Abstract
Purpose
Myeloperoxidase (MPO) generates reactive oxygen species and also activates xenobiotics. In a rigorous clinical trial (Southwest Oncology Group SWOG-8897), we examined relationships between genotypes and disease-free survival (DFS) among women treated for breast cancer, as well as those who did not receive adjuvant chemotherapy.
Patients and Methods
Patients were assigned to risk groups according to standard prognostic features; the low-risk group (n = 753 genotyped) received follow-up only, and the high-risk group (n = 401 genotyped) was randomly assigned to adjuvant cyclophosphamide, methotrexate, and fluorouracil (CMF) or cyclophosphamide, doxorubicin, and fluorouracil (CAF), with or without tamoxifen. DNA from archived normal lymph node tissue was genotyped, and Cox proportional hazard models were used to calculate DFS associated with MPO genotypes.
Results
Among women in the treatment arm, those with MPO G alleles had more than a two-fold reduction in hazard of recurrence (adjusted hazard ratio [HR] for GA genotypes, 0.51; 95% CI, 0.21 to 0.99; HR for GG genotypes, 0.41; 95% CI, 0.21 to 0.77). Effects were greatest among women who were further randomly assigned to tamoxifen (HR for GA genotypes, 0.28; 95% CI, 0.12 to 0.69; HR for GG genotypes, 0.19; 95% CI, 0.08 to 0.45). There were no significant associations between genotypes and DFS among women in the untreated arm, and relationships between genotypes and DFS did not differ by CAF or CMF.
Conclusion
These results, observed in two independent study populations, indicate that high-activity MPO genotypes are associated with better survival among women receiving cyclophosphamide-containing therapy, particularly when followed by tamoxifen therapy. MPO can be inhibited and/or upregulated by commonly used drugs; thus, our findings merit further investigation for optimization of therapeutics for breast cancer.
INTRODUCTION
Although adjuvant chemotherapy has greatly increased breast cancer survival, the benefits are not universal. Progress has been made in identifying predictive somatic markers;1–3 however, germline host factors may also modify the efficacy of cancer therapy. Studies4 evaluating genetic single-nucleotide polymorphisms in enzymes that metabolize commonly used drugs for breast cancer treatment, such as cyclophosphamide, have yielded inconsistent results. These inconsistent findings may be due to heterogeneity based on the number of drugs commonly used together to treat breast cancer or they may be due to the number of enzymes involved in the metabolic pathways of many chemotherapeutic drugs. Because of this lack of specificity for metabolic pathways in combinations of drugs commonly used to treat breast cancer, a more global approach to studying modifiers of cancer treatment outcomes may be warranted. Because oxidative stress is a common cytotoxic mechanism for many agents used to treat breast cancer, we have been focusing on genes in that pathway.
Myeloperoxidase (MPO) may play a role in response to treatment for breast cancer, in part because of the prominent role it likely plays in breast physiology, primarily as an antibacterial enzyme to protect human breast milk during lactation.5 MPO also activates a number of drugs and procarcinogens6 and produces highly cytotoxic hypochlorous acid,7 resulting in oxidative stress–mediated apoptosis.8 MPO is released by neutrophils and macrophages, which are known to invade and infiltrate tumors and may participate in killing surviving or recurrent cancer cells during and following chemotherapy.
MPO also appears to interact with steroid hormones. Both estrogen and tamoxifen bind to the estrogen receptor (ER), resulting in dimerization and binding to target DNA sites9 where they recruit coactivators or corepressors, depending on the tissue or promoter complex. Estrogen inhibits rather than activates the MPO gene,10 and it is possible that tamoxifen recruits coactivator complexes to the ER, allowing transcriptional activation of the MPO gene.
A functional promoter polymorphism in MPO results in higher transcription in G allele carriers and also creates a stronger ER binding site on the −463G/A allele.10–12 The G allele is more highly expressed than the A allele in human monocyte-macrophages, resulting in higher levels of MPO expression.10,11 We previously investigated relationships between MPO genotypes and disease-free survival (DFS) in a relatively small heterogeneous sample of women who received chemotherapy for breast cancer and found that those with GG genotypes had almost a two-fold reduction in hazard of death (hazard ratio [HR], 0.60; 95% CI, 0.38 to 0.95).13
To follow up on these intriguing findings and to address the limitations of that earlier study, we initiated an ancillary study in the context of a large clinical trial—Southwest Oncology Group SWOG-8897—of adjuvant chemotherapy and tamoxifen for node-negative breast cancer. We hypothesized that patients with MPO G alleles with greater MPO enzymatic activity would have better outcomes due to greater chemotherapy-induced cancer cytotoxicity. We were also interested in the potential modifying effects of tamoxifen treatment on these relationships. To determine whether MPO is expressed in human breast tissue, we also performed immunohistochemistry on those tissues.
