Abstract
Purpose
E2100, an open-label, randomized, phase III trial conducted by the Eastern Cooperative Oncology Group (ECOG), demonstrated a significant improvement in progression-free survival (PFS) and overall response rate (ORR) with paclitaxel plus bevacizumab compared with paclitaxel alone as initial chemotherapy for patients with HER2-negative metastatic breast cancer.
Methods
An independent, blinded review of radiologic and clinical data was performed, assessing progression and response according to Response Evaluation Criteria in Solid Tumors. In addition, ECOG's investigator assessments were reanalyzed using the same methods applied to the independent review. The primary end point was PFS as assessed by an independent review facility (IRF).
Results
The addition of bevacizumab to paclitaxel resulted in a statistically significant improvement in PFS using both the IRF and investigator assessments. Hazard ratios for PFS (0.48, 95% CI, 0.385 to 0.607; P < .0001 for the IRF v 0.42, 95% CI, 0.34 to 0.52; P < .0001 for ECOG investigators) and the improvement in median PFS (11.3 v 5.8 months for the IRF v 11.4 v 5.8 months for ECOG investigators) were similar. Among patients with measurable disease at baseline, the IRF-assessed ORR was significantly higher in patients treated with paclitaxel and bevacizumab (48.9% v 22.2%; P < .0001).
Conclusion
The risk of progression was reduced by more than half and the ORR more than doubled with the addition of bevacizumab to weekly paclitaxel in both analyses, confirming a substantial and robust bevacizumab treatment effect. The consistency between the IRF and ECOG analyses validates the original data previously reported by ECOG in this open-label trial.
INTRODUCTION
E2100 was an open-label, multicenter, randomized, phase III trial conducted by the Eastern Cooperative Oncology Group (ECOG), comparing paclitaxel plus bevacizumab with paclitaxel alone as initial chemotherapy for patients with human epidermal growth factor receptor 2 (HER2)–negative metastatic or locally recurrent breast cancer. The primary end point was progression-free survival (PFS) based on the final ECOG-reviewed investigator assessment (henceforth, ECOG investigator). An independent data monitoring committee declared the study positive and released the results at the first planned interim efficacy analysis in April 2005. Recently, the final analysis confirmed a significant improvement in PFS and overall response rate (ORR) with paclitaxel plus bevacizumab compared with paclitaxel alone.1
Because E2100 was an open-label study and enrolled patients without measurable disease, we conducted a retrospective, independent, and blinded review of response and progression. Here we report the results of this independent review and compare them with a reanalysis of the ECOG investigator assessment of response and progression using the same statistical methodology that was applied to the independent review. We also compare the results from the independent review of E2100 with several other recently reported phase III trials in metastatic breast cancer (MBC) that incorporated prospective, independent reviews.2,3
METHODS
Patient Eligibility and Trial Design
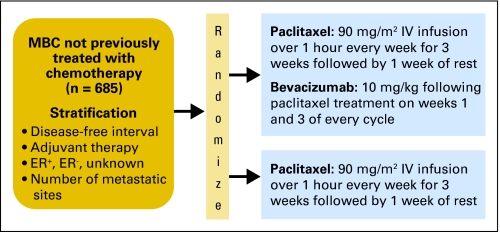
The E2100 study design (Fig 1), major inclusion and exclusion criteria, and final efficacy analyses have been previously reported.1 This study was approved by institution review boards at all participating sites, and informed consent was obtained from all participants.
Fig 1.
Study schema. MBC, metastatic breast cancer; ER, estrogen receptor; IV, intravenous.
ECOG Assessment of Response and Progression
According to protocol, response and progression were to be determined using Response Evaluation Criteria in Solid Tumors (RECIST). Target lesions were identified at baseline, and the status of all lesions was reported at each follow-up assessment. The disease assessment data submitted to ECOG included individual lesion measurements for target lesions and individual lesion assessment for nontarget lesions, but they did not include central review of scans or radiology reports. Data specialists at the ECOG Data Management Office reviewed disease assessment data to ensure that all baseline target and nontarget lesions were reported and that consistent methods of evaluation were used at each assessment, as required by RECIST. The ECOG data specialists then reviewed the assessment data to determine whether RECIST for response and progression had been met. After all data had been submitted, reviewed, and any discrepancies clarified, the disease assessment was reviewed by the study chair for final determination. If unequivocal progression in nontarget lesions was the only evidence of progression, the institution provided additional supporting clinical information for the study chair to review. In addition, approximately 10% of cases were randomly selected for an onsite quality and compliance audit, including review of radiology scans and reports. Audit findings were incorporated into the final disease assessment when appropriate.
