to 0.96 |
| Progesterone positive, yes v no | .4286 | 0.84 | 0.54 to 1.30 |
Abbreviation: DCIS, ductal carcinoma in situ.
Table 5.
Multivariable Analysis of Time to Local Relapse for All Patients
| Parameter | P | Hazard for Local Failure |
|
|---|---|---|---|
| Estimate | 95% CI | ||
| Randomized treatment 50 Gy WBI, 0 Gy v 16 Gy | .0006 | 0.47 | 0.31 to 0.73 |
| Age, > 50 v ≤ 50 years | < .0001 | 0.42 | 0.28 to 0.65 |
| Systemic treatment, yes v no | .088 | 0.66 | 0.41 to 1.06 |
| Differentiation grade of the invasive tumor, high v low/intermediate | .026 | 1.67 | 1.06 to 2.62 |
| Differentiation grade of DCIS | |||
| High v low/intermediate | .96 | 1.02 | 0.54 to 1.93 |
| No DCIS v high | .39 | 0.80 | 0.48 to 1.33 |
| Margin of invasive tumor, not involved v involved | .33 | 0.78 | 0.49 to 1.27 |
Abbreviations: WBI, whole breast irradiation; DCIS, ductal carcinoma in situ.
Final Margin Status, Age, and Grade of the Tumor
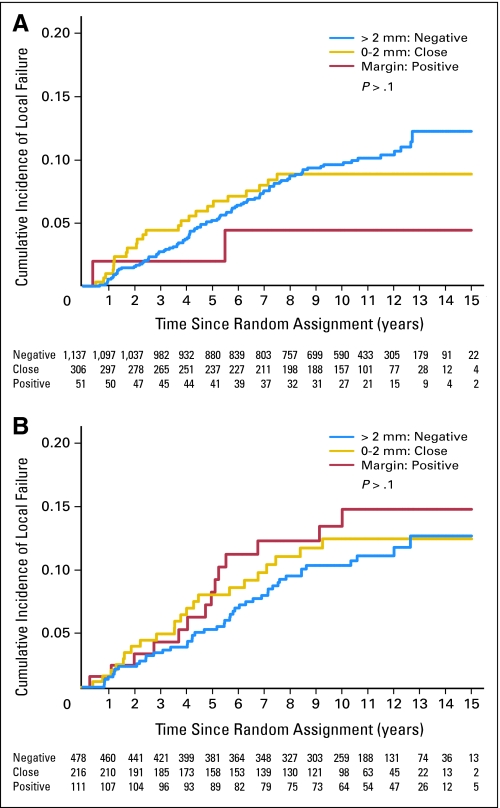
The cumulative local relapse rate as first event at 10 years was not significantly influenced by the margin status (negative, close, or positive) for invasive tumor or DCIS (Fig 1). The cumulative incidence of local relapse at 10 years was higher for patients younger than 50 years old (14.8%; 95% CI, 11.6 to 18.3) compared with patients who were 50 years or older (6.2%; 95% CI, 4.5 to 8.0; P < .001). The cumulative incidence of local relapse at 10 years was 7.3% (95% CI, 5.2 to 9.5), 8.4% (95% CI, 5.3 to 12.0), and 13.7% (95% CI, 9.7 to 17.9) for low-, intermediate-, and high-grade invasive ductal cancer, respectively (P = .0003).
Fig 1.
Cumulative incidence of local failure as first event according to the margins for (A) invasive tumor and for (B) ductal carcinoma in situ.
Influence of the 16-Gy Boost Dose and the Final Margin Status
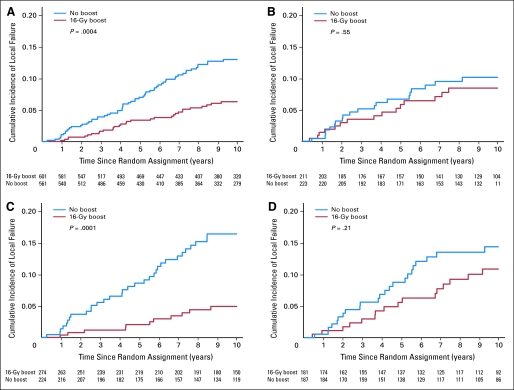
In patients with no invasive tumor at the margin and in those with no DCIS at the margin, the additional boost of 16 Gy significantly reduced the local relapse rate (P = .0004; HR, 0.47; P = .0003; HR, 0.30), while for patients with close or positive margins the effect of the boost was not significant (P = .65, P = .25, respectively for invasive cancer and DCIS).