PATIENTS AND METHODS
Patient Selection
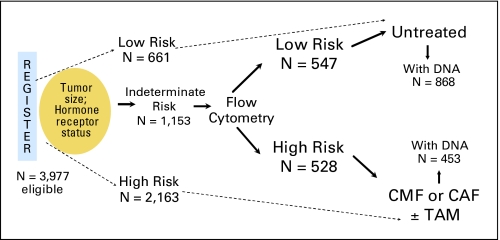
Data and samples were from a completed clinical trial led by SWOG for The Breast Cancer Intergroup of North America (SWOG-8897, Intergroup Protocol INT-0102). Details regarding the eligibility criteria, treatment, and outcomes were previously reported14; the schema is summarized in Figure 1. Briefly, women with node-negative breast cancer were eligible and, once enrolled, were placed into one of three categories of presumed recurrence risk (high, low, indeterminate). Patients were designated as high risk if their tumors were more than 2 cm or were hormone receptor–negative (both ER-negative and progesterone-negative), as determined at the local institution. This group of patients was not included in these analyses because of the lack of available tissue for genotyping (not mandated for this group in the protocol). The initially designated low-risk group consisted of women with tumors too small (usually < 1 cm) to perform hormone receptor assays by the dextran-coated charcoal methodology in place in that era.
Fig 1.
Schema of trial design for Southwest Oncology Group SWOG-8897 and tissues available for genotyping. CMF, cyclophosphamide, methotrexate, and fluorouracil; CAF, cyclophosphamide, doxorubicin, and fluorouracil; TAM, tamoxifen.
The indeterminate-risk group was composed of women with tumors less than 2 cm who were positive for hormone receptor. Flow cytometry was performed on their tumors; if the primary cancer was determined to have a high S-phase fraction (> 4.4% S phase for diploid tumors or > 7% for aneuploid tumors), these patients were considered high risk, while those with lower S-phase fraction were placed in the low-risk category.
Patients in the low-risk category (either initially or after flow cytometry determination) comprised an observational cohort that did not receive adjuvant endocrine therapy or chemotherapy. Women in the high-risk category were randomly assigned to adjuvant chemotherapy consisting of six cycles of oral cyclophosphamide, intravenous methotrexate, and fluorouracil (CMF), or six cycles of oral cyclophosphamide, intravenous doxorubicin, and fluorouracil (CAF). Patients in the high-risk categories were also randomly assigned to 5 years of oral tamoxifen or not, initiated after the chemotherapy.
In total, 3,977 women were registered to SWOG-8897, of whom 1,208 were considered low risk and did not receive any adjuvant systemic therapy. The treated patients potentially eligible for analysis in this study (n = 1,153) were those who were initially categorized as indeterminate risk and then assigned to the high-risk category on the basis of flow cytometry results, as shown in Figure 1. Distributions of age, race, and type of primary surgery were similar among all treatment groups. The median follow-up time was 10.8 years for patients still alive, and mature results of outcomes showed that CAF was not significantly better than CMF for the protocol-specified primary end point of DFS.14
Tissue Collection and Processing
Because protocol-mandated tissue procurement was restricted to the low-risk and indeterminate-risk patients, those included in this analysis had smaller tumors and were either hormone receptor–positive or had tumors that were too small to evaluate. Patients consented to use of their tissue for ancillary research studies, and this study was approved by the institutional review board at Roswell Park Cancer Institute and at Mount Sinai School of Medicine where the study was initially begun. Two 5-μm slides of normal lymph node tissue were selected from the SWOG tissue bank housed in San Antonio for patients in these categories, tissues were deparaffinized and removed from slides, and DNA was extracted as previously described.15 DNA samples were plated in a blinded fashion for genotyping, with duplicates from 5% of the samples included for quality control. Genotyping was performed using high-throughput matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Sequenom, San Diego, CA). After analyzing sufficient amounts of amplifiable DNA, MPO genotype data were available for 401 and 753 treated and untreated women, 76% and 62% of those accrued to these two groups, respectively.