Radiographic and Clinical Data Collection for Independent Review
Between December 2001 and May 2004, ECOG, together with all of the major North American cooperative groups, enrolled 722 patients at 258 centers in the United States, Canada, Peru, and South Africa. The original data cutoff for the first planned interim efficacy analysis (February 9, 2005, henceforth “the cutoff date”) was used for all efficacy analyses described here.
An independent, blinded review of all 722 patients was conducted by the independent review facility (IRF; RadPharm, Princeton, NJ). Scan collection for the review began in July 2006 and was facilitated by ECOG, Alpha Oncology (the contract research organization for ECOG), and RadPharm. Sites were instructed to send images and any other tumor assessments used to assess outcome as specified by the E2100 protocol up to the data cutoff date directly to RadPharm. Clinical information, including assessment of lesions by physical examination, cytology results, and free text comments recorded on the ECOG tumor assessment Case Report Forms were extracted from the database, any mention of treatment assignment or ECOG investigator assessment was redacted, and these data were provided to RadPharm for incorporation into the independent review.
Interpretation of the Radiographic and Clinical Data by the IRF
Before data review, Genentech and RadPharm developed an IRF Charter specifying the independent review process, including details of the communication between trial sites and RadPharm, and the technical aspects of data submission. On receipt of radiographic data, the contents and readability of the data were verified, the quality of the images assessed, and the images transferred into RadPharm's digital image database for interpretation. All sites of disease were identified and prospectively categorized as either target or nontarget lesions on baseline images.
Two radiologists independently interpreted all images for each patient and made progression and response assessments according to RECIST, with the exception that baseline scans were allowed to be more than 4 weeks before random assignment. If the two radiologists agreed on the best response, the presence or absence of progression, and the date of progression, an oncologist performed a final review of all available data and made the final determination. If the two radiologists disagreed, adjudication was performed by a third radiologist, and the read was then reviewed by the RadPharm oncologist, who made the final determination.
Analysis Methods
In this article, we report the IRF analysis of efficacy end points and compare them with a reanalysis of the ECOG investigator assessment of response and progression using the same statistical methodology that was applied to the independent review. The methods used here and those used in the ECOG analysis as reported by Miller et al1 are summarized in Table 1.
Table 1.
Comparison of Methods Used in the ECOG and IRF Analyses
| Criterion | ECOG Analysis1 | Current Analysis |
|---|---|---|
| Analysis population for efficacy | Eligible patients (n = 673) | Randomly assigned patients (n = 722*) |
| PFS based on tumor assessments by: | ECOG review of investigator assessment | Blinded, independent review and reanalysis of investigator assessments |
| Database cutoff | November 15, 2006 | February 9, 2005 |
| PFS definition | All deaths | On study deaths† |
| NPT | No censoring for NPT | Censoring for NPT |
| PFS analysis | Stratified by disease-free interval and prior adjuvant therapy | Stratified by disease-free interval, number of metastatic sites, prior adjuvant therapy, and ER status (same strata used for randomization) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IRF, independent review facility; PFS, progression-free survival; NPT, nonprotocol therapy.
PFS was censored at day 1 for 97 patients.
Within 84 days of the last protocol therapy.
All randomly assigned patients were included in the primary IRF analysis of PFS. The primary end point was PFS as assessed by the IRF, defined as the time from random assignment until disease progression or on-study death from any cause. On-study death was defined as death occurring before 84 days after the last protocol therapy. PFS was censored at the date of last adequate tumor assessment (or if no tumor assessments were performed after the baseline visit, at the time of random assignment plus 1 day) for patients who had not experienced disease progression or death before the data cutoff date, including those patients removed from treatment because of progressive disease or worsening symptoms, if progression was not confirmed by the IRF. Data from patients who died after the data cutoff date without progressive disease were censored at the last tumor assessment before the cutoff date. Data from patients who received non–protocol-specified anticancer therapy before documented disease progression were censored at the time of the last tumor assessment before receiving non–protocol-specified therapy. Patients without any imaging or pertinent medical information available for IRF review were censored at the date of random assignment, effectively removing them from the analysis. Patients with follow-up but who had no baseline tumor imaging available for IRF review were recorded as having progressive disease at their first postbaseline scan documenting disease, under the conservative assumption that new lesions might be present.