Influence of the 16-Gy Boost in High-Risk Patients
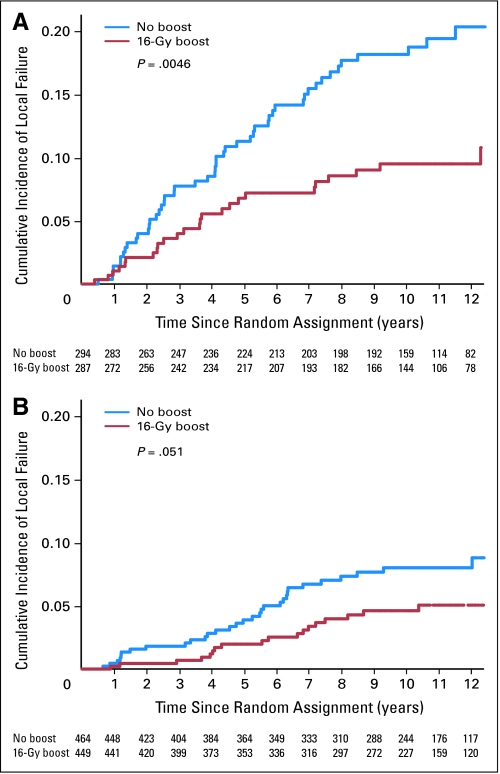
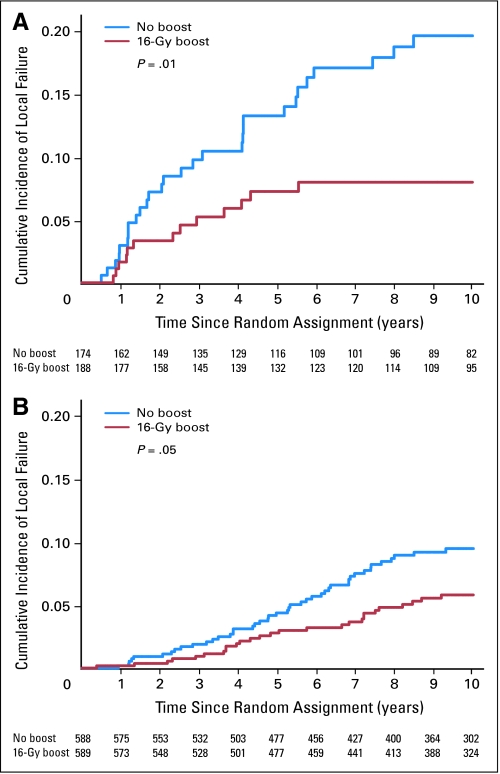
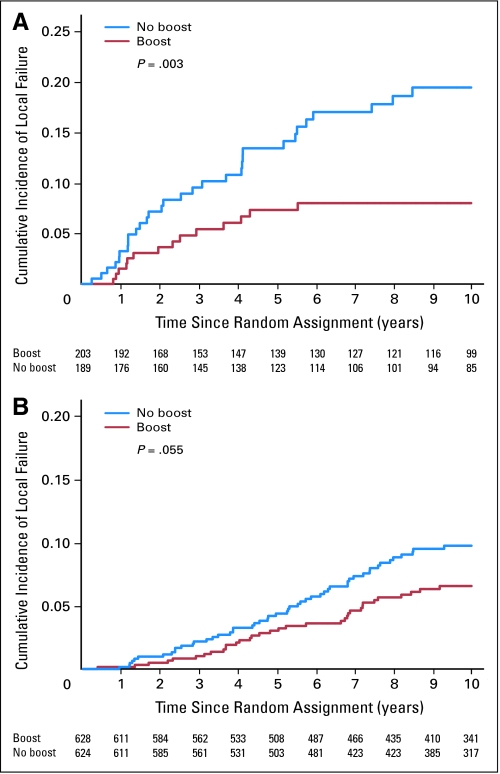
The multivariate analysis indicated that both young patients and high-grade invasive cancer were independently associated with an increased risk of local relapse. For patients younger than 50 years, the boost dose reduced the 10-year cumulative local relapse rate from 19.4% to 11.4% (P = .0046; HR, 0.51; Fig 2). For patients with high-grade invasive ductal carcinoma, the boost dose reduced the cumulative 10-year local relapse rate from 18.9% to 8.6% (P = .01; HR, 0.42; Fig 3).
Fig 2.
Cumulative incidence of local failure by boost group and age. (A) Patients younger than 51 years (P = .0046). (B) Patients older than 50 years (P = .051).
Fig 3.
Cumulative incidence of local failure by boost group and grading of invasive ductal carcinoma. (A) High grade of invasive carcinoma (P = .01). (B) Low/intermediate grade of invasive carcinoma (P = .05).
Additional Analysis of the Entire Central Pathology Review Cohort
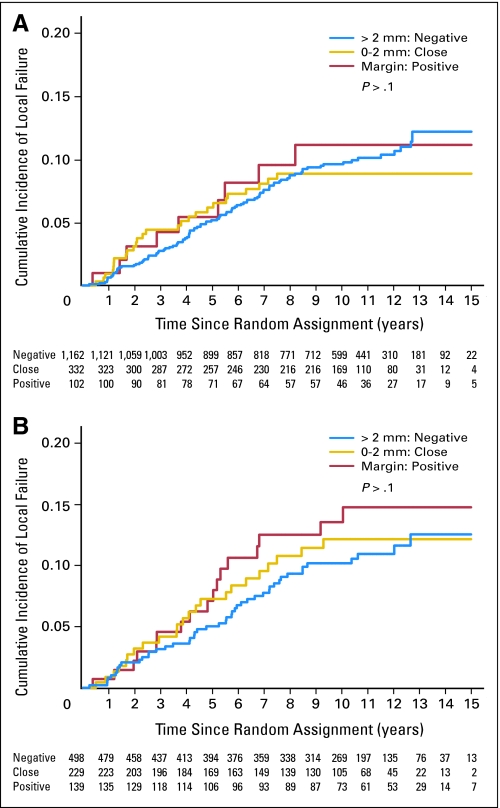
Of note, 108 patients who had an incomplete tumor resection, and therefore were included in the 10 Gy versus 26 Gy boost section of the boost no boost trial, were also part of the central pathology review, but were not included in the analysis presented. A secondary analysis including the entire cohort of 1,724 (1,616 completely resected patients and 108 incomplete resected patients, according to the local pathologist) did not change the major findings (Appendix Tables A1 to A4 and Appendix Figs A1 to A3, online only).