Statistical Analyses
The end point assessed in this study was the impact of genotype on DFS, defined per the protocol as the time from the date of being randomly assigned to the date of first breast cancer recurrence or death due to any cause. The effects of genotypes were evaluated by contrasting women having genotypes for higher transcription alleles (AG and GG) with women having low transcription (AA) genotypes. Kaplan-Meier estimates of DFS for the subgroups of patients with common and variant MPO alleles were generated, and differences were determined using log-rank tests. Cox regression models were used to adjust for menopausal status and time from initial surgery, as well as type of treatment in the treated group. The proportional hazards assumption for the Cox regression model was tested in each model. The primary hypothesis was that MPO genotypes may have an impact on DFS in patients treated with CAF or CMF by virtue of enhanced activation of cyclophosphamide or indirect effects on treatment-induced cytotoxicity but have no effects among untreated patients. Thus, we performed separate analyses for the treated and untreated groups, and we also used a composite model that tested an explicit interaction of treatment and MPO genotypes on outcome. We performed further tests to determine if relationships varied by whether women received CAF or CMF or whether they were assigned to tamoxifen or not. Specimen selection and data analysis are reported according to REMARK criteria (Reporting Recommendations for Tumor Marker Prognostic Studies).16
Immunohistochemistry Analysis
We tested breast cancer tissue for the presence of MPO, to support the notion that MPO-generated oxidants could have a direct impact on survival of residual tumor cells in patients undergoing treatment. Breast cancer tissues from the Sidney Kimmel Cancer Center Tumor Bank were deparaffinized in citrate buffer before antigen retrieval. Sections were blocked in 10% serum before incubation with rabbit polyclonal antibodies to human MPO (1:1,000; BioDesign, Saco, ME) overnight, followed by biotinylated secondary antibodies, and avidin-conjugated horseradish peroxidase (Vector Laboratories, Burlingame, CA), before development with SG substrate (Vector Laboratories).
RESULTS
Patient Characteristics
The characteristics of the study population with sufficient tissue for genotyping in both the treated and untreated arms are listed in Table 1. As expected, women in the low-risk category (no systemic adjuvant therapy) tended to be slightly older and postmenopausal, to have smaller tumors, and to receive breast-conserving surgery rather than mastectomy. Among the women who were randomly assigned to treatment, there were 112 events (recurrences or deaths); among those in the low-risk untreated arm, there were 215 events.
Table 1.
Demographic and Clinical Characteristics of Patients Participating in Southwest Oncology Group SWOG-8897 From Whom DNA Was Available
| Characteristic | % Treated Group (n = 453) | % Untreated Group (n = 868) |
|---|---|---|
| Age, years | ||
| < 40 | 15 | 8 |
| 40-49 | 37 | 31 |
| 50-59 | 23 | 25 |
| 60-69 | 19 | 24 |
| ≥ 70 | 6 | 12 |
| Median | 49 | 54 |
| Range | 27-85 | 24-89 |
| Race | ||
| White (non-Hispanic) | 88 | 92 |
| Black (non-Hispanic) | 6 | 4 |
| Hispanic | 2 | 2 |
| Other | 4 | 2 |
| Menopausal status | ||
| Premenopausal | 50 | 38 |
| Postmenopausal | 50 | 62 |
| Postmenopausal estrogen | ||
| Yes | 14 | 18 |
| No | 86 | 82 |
| Primary treatment | ||
| BCS, delayed RT | 18 | 23 |
| BCS, RT prior to registration | 12 | 13 |
| Mastectomy | 70 | 64 |
| Tumor size, cm | ||
| ≤ 1 | 25 | 59 |
| 1.1-1.9 | 75 | 40 |
| MPO genotypes | ||
| AA | 5 | 3 |
| GA | 30 | 33 |
| GG | 65 | 64 |
Abbreviations: BCS, breast-conserving surgery; RT, radiation therapy; MPO, myeloperoxidase.