PFS was compared between the treatment arms using a two-sided stratified log-rank test. The overall type I error rate for the two-sided test for the primary end point was controlled at α = 0.05. The stratification factors consisted of the same four stratification factors used for patient randomization: disease-free interval (≤ 24 months, > 24 months), number of metastatic sites (< 3, ≥ 3), adjuvant chemotherapy (yes, no), and estrogen receptor (ER) status (ER positive, ER negative, ER unknown). Kaplan-Meier methodology was used to estimate median PFS for each treatment arm. Cox proportional hazards methods, with data stratified according to stratification factors, were used to estimate hazard ratios (HRs) and test for the significance of time-to-event variables. The primary efficacy analysis population was the intent-to-treat population, defined as all patients who were randomly assigned to study treatment, irrespective of whether the assigned treatment was received.
The same analysis methods were applied to the ECOG investigator-assessed progression data. In particular, the PFS end point for the ECOG investigator-assessed analysis was defined using the same data cutoff and censoring rules as specified above for the IRF, rather than the PFS definition previously reported by ECOG (Table 1). To investigate the effect of patients whose scans or pertinent clinical data were not available for IRF review, a sensitivity analysis was performed that compared the PFS results in the total population with those for the subset of patients with at least one scan submitted for IRF assessment. PFS based on both the ECOG investigator-assessed as well as the IRF-assessed progression events were analyzed for stratification factors and relevant baseline characteristics.
Objective response was defined as a complete or partial best overall response per RECIST, confirmed by repeat assessment ≥ 4 weeks after the criteria for response were first met. Randomly assigned patients who did not meet this criterion, including patients for whom a postbaseline tumor assessment was not available for IRF review, were considered nonresponders. The primary analysis of ORR included only patients with measurable disease at baseline. ORRs were formally compared between the paclitaxel-alone arm and the paclitaxel plus bevacizumab arm using the stratified Cochran-Mantel-Haenszel test, with the same stratification factors used for patient random assignment. An objective response analysis based on ECOG investigator assessment using the same methodology was also conducted.
RESULTS
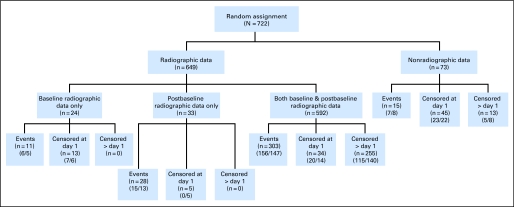
The two groups of patients compared in this analysis were similar at baseline with respect to their demographics and tumor characteristics (Table 2). A flowchart of patient disposition based on the IRF review of all 722 enrolled patients is provided in Figure 2. At least one image was submitted for IRF evaluation for 649 (89.9%) of the 722 patients. The proportion of patients with completely missing radiographic images for the IRF review was comparable between the two treatment arms: 38 patients (10.3%) in the paclitaxel plus bevacizumab arm and 35 patients (9.9%) in the paclitaxel-alone arm. Among the 722 patients, 625 patients (86.6%) in the IRF database had PFS follow-up determined and 97 patients (13.4%) did not: 47 patients (13%) in the paclitaxel plus bevacizumab arm and 50 patients (14%) in the paclitaxel-alone arm. The most common reason for lack of follow-up was completely missing images (6%, Fig 2). The baseline characteristics of the patients with and without radiographic images submitted and the patients with and without PFS follow-up were each compared, and no significant differences were found.
Table 2.