DISCUSSION
This study with central pathology review, suggested that patients younger than 50 years and/or with high-grade breast cancer should be considered at high risk for local relapse. An additional boost dose of 16 Gy however significantly reduced the risk for local relapse in those high-risk patients as well as in other patients with negative margins. Close or positive tumor margin status had no significant effect on local relapse this, however, may in part be explained by the fact that a limited number of patients with involved margins with invasive tumor entered this randomized clinical trial.
Optimizing local control after BCT remains an important clinical issue, especially as the Early Breast Cancer Trialists Collaborative Group showed that reducing local relapse rate reduces breast cancer mortality.14 We have previously reported that an additional boost of 16 Gy to the primary tumor bed significantly decreases the 10-year cumulative local relapse rate from 10.2% to 6.2% (P < .001) in the whole patient population of the EORTC 22881/10882 boost no boost trial.12 In this study, one breast cancer pathologist (H.L.P.) reviewed one third of surgical specimens. This included patients who underwent a complete resection and 50 Gy of WBI, followed by no boost versus an additional boost of 16 Gy to the tumor bed, as well as those who underwent incomplete tumor resection and 50 Gy of WBI followed by an additional dose boost of 10 Gy versus 26 Gy to the tumor bed, the latter data are presented in the Appendix (online only). This centralized pathology review provided a unique opportunity to evaluate the relationship between margin status, tumor type, histologic grade, and other prognostic factors, and to identify a subgroup of patients at high risk for local relapse. In addition to the study of clinical and histopathologic prognostic factors, the value of the boost dose to the tumor bed in the high risk group for local relapse could be assessed.
In our study, the additional boost to the primary tumor bed significantly increased local control in patients with negative margins, which is consistent with our previous publication.12 However, a dose-effect on local control could not be confirmed in the case of positive margins whatever the tumor type (DCIS and/or invasive tumors) found at the resection margin. The extent of margin involvement has been described as a prognostic factor of local relapse risk.19,20 However, this was not determined in this study. Several authors have reported that the additional radiation boost can indeed reduce local relapse rates in margin of positive patients.9,37–41 An adjusted radiotherapeutic approach, in which the boost dose is tailored to the margin status, might be expected to optimize local control. Smitt et al18 showed a dose-response effect for doses greater than 66 Gy. Of note, Wazer et al20 noted a lower recurrence rate for higher doses of radiation in case of margin involvement. Pezner et al,42 Vicini et al,43 and Perez et al37 also found that, if close attention is paid to the pathologic assessment of the margins, increasing doses of irradiation for patients with close or positive margins reduced the local relapse rate to those with negative margins. These long-term results indicate that the prognostic significance of the surgical margins status might be negated by margin directed tumor bed dose escalation.13,20,40,44 Based on these previously published results, the EORTC boost trial 22881/10882 evaluated in a separate stratum of the trial those patients who underwent incomplete tumor resection (positive margins) followed by 50 Gy of WBI who were randomly assigned to a boost dose of 10 versus 26 Gy. Although the P value was not significant (probably due to a small sample size), the cohort of patients that received a higher boost dose to the tumor bed seemed to have a lower rate of local relapse than those receiving the lower boost dose of radiation.34
As reported by other authors the identification of risk factors for local relapse among patients after a microscopically complete tumorectomy is of particular interest and importance.21,38 In this study, histologic grade for invasive tumor, as well as age were independent risk factors for the local relapse risk. This is consistent with other studies reporting that patients with high-grade tumors have a worse local control.45,46 A remarkable finding in our study was the ability of the boost to effectively reduce the risk of local relapse in patients with high grade invasive tumors and to a lesser extent in terms of absolute reduction in low or intermediate grade tumors (Fig 3).
The effect of young age on the risk of local relapse for patients treated with BCT has been recognized in many studies using various age cut points.15,46–48 This analysis and previously published result of the central pathology review demonstrated that high grade invasive tumors were more common in the young patients.45 There is also now a suggestion that young breast cancer patients have a different biologic entity.49 The 10-year results of our EORTC 22881/10882 trial demonstrated that the additional dose of 16 Gy had to the largest absolute benefit on local control in young patients.12,48
We fully acknowledge that this analysis is a subset analysis and is beset by all the caveats associated with a subset analysis. Despite biases, we believe that these results have important implications for clinical practice, and may guide in the decision which patients will benefit from a boost dose of 16 Gy. The absolute gain will notably be highest in patients with high-risk factors for local relapse (ie, younger age and high tumor grade). Even for patients older than 60 years having high grade invasive tumor the boost dose does reduce the 10-year cumulative local relapse rate from 10.8% to 4% at (Appendix Table A5, online only). However, the benefit of the boost dose in reducing local relapse has to be weighted against the adverse effects. Our previous study has shown that fibrosis, the major adverse effect of the boost dose, was independent of age. To tailor locoregional treatment, Collette et al50 recently developed a nomogram to assess the risk of fibrosis development for a single patient based on a number of patient-, tumor-, and treatment-related factors.
In summary, the EORTC 22881/10882 trial has demonstrated the benefit of a 16 Gy boost dose of radiation in all patients, irrespective of age.12 The present subset analysis suggests that young age and high-grade invasive ductal cancer were the most important risk factors for local relapse, while margin status had no significant influence. An additional dose of 16 Gy to the tumor bed significantly reduced the negative effects of both young age and high grade invasive tumor.