Association of MPO Genotype and Chemotherapy
There was 100% concordance for the 41 pairs of duplicates that were randomly distributed in the DNA plates for genotyping. There was a 13% call-rate failure occurring in the samples with the lowest amounts of DNA; these results were excluded from the analyses. The distributions of genotypes were similar to those in previous studies, with AA genotypes present in approximately 6% of the population, and AG present in 30%. When the effects of variant MPO alleles were evaluated among women who received CAF or CMF, we noted significant associations between genotypes and DFS (log-rank test comparing the three groups, P = .02), as shown in Figure 2A. When each genotype was compared with the referent AA group, there were significant associations for both GG (P = .006) and AG (P = .02); these P values should be compared with a P value of .0167 to accommodate multiple comparisons. When Cox proportional hazard models were evaluated to control for potential confounders (Table 2), we observed a reduction in risk of recurrence among women who were heterozygotes (adjusted HR for AG genotypes, 0.53; 95% CI, 0.27 to 1.05; P = .07) and for women homozygous for G alleles (adjusted HR for GG genotypes, 0.41; 95% CI, 0.22 to 0.78; P = .007). Because of the relatively small number of women in the reference group (with AA genotypes), we also evaluated associations with those with GG and GA genotypes combined as the referent (n = 379). Using this approach, women with AA genotypes had a more than two-fold increase in hazard of recurrence (HR, 2.28; 95% CI, 1.22 to 4.26).
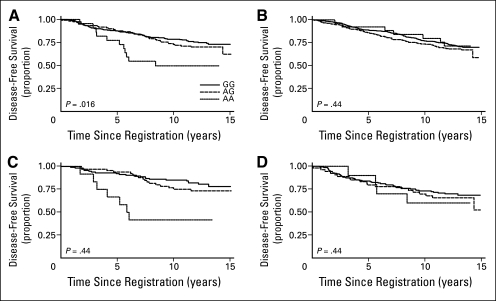
Fig 2.
Disease-free survival by myeloperoxidase (MPO) genotypes among women who (A) received adjuvant therapy, (B) did not receive adjuvant therapy, (C) received adjuvant cyclophosphamide-containing chemotherapy with further assignment to tamoxifen, and (D) received adjuvant cyclophosphamide-containing chemotherapy and no tamoxifen therapy. S8897, Southwest Oncology Group SWOG-8897 trial. Key in (A) also applies to parts (B), (C), and (D).
Table 2.
Disease-Free Survival Hazard Ratios for MPO Genotypes Among Women in Treated and Untreated Arms Controlling for Menopausal Status, Time Between Surgery and Registration, and Type of Randomized Treatment
| Treatment and MPO Genotype | Total No. of Patients | Failures | Adjusted HR | 95% CI | P |
|---|---|---|---|---|---|
| CAF or CMF | |||||
| AA, referent | 22 | 11 | 1.00 | ||
| AG | 120 | 36 | 0.53 | 0.27 to 1.05 | .07 |
| GG | 259 | 65 | 0.41 | 0.22 to 0.78 | .007 |
| CAF or CMF with no adjuvant therapy | |||||
| AA, referent | 26 | 7 | 1.00 | ||
| AG | 246 | 77 | 1.27 | 0.59 to 2.76 | .54 |
| GG | 481 | 131 | 1.08 | 0.50 to 2.31 | .84 |
| Treated with CAF or CMF plus tamoxifen | |||||
| AA, referent | 12 | 7 | 1.00 | ||
| AG | 66 | 17 | 0.28 | 0.12 to 0.69 | < .01 |
| GG | 115 | 22 | 0.19 | 0.08 to 0.45 | < .000 |
| Treated with CAF or CMF with no tamoxifen | |||||
| AA, referent | 10 | 4 | 1.00 | ||
| AG | 54 | 19 | 0.92 | 0.31 to 2.71 | .88 |
| GG | 144 | 43 | 0.76 | 0.27 to 2.12 | .60 |
Abbreviations: MPO, myeloperoxidase; HR, hazard ratio; CAF, cyclophosphamide, doxorubicin, and fluorouracil; CMF, cyclophosphamide, methotrexate, and fluorouracil.