Demographic and Disease Characteristics of Randomly Assigned Patients
| Characteristic | PAC/BEV (n = 368) |
PAC (n = 354) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 56 | 55 | ||
| Range | 29-84 | 27-85 | ||
| Estrogen receptor status | ||||
| Positive | 223 | 60.6 | 223 | 63.0 |
| Negative | 138 | 37.5 | 127 | 35.9 |
| Unknown | 7 | 1.9 | 4 | 1.1 |
| Progesterone receptor status | ||||
| Positive | 166 | 45.1 | 158 | 44.6 |
| Negative | 184 | 50.0 | 182 | 51.4 |
| Unknown | 18 | 4.9 | 14 | 4.0 |
| HER2 status | ||||
| Positive | 9 | 2.4 | 6 | 1.7 |
| Negative | 334 | 90.8 | 316 | 89.3 |
| Unknown | 25 | 6.8 | 32 | 9.0 |
| Previous adjuvant chemotherapy | ||||
| None | 124 | 33.7 | 123 | 34.7 |
| Anthracycline but not taxane | 115 | 31.2 | 114 | 32.2 |
| Taxane but not anthracycline | 5 | 1.4 | 2 | 0.6 |
| Anthracycline and taxane | 69 | 18.8 | 66 | 18.6 |
| Other | 55 | 14.9 | 49 | 13.8 |
| Disease-free interval | ||||
| ≤ 24 months | 150 | 40.8 | 146 | 41.2 |
| > 24 months | 218 | 59.2 | 208 | 58.8 |
| Extent of disease | ||||
| ≥ 3 sites | 160 | 43.5 | 170 | 48.0 |
| < 3 sites | 208 | 56.5 | 184 | 52.0 |
| Location of disease* | ||||
| Visceral disease | 224 | 60.9 | 225 | 63.4 |
| Bone only | 36 | 9.8 | 27 | 7.6 |
| Disease evaluation* | ||||
| Measurable | 229 | 62.2 | 243 | 68.6 |
| Nonmeasurable | 139 | 37.8 | 111 | 31.4 |
NOTE. Because of rounding, percentages may not sum to 100.
Abbreviations: PAC, paclitaxel; BEV, bevacizumab; HER2, human epidermal growth factor receptor 2.
As determined by independent review facility review.
Fig 2.
Patient disposition, E2100 independent review facililty analysis. n, number of patients. (x/y), total number of patients within paclitaxel and bevacizumab/paclitaxel arms.
Efficacy
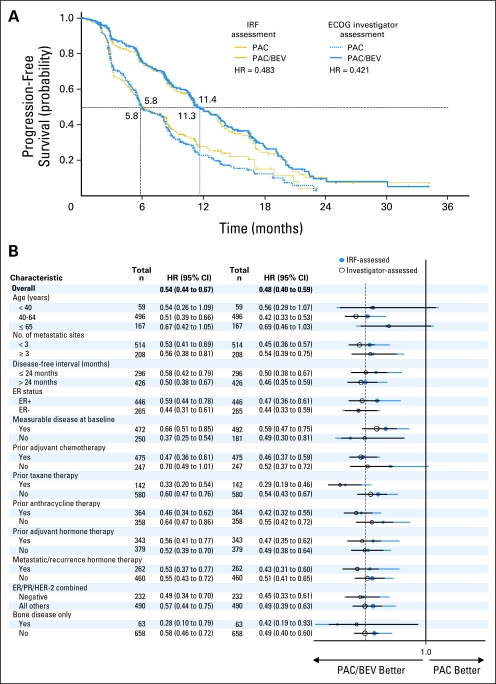
According to IRF review, 357 PFS events occurred: 173 in the paclitaxel plus bevacizumab arm and 184 in the paclitaxel-alone arm, The addition of bevacizumab to paclitaxel resulted in a statistically significant (P < .0001) prolongation of PFS (Table 3). Median PFS was 11.3 months in the paclitaxel plus bevacizumab arm compared with 5.8 months in the paclitaxel-alone arm, with a stratified HR of 0.48 (95% CI, 0.39 to 0.61; Fig 3A).
Table 3.