Acknowledgment
We acknowledge and thank the following for their active participation: R.P. Müller, Cologne, Germany; J. Kurtz, Geneva, Switzerland; D.A.L. Morgan, Nottingham, Great Britain; J.B. Dubois, Montpellier, France; E. Salamon, Namur, Belgium; R.O. Mirimanoff, Lausanne, Switzerland; J.W.H. Leer, Nijmegen, the Netherlands; M. Bolla, Grenoble, France; A. Kuten, Haifa, Israel; A. Renaud, La Louviere, Belgium; U. Schulz, Krefeld, Germany; P.C.M. Koper, Rotterdam, the Netherlands; D. Van den Weyngaert, Antwerp, Belgium; G.A. Storme, Brussels, Belgium; G.H.M. Calitchi, Creteil, France; W. Budach, Berlin, Germany; S. Roth, Dusseldorf, Germany; M. Poulsen, Brisbane, Australia; M.A. Dominguez, Pamplona, Spain; E. Monpetit, Vannes, France; F. Kovner, Tel Aviv, Israel; A. Biete Sola, Barcelona, Spain; Calvo, Madrid, Spain; I. Barillot, Tours, France; J. Borger, Maastricht, the Netherlands.
Appendix
Table A1.
Population Characteristics by Margins for Invasive Cancer According to Central Pathology Review for All Patients
| Parameter | Margin for Invasive Tumor |
P | |||||
|---|---|---|---|---|---|---|---|
| Positive |
Close |
Negative |
|||||
| No. | % | No. | % | No. | % | ||
| No. of patients | 102 | 332 | 1,162 | ||||
| Complete WBI 50 Gy | |||||||
| No boost | 27 | 4 | 160 | 22 | 549 | 75 | |
| Boost 16 Gy | 24 | 3 | 146 | 19 | 588 | 78 | |
| Incomplete WBI 50 Gy and 10 Gy | |||||||
| No extra boost | 27 | 56 | 9 | 19 | 12 | 25 | |
| Extra boost (16 Gy) | 24 | 44 | 17 | 31 | 13 | 24 | |
| Volume of excision biopsy specimen, cm3 | |||||||
| Median | 90 | 105 | 120 | .0038 | |||
| Range | 7.9-540 | 4.5-1,800 | 1.3-1,680 | ||||
| Largest diameter, mm | |||||||
| Median | 15.0 | 17.0 | 15.0 | .00131 | |||
| Range | 5.0-45.0 | 4.0-50.0 | 2.0-50.0 | ||||
| Age at random assignment, years | |||||||
| Median | 54 | 51 | 55 | .0068 | |||
| Range | 29-70 | 27-71 | 28-76 | ||||
| Younger than 50 | 36 | 35 | 145 | 44 | 384 | 33 | .0029 |
| 50-60 | 35 | 34 | 115 | 35 | 419 | 36 | |
| Older than 60 | 31 | 30 | 72 | 22 | 359 | 31 | |
| N+ according to local pathology | 36 | 35 | 91 | 28 | 250 | 22 | .0014 |
| Postmenopausal | 66 | 65 | 175 | 53 | 736 | 63 | .0017 |
| Receptor status | |||||||
| Estrogen+ | 51 | 76 | 160 | 66 | 637 | 73 | .080 |
| Progesterone+ | 44 | 72 | 140 | 66 | 485 | 62 | .21 |
| Systemic treatment | 53 | 52 | 138 | 42 | 392 | 34 | .0001 |
| Mitotic activity index | |||||||
| < 10 | 79 | 78 | 215 | 65 | 847 | 73 | .023 |
| 10-19 | 11 | 11 | 58 | 18 | 147 | 13 | |
| ≥ 20 | 11 | 11 | 58 | 18 | 160 | 14 | |
| Histology | |||||||
| Ductal | 61 | 60 | 224 | 68 | 861 | 75 | < .0001 |
| Lobular | 19 | 19 | 22 | 7 | 57 | 5 | |
| Mixed pattern | 18 | 18 | 63 | 19 | 163 | 14 | |
| Other | 3 | 3 | 22 | 7 | 74 | 6 | |
| Differentation of the invasive tumor | |||||||
| Low | 52 | 54 | 150 | 46 | 573 | 51 | .29 |
| Intermediate | 22 | 23 | 83 | 26 | 296 | 26 | |
| High | 22 | 23 | 92 | 28 | 258 | 23 | |
| Extensive intraductal component | 13 | 13 | 47 | 14 | 121 | 10 | .15 |
| Differentiation of DCIS | |||||||
| Low | 19 | 19 | 77 | 23 | 190 | 16 | .036 |
| Intermediate | 19 | 19 | 85 | 26 | 299 | 26 | |
| High | 12 | 12 | 46 | 14 | 155 | 13 | |
| No DCIS | 52 | 51 | 124 | 37 | 518 | 45 | |
| Vascular invasion | |||||||
| None | 77 | 76 | 236 | 71 | 884 | 77 | .096 |
| Present | 19 | 19 | 58 | 18 | 158 | 14 | |
| Doubtful | 5 | 5 | 37 | 11 | 108 | 9 | |
NOTE. Randomized according to local pathology.
Abbreviations: WBI, whole breast irradiation; DCIS, ductal carcinoma in situ.
Table A2.