There was no significant interaction between MPO genotypes and the type of chemotherapy to which patients were randomly assigned (CAF v CMF) on DFS (P = .98). There were no associations between genotypes and DFS among women in the low-risk, untreated group (Fig 2B) (log-rank P = .44), with adjusted models showing no associations between G alleles and risk of recurrence among heterozygotes (HR for AG genotypes, 1.27; 95% CI, 0.59 to 2.76; P = .54) or among homozygotes for G alleles (HR for GG genotypes, 1.08, 95% CI, 0.50 to 2.31; P = .84) (Table 2).
Association Between MPO Genotype and Tamoxifen
Among women who were randomly assigned to adjuvant tamoxifen, there were notable differences in DFS by MPO genotype (Figs 2C and 2D). As shown in Table 2, women who were heterozygous for G alleles and received tamoxifen had a two-thirds reduction in hazard of recurrence (HR, 0.31; 95% CI, 0.13 to 0.74; P < .001), and those who were homozygous had an 80% reduction in risk (HR, 0.21; 95% CI, 0.09 to 0.49; P < .005). Weaker, nonsignificant reductions in DFS were noted among women who did not receive tamoxifen (Table 2). There were no violations of the proportional hazards assumptions in the Cox model.
Immunohistochemistry Results
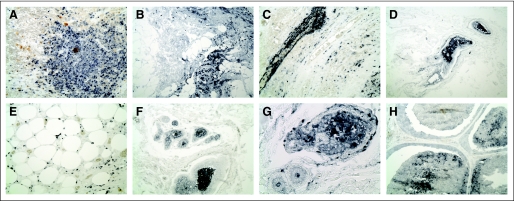
Immunohistochemical staining of breast cancer tissue revealed the presence of significant levels of MPO in infiltrating macrophages and neutrophils (Figs 3A-C). MPO was also seen in adipose-associated macrophages in adjacent areas (Fig 3E) and was detected in ductal lumens associated with dead cells and detritus (Figs 3D, F, G, and H), demonstrating the presence of MPO in breast tumor tissues.
Fig 3.
Myeloperoxidase (MPO) immunostaining in breast cancer tissues. Sections from paraffin-embedded ductal carcinoma tissues were immunostained with polyclonal antibodies against human MPO. MPO protein is detected in invading macrophages and neutrophils (A-C, ×20) and in dead cells and detritus in the lumen of ducts (D, F, G, H), as well as in (E) macrophages associated with adjacent adipose cells.
DISCUSSION
In this ancillary study to a completed clinical trial, we found that genotypes related to higher transcription of myeloperoxide, a cytotoxic antibacterial enzyme, were associated with more than a two-fold reduction in hazard of breast cancer recurrence among women treated with CAF or CMF, replicating our earlier findings.13 The replication of findings in both studies, and the strength of the associations, provides confidence that the associations are not likely to be spurious. The fact that there was no effect of MPO genotypes on DFS among women who did not receive adjuvant therapy suggests that MPO is predictive of chemotherapeutic efficacy. The effects of MPO genotypes among treated patients were observed primarily among those who were also randomly assigned to tamoxifen, and it is unclear whether associations are specific to cyclophophosphamide-containing therapy, tamoxifen treatment, or a combination of the two.
The MPO polymorphism evaluated has known functional significance. In cultured human monocyte-macrophages, the MPO GG genotype has expression several-fold higher than GA or AA genotypes.10 Similarly, in transgenic mice expressing the native human MPO alleles, mRNA expression and protein levels are several-fold higher with G than with A alleles.10 In human studies, levels of extracellular MPO activity in bronchoalveolar lavage fluid from lung cancer patients17 were lower in MPO AA than in AG or GG individuals, with a dose-response increase in DNA adducts in bronchoalveolar lavage cells with G alleles. These data clearly demonstrate the functional importance of the MPO −463 polymorphism and provide a biologic rationale for increased DFS among women with high-activity GG genotypes.
Although a number of studies have noted increases in cancer risk with MPO high-activity genotypes, particularly for lung cancer18 and more recently for breast cancer,19,20 there have been few studies examining effects of MPO genotypes on survival among cancer patients. Wu et al21 noted that MPO low-activity genotypes were associated with a higher risk of recurrence among esophageal cancer patients receiving cisplatin, similar to our findings. In 95 patients with metastatic breast cancer who received high-dose chemotherapy and autologous stem cell transplantation,22 MPO genotypes were not associated with progression-free survival, but the population was small and not representative of breast cancer patients receiving standard chemotherapy.