Selected Efficacy End Points Based on IRF and ECOG Investigator Assessments
| End Point | ECOG Investigator Assessment |
IRF Assessment |
||
|---|---|---|---|---|
| PAC/BEV (n = 368) | PAC (n = 354) | PAC/BEV (n = 368) | PAC (n = 354) | |
| Patients with PFS event | ||||
| No. | 201 | 244 | 173 | 184 |
| % | 54.6 | 68.9 | 47.0 | 52.0 |
| Earliest contributing event | ||||
| Disease progression | ||||
| No. | 192 | 236 | 158 | 166 |
| % | 95.5 | 96.7 | 91.3 | 90.2 |
| On-study death | ||||
| No. | 9 | 8 | 15 | 18 |
| % | 4.7 | 3.3 | 9.5 | 10.8 |
| Median PFS, months | 11.4 | 5.8 | 11.3 | 5.8 |
| Stratified analysis, PFS* | ||||
| HR | 0.421 | 0.483 | ||
| 95% CI | 0.343 to 0.516 | 0.385 to 0.607 | ||
| Log-rank test P | < .0001 | < .0001 | ||
| Objective response rate† | ||||
| No. of patients | 252 | 273 | 229 | 243 |
| % with an objective response | 48.0 | 23.4 | 48.9 | 22.2 |
| Difference in rates | 24.6 | 26.7 | ||
| 95% CI | 16.6 to 32.5 | 18.4 to 35.0 | ||
| P | < .0001 | < .0001 | ||
Abbreviations: IRF, independent review facility; ECOG, Eastern Cooperative Oncology Group; PAC, paclitaxel; BEV, bevacizumab; PFS, progression-free survival; HR, hazard ratio.
Relative to paclitaxel.
Limited to patients with measurable disease at baseline.
Fig 3.
(A) Comparison of independent review facility (IRF) –assessed and investigator-assessed progression-free survival (PFS). (B) Side-by-side comparison of progression-free survival hazard ratios (HRs) with 95% CIs for subgroups as assessed by the independent review facility (IRF) and investigators. All HRs and CIs are based on an unstratified Cox regression model. ECOG, Eastern Cooperative Oncology Group; PAC, paclitaxel; BEV, bevacizumab; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
In the ECOG investigator review, there were 445 PFS events: 201 in the paclitaxel plus bevacizumab arm and 244 in the paclitaxel-alone arm. The stratified PFS HR was 0.42 (95% CI, 0.34 to 0.52; P < .0001, Table 3), and median PFS times, approximately 11.4 months in the paclitaxel plus bevacizumab arm and 5.8 months in the paclitaxel-alone arm (Fig 3A), were nearly identical to the IRF.
PFS events were included in both the IRF and ECOG analyses for 314 patients (43.5%); 234 patients (32.4%) did not have a PFS event in either. For 43 patients (6%), only the IRF review documented a PFS event, whereas for 131 patients (18.1%), only the ECOG analysis identified a PFS event. Of the 131 patients with a PFS event that was determined only by ECOG review, 49 patients (37.4%) did not have scans submitted for the IRF or were missing scans at the time point that PFS was identified in the ECOG review. Of the 314 patients with PFS events in both analyses, the date of PFS was the same for 171 patients (54.5%) and within 6 weeks for 221 patients (70.4%). Agreement was evenly distributed across the two treatment arms. The benefit associated with the addition of bevacizumab to paclitaxel was robust, consistent across various patient subgroups, and consistent between the ECOG-based assessment and the IRF-based assessment, with overlapping 95% CIs between the two analyses across the various subgroups (Fig 3B).
To understand the effect on PFS of patients whose images were not submitted for IRF review, we conducted a sensitivity analysis including only the patients (n = 649) who had at least one radiographic image submitted for IRF assessment. This analysis again demonstrated a statistically significant benefit of adding bevacizumab to paclitaxel, with an HR of 0.50 (95% CI, 0.40 to 0.63; P < .0001) for PFS by IRF and an HR of 0.42 (95% CI, 0.34 to 0.52) for PFS by the ECOG investigators.
Among patients with measurable disease at baseline (paclitaxel plus bevacizumab, n = 229; paclitaxel alone, n = 243), the IRF-assessed ORR was significantly higher in the paclitaxel plus bevacizumab arm than in the paclitaxel-alone arm (48.9% v 22.2%; P = .0001; Table 3). By ECOG investigator assessment, the difference in ORR for patients with baseline measurable disease (paclitaxel plus bevacizumab, n = 252; paclitaxel alone, n = 273) was also statistically significant (48.0% v 23.4%; P < .0001; Table 3).