Population Characteristics by Margins for DCIS According to Central Pathology Review for All Patients
| Parameter | Margin for DCIS |
P | |||||
|---|---|---|---|---|---|---|---|
| Positive |
Close |
Negative |
|||||
| No. | % | No. | % | No. | % | ||
| No. of patients | 139 | 229 | 498 | ||||
| Volume of excisional biopsy specimen, cm3 | |||||||
| Median | 102.3 | 120 | 118.5 | .48 | |||
| Range | 4.5-614.1 | 5.6-1,800 | 1.3-1,584 | ||||
| Largest diameter, mm | |||||||
| Median | 15.0 | 15.0 | 15.0 | .21 | |||
| Range | 4.0-40.0 | 4.0-40.0 | 4.0-40.0 | ||||
| Age at random assignment, years | |||||||
| Median | 51 | 52 | 54 | .014 | |||
| Range | 28-70 | 27-69 | 28-76 | ||||
| Younger than 50 | 62 | 45 | 92 | 40 | 163 | 33 | |
| 50-60 | 54 | 39 | 81 | 35 | 192 | 39 | |
| Older than 60 | 23 | 17 | 56 | 24 | 143 | 29 | |
| N+ according to local pathology | 40 | 29 | 63 | 28 | 126 | 26 | .67 |
| Postmenopausal | 74 | 53 | 130 | 57 | 303 | 61 | .22 |
| Receptor status | |||||||
| Estrogen+ | 66 | 67 | 113 | 65 | 292 | 78 | .0029 |
| Progesterone+ | 54 | 61 | 101 | 64 | 233 | 69 | .32 |
| Systemic treatment | 63 | 45 | 96 | 42 | 176 | 35 | .051 |
| Mitotic activity index | |||||||
| < 10 | 94 | 68 | 139 | 61 | 391 | 79 | < .0001 |
| 10-19 | 21 | 15 | 56 | 25 | 64 | 13 | |
| ≥ 20 | 24 | 17 | 33 | 14 | 41 | 8 | |
| Histology | |||||||
| Ductal | 110 | 79 | 188 | 82 | 408 | 82 | .73 |
| Mixed pattern | 29 | 21 | 41 | 18 | 90 | 18 | |
| Extensive intraductal component | 71 | 51 | 50 | 22 | 50 | 10 | < .0001 |
| Differentiation of the invasive tumor | |||||||
| Low | 64 | 47 | 87 | 39 | 253 | 53 | < .0001 |
| Intermediate | 27 | 20 | 76 | 34 | 141 | 29 | |
| High | 46 | 34 | 59 | 27 | 87 | 18 | |
| Differentiation of DCIS | |||||||
| Low | 63 | 45 | 80 | 35 | 130 | 26 | < .0001 |
| Intermediate | 37 | 27 | 96 | 42 | 254 | 51 | |
| High | 39 | 28 | 53 | 23 | 112 | 23 | |
| Vascular invasion | |||||||
| None | 100 | 72 | 150 | 66 | 364 | 73 | .20 |
| Doubtful | 11 | 8 | 30 | 13 | 49 | 10 | |
| Present | 28 | 20 | 49 | 21 | 83 | 17 | |
Abbreviation: DCIS, ductal carcinoma in situ.
Table A3.
Univariable Analysis of Ipsilateral Breast Cancer Recurrence for All Patients Stratified by Randomized Treatment
| Parameter | P | Hazard for Local Failure |
|
|---|---|---|---|
| Estimate | 95% CI | ||
| Complete: WBI 50 Gy, 0 Gy v 16 Gy boost | .0008 | 0.54 | 0.37 to 0.77 |
| Incomplete: WBI 50 Gy and 10 Gy, 0 Gy v 16 Gy extra boost | .62 | 0.74 | 0.23 to 2.42 |
| Both complete and incomplete, 0 and 10 Gy v 16 and 26 Gy | .0008 | 0.55 | 0.39 to 0.780 |
| Age, > 50 v ≤ 50 years | < .0001 | 0.40 | 0.28 to 0.56 |
| Nodal status, N+ v N− | .52 | 0.87 | 0.57 to 1.32 |
| Systemic treatment (chemotherapy or tamoxifen), yes v no | .012 | 0.61 | 0.41 to 0.89 |
| Volume of excisional biopsy specimen, cm3 | .13 | 0.990 | 0.998 to 1.00 |
| Estrogen receptor, + v − | .083 | 0.70 | 0.46 to 1.05 |
| Progesterone receptor, + v − | .52 | 0.87 | 0.57 to 1.33 |
| Angioinvasive growth, yes v no | .86 | 1.04 | 0.69 to 1.55 |
| Extensive intraductal component, yes v no | .39 | 1.24 | 0.77 to 2.00 |
| Histology invasive tumor | |||
| Lobular v ductal | .65 | 0.85 | 0.41 to 1.75 |
| Mixed v ductal | .32 | 0.78 | 0.47 to 1.28 |
| Other v ductal | .66 | 0.85 | 0.41 to 1.75 |
| Ductal component, yes v no | .33 | 1.23 | 0.81 to 1.90 |
| Lobular component, yes v no | .33 | 0.81 | 0.52 to 1.24 |
| Differentiation grade of the invasive tumor | |||
| Intermediate v high | .027 | 0.60 | 0.38 to 0.94 |
| Low v high | .0004 | 0.49 | 0.33 to 0.72 |
| Differentiation grade of DCIS | |||
| Intermediate v high | .057 | 1.75 | 0.98 to 3.12 |
| Low v high | .55 | 1.19 | 0.67 to 2.11 |
| No DCIS v high | .70 | 0.89 | 0.50 to 1.60 |
| Mitotic activity index, < 10 v ≥ 10 | .069 | 1.401 | 0.97 to 2.01 |
| Invasive tumor at the margin | |||
| Close v positive | .99 | 1.00 | 0.44 to 2.28 |
| Negative v positive | .69 | 1.17 | 0.53 to 2.54 |
| Not involved v involved | .45 | 1.17 | 0.78 to 1.74 |
| DCIS at the margin | |||
| No DCIS v positive | .15 | 0.54 | 0.24 to 1.48 |
| Negative v positive | .22 | 0.50 | 0.17 to 1.51 |
| Close v positive | .23 | 0.43 | 0.11 to 1.73 |
| Not involved v involved | .52 | 0.87 | 0.57 to 1.34 |
| DCIS and/or invasive at the margin, involved v not involved | .63 | 0.92 | 0.64 to 1.30 |
NOTE. Randomized according to local pathology.