The mechanism whereby MPO genotypes may potentiate the effects of chemotherapy is not known, but there are several possible scenarios. MPO is known to be extremely important in drug metabolism, oxidizing a wide variety of compounds and a broad range of functional groups.6 Cyclophosphamide, used in both arms of the trial, is activated via pathways similar to those for a number of chemical carcinogens,6,23,24 of which MPO is known to participate in oxidative activation. Similar to findings that lower MPO activity genotypes (A alleles) were associated with lower levels of polycyclic aromatic hydrocarbon–DNA adducts,17 presumably due to lesser activation of polycyclic aromatic hydrocarbons to reactive intermediates, higher levels of MPO may result in greater activation of cyclophosphamide, and hence, greater tumor-cell kill and better survival.
MPO may also enhance tumor-cell kill through stimulation of increased production of MPO-derived oxidants by chemotherapy, which may directly result in additional damage to tumor cells7,25–27 or may induce apoptosis,8 thereby potentiating the effects of chemotherapy.
The effects of MPO genotypes on DFS only among women receiving chemotherapy were observed primarily among women who were further randomly assigned to tamoxifen, with slight, nonsignificant increased survival for women with G alleles who did not receive tamoxifen. Potential mechanisms are shown in Figure 4. It is possible that the effects of MPO genotypes on DFS are through potentiation of cytotoxicity of cyclophosphamide, either through greater activation of the drug, or through oxidative stress–related cell death, as illustrated in Fig 4A. It is also possible that the effects are primarily due to adjuvant therapy with tamoxifen (Fig 4B). There are known relationships between MPO activity and estrogens and differential effects on expression depending on genotype.28 It has been noted that Sp1, a transcription factor binding preferentially to the G allele, can enhance binding by the ER,12 and Norris et al29 suggested that the hormone response element at the −463 polymorphic site can serve as an estrogen response element. If, in fact, the MPO polymorphism alters ER binding, then the effects of tamoxifen treatment on breast cancer recurrence may clearly differ by MPO genotypes. It is also possible that, because MPO is inhibited by estrogens, blocking the binding of estrogen to ERs by tamoxifen removes this inhibition, further enhancing the effects of MPO on survival among those treated with the antiestrogen. However, because of the limited sample size in the analyses stratified by tamoxifen therapy, the role of chance cannot be excluded. Follow-up of these findings in a tamoxifen trial would shed more light on these potential relationships.
Fig 4.
Potential mechanisms of action for associations between myeloperoxidase (MPO) genotypes and disease-free survival through (A) effects of cyclophosphamide (CPA) and (B) interactions with tamoxifen. ER, estrogen receptor.
The observed dramatic differences in treatment outcomes by genotype indicate that MPO may greatly contribute to treatment efficacy and suggest that these relationships, now observed in two independent study populations, merit further investigation for optimization of therapeutics for breast cancer, with implications that regulating the expression of MPO might benefit patients during treatment for breast cancer. Peroxisome proliferator–activated receptor gamma (PPARγ) agonists such as rosiglitazone have been found to sharply increase MPO expression in macrophages10 and could be used to complement adjuvant therapy, thus increasing treatment efficacy. However, additional replication of these findings in other trials and clarification of the association with tamoxifen are likely needed before application of the findings to the clinic, with trials to upregulate MPO expression. As the field of pharmacogenetics evolves, stratification of patients according to genotypes for dosing and/or addition of targeted therapies for upregulation or inhibition of key enzymes may result in more individualized therapy, similar to that based on tumor characteristics, and thus lead to enhanced treatment efficacy for breast cancer patients.
Acknowledgment
Martin Abeloff, MD, of the Sidney Kimmel Comprehensive Cancer Center at The Johns Hopkins University, Baltimore, MD, now deceased, was principal investigator of the parent clinical trial for the Eastern Cooperative Oncology Group.
Glossary Terms
- Xenobiotic:
A chemical found in the body that is not normally produced or expected to be there; substances foreign to a biologic system.
- Pharmacogenetic:
A branch of pharmacology dedicated to understanding the hereditary basis for drug responses that are idiosyncratic in nature. Although inborn errors of metabolism also have a genetic basis, pharmocogenetic disorders may never manifest if the drug is never introduced in the host.