DISCUSSION
This retrospective, independent, blinded review of radiologic and clinical data from the E2100 study validates the results originally reported by ECOG after the first interim efficacy analysis and were recently confirmed in the final analysis. The addition of bevacizumab to weekly paclitaxel as initial chemotherapy for women with metastatic or locally recurrent HER2-negative breast cancer results in a statistically significant and clinically meaningful improvement in PFS and ORR. The consistency in outcomes between the IRF and ECOG assessments for the primary and secondary efficacy end points demonstrates that the ECOG results were not the result of systematic bias in an open-label study.
The IRF analysis included 357 PFS events, whereas the ECOG investigator analysis included 445 PFS events. The major reasons for censoring by the IRF were differences in lesion selection or radiographic interpretation leading to disagreement on the presence or absence of progression, and scans being completely missing for IRF evaluation. When an ECOG investigator determined that a PFS event had occurred, the patient was taken off study and no further images or clinical data were collected. Consequently, if an ECOG investigator-determined PFS event was not confirmed by IRF review, subsequent images were usually not available. Despite the difference in the number of PFS events, the PFS medians, HRs, and ORRs were nearly identical.
Collecting images for central review is always challenging, and we expected even more difficulty in obtaining images for this retrospective review. Remarkably, at least one image was available for approximately 90% of patients. The amount of data missing for IRF review was comparable across the arms, and a sensitivity analysis to assess the effect of patients with missing radiographic data retained the significant treatment effects for the paclitaxel-bevacizumab combination. Patient demographics and baseline disease characteristics were comparable between the treatment arms for the 73 patients whose images were not submitted for IRF assessment. Moreover, the baseline disease characteristics and the demographics were similar between the patients with and without scans for IRF evaluation.
One area of particular interest for trials using independent review is the concordance between IRF and ECOG investigator assessments. In E2100, the IRF and the ECOG investigators agreed on PFS for 76% of patients and agreed on best response for 80%. Because change in disease status is a continuous variable on which arbitrary categories have been imposed, some disagreement for patients whose true disease status is near these arbitrary cutoffs should be expected. In addition, only measurement of one dimension is recorded, but metastatic lesions are three dimensional and frequently seen in multiple images. Some disagreement should be expected based on which lesions or which images of the same lesions are selected for measurement. For example, 295 (45.5%) of the 649 patients with scans available for IRF review required adjudication by a third radiologist because of disagreement in at least one of the efficacy variables (date of progression, best overall response, and date of first response) among the original two radiologist reviewers. In 289 of these cases, the adjudicator agreed with one of the original radiologists on all three parameters. For six patients, the adjudicator disagreed with both of the original radiologist and provided his/her own interpretation. The strongest indicator, however, for the overall agreement between the IRF- and ECOG investigator–based assessments is demonstrated by the Kaplan-Meier curves for PFS shown in Figure 3A. Any lack of concordance at the individual patient level did not alter the shape of the Kaplan-Meier PFS curves, the HRs, or the medians.
Independent reviews performed retrospectively (as in E2100), or prospectively, have been conducted as part of the analysis of other oncology trials. Two large, open-label, phase III trials in patients with MBC, one involving ixabepilone another lapatinib, incorporated prospective independent review.2,3 The results from E2100 and the lapatinib and ixabepilone trials are presented in Table 4. There are myriad differences between these trials, including the sponsor (National Cancer Institute/cooperative group v industry), patient populations (HER2 negative v HER2 positive), extent of prior treatment (first-line chemotherapy for metastatic disease v later line), type of therapy being studied (antibody v small-molecule tyrosine kinase inhibitor v cytotoxic), prognosis, and inclusion of patients with nonmeasurable disease (patients with nonmeasurable disease were included in E2100). Despite these differences, the three trials demonstrate comparable results for PFS and response rate as determined by the IRF and the investigator (ECOG in the case of E2100). Any differences between the local and central reviews did not alter the conclusions about the efficacy of treatment.
Table 4.