Abbreviations: WBI, whole breast irradiation; DCIS, ductal carcinoma in situ.
Table A4.
Multivariable Analysis of Time to Local Relapse for All Patients
| Parameter | P | Hazard for Local Failure |
|
|---|---|---|---|
| Estimate | 95% CI | ||
| Randomized treatment 50 Gy WBI | |||
| 0 Gy v 16 Gy | .0007 | 0.48 | 0.31 to 0.73 |
| 50 Gy WBI and 10 Gy, 0 Gy v 16 Gy | .26 | 0.50 | 0.13 to 1.87 |
| 16 and 26 Gy v 0 and 10 Gy | .0005 | 0.49 | 0.32 to 0.73 |
| Age > 50 v ≤ 50 years | < .0001 | 0.40 | 0.26 to 0.60 |
| Systemic treatment, yes v no | .042 | 0.63 | 0.40 to 0.98 |
| Differentiation grade of the invasive tumor, high v low/intermediate | .024 | 1.65 | 1.07 to 2.55 |
| Differentiation grade of DCIS | |||
| High v low/intermediate | .98 | 1.01 | 0.540 to 1.86 |
| No DCIS v high | .45 | 0.83 | 0.51 to 1.34 |
| Margin of invasive tumor, not involved v involved | .36 | 0.82 | 0.52 to 1.29 |
Abbreviations: WBI, whole breast irradiation; DCIS, ductal carcinoma in situ.
Table A5.
Relation Between the Effect of the Boost on the 10-Year Ipsilateral Breast Cancer Recurrence Rate in High-Grade Invasive Ductal Cancer According to Age, With Cutoff Level of 60 Years for All Patients
| Local Relapse at 10 Years | High Grade, No Boost |
High Grade, 16 Gy Boost |
Overall |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | No. | % | 95% CI | |
| Age < 60 years | 156 | 21.1 | 14.1 to 28.7 | 138 | 9.9 | 4.6 to 16.2 | 294 | 15.9 | 11.3 to 20.8 |
| Age ≥ 60 years | 33 | 10.8 | 0.0 to 26.1 | 65 | 4.0 | 0.0 to 10.6 | 98 | 6.3 | 0.8 to 13.1 |
| Overall | 189 | 19.5 | 13.2 to 26.1 | 203 | 8.0 | 4.0 to 12.7 | |||
Fig A1.
Cumulative incidence of local relapse as first event according to the margins for (A) invasive tumor and (B) ductal carcinoma in situ for all patients.
Fig A2.
(A-D) Cumulative incidence of local failure according to the margin status for invasive tumor and ductal carcinoma in situ (DCIS), split according to boost group for all patients. (B, D) Involved margins combines close and positive margins. (A) Margin not involved with invasive carcinoma (P = .0004). (B) Close or involved margin with invasive carcinoma (P = .55). (C) Margin not involved with DCIS (P = .0001). (D) Close or involved margin with DCIS (P = .21).
Fig A3.
(A, B) Cumulative incidence of local failure by boost group and grade of invasive ductal carcinoma. (A) High grade of invasive carcinoma (P = .0030). (B) Low/intermediate grade of invasive carcinoma (P = .055).
Footnotes
European Organisation for Research and Treatment of Cancer was supported by Grants No. 5R10-CA11488-11 through 5U10-CA11488-38 from the National Cancer Institute (Bethesda, MD) for conducting trial 22881/10882.
The contents of this article are solely the responsibility of the authors and do not necessarily reflect the official views of the National Cancer Institute.
Written on behalf of the European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
See accompanying editorial on page 4929
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Harry Bartelink
Provision of study materials or patients: Johannes L. Peterse, Jean-Claude Horiot, Françoise Collin, Philip M. Poortmans, S. Bing Oei, Alain Fourquet, Jos J. Jager, Dominic A.X. Schinagl, Carla C. Wárlám-Rodenhuis, Harry Bartelink
Collection and assembly of data: Laurence Collette, Harry Bartelink
Data analysis and interpretation: Heather A. Jones, Ninja Antonini, Augustinus A.M. Hart, Laurence Collette, Harry Bartelink
Manuscript writing: Heather A. Jones, Harry Bartelink
Final approval of manuscript: Heather A. Jones, Ninja Antonini, Augustinus A.M. Hart, Johannes L. Peterse, Jean-Claude Horiot, Françoise Collin, Philip M. Poortmans, S. Bing Oei, Laurence Collette, Henk Struikmans, Walter F. Van den Bogaert, Alain Fourquet, Jos J. Jager, Dominic A.X. Schinagl, Carla C. Wárlám-Rodenhuis, Harry Bartelink
REFERENCES
- 1.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow- up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 4.Whelan TJ, Lada BM, Laukkanen E, et al. Breast irradiation in women with early stage invasive breast cancer following breast conservation surgery. Cancer Prev Control. 1997;1:228–240. [PubMed] [Google Scholar]
- 5.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organisation for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 6.Arriagada R, Le MG, Rochard F, et al. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data: Institute Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–1564. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 7.Touboul E, Buffat L, Belkacemi Y, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys. 1999;43:25–38. doi: 10.1016/s0360-3016(98)00365-4. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332:907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 9.Haffty BG, Reiss M, Beinfield M, et al. Ipsilateral breast tumor recurrence as a predictor of distant disease: Implications for systemic therapy at the time of local relapse. J Clin Oncol. 1996;14:52–57. doi: 10.1200/JCO.1996.14.1.52. [DOI] [PubMed] [Google Scholar]
- 10.Mirza NQ, Vlastos G, Meric F, et al. Predictors of locoregional recurrence among patients with early-stage breast cancer treated with breast-conserving therapy. Ann Surg Oncol. 2002;9:256–265. doi: 10.1007/BF02573063. [DOI] [PubMed] [Google Scholar]
- 11.Bartelink H, Horiot JC, Poortmans P, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 12.Bartelink H, Horiot J, Poortmans P, et al. Impact of a high radiation dose on local control and survival in Breast-conserving therapy for early stage breast cancer: 10–year results of the randomized boost versus no boost EORTC 22881-10882. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 13.Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-Gy boost in the conservative treatment of early breast cancer: Results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15:963–968. doi: 10.1200/JCO.1997.15.3.963. [DOI] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists Collaborative Group (EBCTCG): Effects of radiation and differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomized trial. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 15.Jobsen JJ, van der Palen J, Ong F, et al. The value of a positive margin for invasive carcinoma in breast-conservative treatment in relation to local recurrence is limited to young women only. Int J Radiat Oncol Biol Phys. 2003;57:724–731. doi: 10.1016/s0360-3016(03)00644-8. [DOI] [PubMed] [Google Scholar]
- 16.Anscher MS, Jones P, Prosnitz LR, et al. Local failure and margin status in early-stage breast carcinoma treated with conservation surgery and radiation therapy. Ann Surg. 1993;218:22–28. doi: 10.1097/00000658-199307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansfield CM, Komarnicky LT, Schwartz GF, et al. Ten-year results in 1070 patients with stages I and II breast cancer treated by conservative surgery and radiation therapy. Cancer. 1995;75:2328–2336. doi: 10.1002/1097-0142(19950501)75:9<2328::aid-cncr2820750923>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Smitt MC, Nowels KW, Zdeblick MJ, et al. The importance of lumpectomy surgical margin status in long term results of breast conservation. Cancer. 1995;76:259–267. doi: 10.1002/1097-0142(19950715)76:2<259::aid-cncr2820760216>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.DiBiase SJ, Komarnicky LT, Schwartz GF, et al. The number of positive margins influences the outcome of women treated with breast preservation for early stage breast carcinoma. Cancer. 1998;82:2212–2220. [PubMed] [Google Scholar]
- 20.Wazer DE, Schmidt-Ullrich RK, Ruthazer R, et al. Factors determining outcome for breast-conserving irradiation with margin-directed dose escalation to the tumor bed. Int J Radiat Oncol Biol Phys. 1998;40:851–858. doi: 10.1016/s0360-3016(97)00861-4. [DOI] [PubMed] [Google Scholar]
- 21.Park CC, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: Influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18:1668–1675. doi: 10.1200/JCO.2000.18.8.1668. [DOI] [PubMed] [Google Scholar]
- 22.Kreike B, Hart AA, van de Velde T, et al. Continuing risk of ipsilateral breast relapse after breast-conserving therapy at long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;71:1014–1021. doi: 10.1016/j.ijrobp.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein NS, Kestin L, Vicini F. Factors associated with ipsilateral breast failure and distant metastases in patients with invasive breast cancer treated with breast conserving therapy. A clinicopathology study of 607 neoplasms from 583 patients. Am J Clin Pathol. 2003;120:500–527. doi: 10.1309/8941-VDAJ-MKY2-GCLX. [DOI] [PubMed] [Google Scholar]
- 24.Veronesi U, Salvadori B, Luini A, et al. Breast conservation is a safe method in patients with small cancer of the breast: Long-term results of three randomized trials on 1,973 patients. Eur J Cancer. 1995;31A:1574–1579. doi: 10.1016/0959-8049(95)00271-j. [DOI] [PubMed] [Google Scholar]
- 25.van Dongen JA, Bartelink H, Fentiman IS, et al. Factors influencing local relapse and survival and results of salvage treatment after breast-conserving therapy in operable breast cancer: EORTC trial 10801, breast conservation compared with mastectomy in TNM stage I and II breast cancer. Eur J Cancer. 1992;28A:801–805. doi: 10.1016/0959-8049(92)90118-l. [DOI] [PubMed] [Google Scholar]