Comparison of IRF- and Investigator-Based Assessments in Various Studies
| Assessment | Bevacizumab/Study E21001 |
Lapatinib/Study EGF1001512 |
Ixabepilone/Study CA1630463 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECOG |
IRF |
INV |
IRF |
INV |
IRF |
|||||||
| Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | |
| ORR, * | 23 | 48 | 22 | 49 | 17 | 32 | 14 | 24 | 22 | 42 | 14 | 35 |
| Difference in ORR within method, % | 25 | 27 | 15 | 10 | 20 | 21 | ||||||
| Median PFS, months | 5.8 | 11.4 | 5.8 | 11.3 | 4.1 | 5.5 | 4.1 | 6.2 | 3.8 | 5.3 | 4.2 | 5.8 |
| Difference in PFS within method, months† | 5.6 | 5.5 | 1.4 | 2.1 | 1.5 | 1.6 | ||||||
| Hazard ratio | 0.42 | 0.48 | 0.69 | 0.55 | 0.78 | 0.75 | ||||||
| Agreement, PFS status, between INV and IRF, Ctrl/Exp, % | 76/76 | 66/75 | NA/NA | |||||||||
Data are based on the intent-to-treat population, unless otherwise noted. Results for E2100 were taken from the final clinical study report (CSR) or CSR addendum. Results for lapatinib (Study EGF100151) were taken from the medical and statistical review documents obtained through the Freedom of Information Act (FOIA).
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IRF, independent review facility; INV, investigators; Ctrl, control; Exp, experimental arm; ORR, objective response rate; PFS, progression-free survival; NA, not applicable.
Study E2100 allowed entrance of patients without measurable disease. For consistency with the other two studies, E2100 patients without measurable disease at baseline were excluded from the objective response assessments.
For lapatinib (Study EGF100151) results, all results are based on the April 3, 2006, cutoff as available in the lapatinib FOIA review documents. For ixabepilone (Study CA163046) results, all results are based on the ixabepilone FOIA review documents.
IRF analysis was not included in the original E2100 study design, but was implemented after the study was completed, at the US Food and Drug Administration's request, to be included in the registration application. Despite the difficulties inherent in a retrospective IRF, it is notable that the ECOG investigator-assessed response rates in E2100 were nearly identical to the IRF rates, whereas in the other two studies, the investigator-reported rates were higher for both arms than were the IRF rates. This may reflect the contribution of ECOG central review of the individual lesion disease assessment data, which ensured that the RECIST criteria were applied with the same rigor to the investigator assessments as in an IRF review.
The use of IRF assessed PFS in clinical trials in MBC is expensive, time-consuming (therefore potentially delaying the progress of clinical trials), and, most importantly, may not add significant value in describing the treatment benefit over carefully determined investigator-assessed PFS. Other methods to detect bias, including collection of additional scans beyond progression and use of independent review as an auditing tool, as suggested by Dodd et al,4 should be used in future trials.
Footnotes
Supported by Genentech, South San Francisco, CA; by the United States Department of Health and Human Services; and by National Institutes of Health Grants No. CA23318 (to the Eastern Cooperative Oncology Group [ECOG] statistical center), CA66636 (to the ECOG data management center), and CA21115 (to the ECOG coordinating center).
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00028990.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Suman Bhattacharya, Genentech (C); Christopher Bowden, Genentech (C) Consultant or Advisory Role: Robert Gray, Genentech (U); Kathy Miller, Genentech (C), Roche (C) Stock Ownership: Suman Bhattacharya, Genentech; Christopher Bowden, Genentech Honoraria: Kathy Miller, Genentech Research Funding: Robert Gray, Genentech; Kathy Miller, Genentech, Roche; Robert L. Comis, Genentech Expert Testimony: Kathy Miller, Genentech (C) Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Robert Gray, Suman Bhattacharya, Kathy Miller
Administrative support: Robert L. Comis
Provision of study materials or patients: Kathy Miller
Collection and assembly of data: Robert Gray, Suman Bhattacharya, Christopher Bowden, Kathy Miller
Data analysis and interpretation: Robert Gray, Suman Bhattacharya, Christopher Bowden
Manuscript writing: Robert Gray, Suman Bhattacharya, Christopher Bowden, Kathy Miller, Robert L. Comis
Final approval of manuscript: Robert Gray, Suman Bhattacharya, Christopher Bowden, Kathy Miller, Robert L. Comis
REFERENCES
- 1.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 2.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 3.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 4.Dodd L, Korn E, Freidlin B, et al. Blinded independent central review of progression-free survival in phase III clinical trials: Important design element or unnecessary expense? J Clin Oncol. 2008;26:3791–3796. doi: 10.1200/JCO.2008.16.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]