- 26.Gage I, Schnitt SJ, Nixon AJ, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer. 1996;78:1921–1928. doi: 10.1002/(sici)1097-0142(19961101)78:9<1921::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Zhou P, Gautam S, Recht A. Factors affecting outcome for young women with early stage invasive breast cancer treated with breast-conserving therapy. Breast Cancer Res Treat. 2007;101:51–57. doi: 10.1007/s10549-006-9268-y. [DOI] [PubMed] [Google Scholar]
- 28.Ryoo MC, Kagan AR, Wollin M, et al. Prognostic factors for recurrence and cosmesis in 393 patients after radiation therapy for early mammary carcinoma. Radiology. 1989;172:555–559. doi: 10.1148/radiology.172.2.2546175. [DOI] [PubMed] [Google Scholar]
- 29.Spivack B, Khanna MM, Tafra L, et al. Margin status and local recurrence after breast-conserving surgery. Arch Surg. 1994;129:952–957. doi: 10.1001/archsurg.1994.01420330066013. [DOI] [PubMed] [Google Scholar]
- 30.Heimann R, Powers C, Halpern HJ, et al. Breast preservation in stage I and II carcinoma of the breast: The University of Chicago experience. Cancer. 1996;78:1722–1730. doi: 10.1002/(sici)1097-0142(19961015)78:8<1722::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Pezner RD, Terz J, Ben-Ezra J, et al. Now there are two effective conservation approaches for patients with stage I and II breast cancer: How pathological assessment of inked resection margins can provide valuable information for the radiation oncologist. Am J Clin Oncol. 1990;13:175–179. doi: 10.1097/00000421-199004000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Solin LJ, Fowble BL, Schultz DJ, et al. The significance of the pathology margins of the tumor excision on the outcome of patients treated with definitive irradiation for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1991;21:279–287. doi: 10.1016/0360-3016(91)90772-v. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan L, Jewell WR, Krishnan EC, et al. Breast cancer with extensive intraductal component: Treatment with immediate interstitial boost irradiation. Radiology. 1992;183:273–276. doi: 10.1148/radiology.183.1.1549685. [DOI] [PubMed] [Google Scholar]
- 34.Poortmans PM, Collette L, Horiot JC, et al. Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiother Oncol. 2009;90:80–85. doi: 10.1016/j.radonc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histologic grade in breast cancer: Experience from a large study with long-term follow-up. Histopathol. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 36.Holland R, Peterse JL, Millis RR, et al. Ductal carcinoma in situ: A proposal for a new classification. Semin Diagn Pathol. 1994;11:167–180. [PubMed] [Google Scholar]
- 37.Perez C. Conservation therapy in T1–T2 breast cancer: Past, current issues and future challenges and opportunities. Cancer J. 2003:442–453. doi: 10.1097/00130404-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Jobsen JJ, Van der Palen J, Ong F, et al. Differences in outcomes for positive margins in a large cohort of breast cancer patients treated with breast-conserving therapy. Acta Oncolo. 2007;46:172–189. doi: 10.1080/02841860600891325. [DOI] [PubMed] [Google Scholar]
- 39.Cowen D, Houvenaeghel G, Bardou VJ, et al. Local and distant failures after limited surgery with positive margins and radiotherapy for node-negative breast cancer. Int J Radiat Oncol Biol Phys. 2003;47:305–312. doi: 10.1016/s0360-3016(99)00553-2. [DOI] [PubMed] [Google Scholar]
- 40.Peterson ME, Schultz DJ, Reynolds C, et al. Outcomes in breast cancer patients relative to margin status after treatment with breast conserving surgery and radiation therapy: The University of Pennsylvania experience. Int J Radiat Oncol Biol Phys. 1999;43:1029–1035. doi: 10.1016/s0360-3016(98)00519-7. [DOI] [PubMed] [Google Scholar]
- 41.Freedman G, Fowble B, Hanlon A, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys. 1999;44:1005–1015. doi: 10.1016/s0360-3016(99)00112-1. [DOI] [PubMed] [Google Scholar]
- 42.Pezner RD, Wagman LD, Ben-Ezra J, et al. Breast conservation therapy: Local tumor control in patients with pathological clear margins who receive 5000 cGy breast irradiation without local boost. Breast Cancer Res Treat. 1994;32:261–267. doi: 10.1007/BF00666003. [DOI] [PubMed] [Google Scholar]
- 43.Vicini F, Baglan K, Kestin L, et al. The emerging role of brachytherapy in the management of patients with breast cancer. Semin Radiat Oncol. 2002;12:31–39. doi: 10.1053/srao.2002.28662. [DOI] [PubMed] [Google Scholar]
- 44.Santiago RJ, Wu L, Harris E, Fox K, et al. Fifteen-year results of breast-conserving surgery and definitive irradiation for stage 1 and II breast carcinoma: The University of Pennsylvania Experience. Int J Radiat Oncol Biol Phys. 2004;58:233–220. doi: 10.1016/s0360-3016(03)01460-3. [DOI] [PubMed] [Google Scholar]
- 45.Vrieling C, Collette L, Fourquet A, et al. Can patients–treatment and pathological related characteristic explain the high local recurrence rate following breast conserving therapy in young patients? Eur J Cancer. 2003;39:932–944. doi: 10.1016/s0959-8049(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 46.Voogd AC, Nielsen Peterse JL, et al. Differences in risk factor for local and distant recurrence after breast conserving therapy or mastectomy for stage I or II breast cancer: Pooled result of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 47.Bollet MA, Brigitte S-Z, Mazeau V, et al. Age remains the first prognostic factor for loco-regional breast recurrence in young (< 40 years) women treated with breast barest conserving surgery first. Radiother Oncol. 2007;82:272–280. doi: 10.1016/j.radonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Antonini N, Jones H, Horiot JC, et al. Effect of age radiation dose on local control after breast conserving treatment: EORTC trial 22881-10882. Radiother Oncol. 2007;82:265–271. doi: 10.1016/j.radonc.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 50.Collette S, Collette L, Budiharto T, et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: A study based on the EORTC trial 22881-10882 ‘boost versus no boost’. Eur J Cancer. 2008;44:2587–2599. doi: 10.1016/j.ejca.2008.07.032. [DOI] [PubMed] [Google Scholar]