- PPARγ:
An orphan nuclear receptor belonging to the nuclear hormone receptor family termed peroxisome proliferator activated receptors (PPARs), which are ligand-dependent proteins that stimulate gene transcription. PPARs are activated by peroxisome proliferators (eg, clofibric acid, nafenopin, and some fatty acids). Like other nuclear hormone receptors, PPARγ isoforms heterodimerize with the RXR (retinoid X receptor) alpha receptor. Synthetic PPARγ ligands include thiazolidinedones, which are known to improve insulin sensitivity.
- Polymorphism:
See single nucleotide polymorphism.
- Oxidative stress:
Caused by an imbalance between reactive oxygen species and the body's ability to detoxify reactive intermediates and can result in cellular damage as well as programmed cell death.
- REMARK criteria:
Guidelines for reporting tumor marker studies, which include statement of objectives, description of patient population and treatments received, biologic materials, and assay methods. Criteria also include guidelines for reporting data, results, and discussion.
- DNA adduct:
An addition product with a chemical or metabolite bonded to DNA. DNA adducts can result in carcinogenesis.
Footnotes
Supported in part by the Department of Health and Human Services, National Cancer Institute R01 and Public Health Service Cooperative Agreement Grants No. CA095222, CA32102, CA38926, CA02599, CA13612, CA22433, CA27057, CA37981, CA46282, CA20319, CA35431, CA76447, CA45560, CA12644, CA14028, CA58416, CA04919, CA35090, CA35176, CA58686, CA58861, CA46113, CA58882, CA35128, CA74647, CA46136, CA45450, CA35261, CA35192, CA12213, CA16385, CA58658, CA46441, CA58723, CA45377, CA35119, CA42777, CA73590, CA114558-02, CA35178, and CA35262. C.B.A., J.M.R., and D.F.H. are recipients of funding from the Breast Cancer Research Foundation.
Presented in part at the 29th Annual San Antonio Breast Cancer Symposium, December 14-17, 2006, San Antonio, TX.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Christine B. Ambrosone, William E. Barlow, I-Tien Yeh, Laura R. Hutchins, Peter M. Ravdin, Silvana Martino, Kathy S. Albain
Administrative support: Christine B. Ambrosone, Silvana Martino
Provision of study materials or patients: I-Tien Yeh, Warren Davis, James M. Rae, Laura R. Hutchins, Silvana Martino, Kathy S. Albain
Collection and assembly of data: William E. Barlow, Wanda Reynolds, Warren Davis, James M. Rae, Laura R. Hutchins, Silvana Martino, Daniel F. Hayes
Data analysis and interpretation: Christine B. Ambrosone, William E. Barlow, Wanda Reynolds, Robert B. Livingston, I-Tien Yeh, Ji-Yeob Choi, Warren Davis, James M. Rae, Li Tang, Laura R. Hutchins, Peter M. Ravdin, Silvana Martino, C. Kent Osborne, Alan P. Lyss, Daniel F. Hayes, Kathy S. Albain
Manuscript writing: Christine B. Ambrosone, William E. Barlow, Wanda Reynolds, Robert B. Livingston, I-Tien Yeh, Ji-Yeob Choi, Warren Davis, James M. Rae, Li Tang, Laura R. Hutchins, Peter M. Ravdin, Silvana Martino, C. Kent Osborne, Alan P. Lyss, Daniel F. Hayes, Kathy S. Albain
Final approval of manuscript: Christine B. Ambrosone, William E. Barlow, Wanda Reynolds, Robert B. Livingston, I-Tien Yeh, Ji-Yeob Choi, Warren Davis, James M. Rae, Li Tang, Laura R. Hutchins, Peter M. Ravdin, Silvana Martino, C. Kent Osborne, Alan P. Lyss, Daniel F. Hayes, Kathy S. Albain
REFERENCES
- 1.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 4.Choi JY, Nowell SA, Blanco JG, et al. The role of genetic variability in drug metabolism pathways in breast cancer prognosis. Pharmacogenomics. 2006;7:613–624. doi: 10.2217/14622416.7.4.613. [DOI] [PubMed] [Google Scholar]
- 5.Josephy PD. The role of peroxidase-catalyzed activation of aromatic amines in breast cancer. Mutagenesis. 1996;11:3–7. doi: 10.1093/mutage/11.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Hofstra AH, Uetrecht JP. Myeloperoxidase-mediated activation of xenobiotics by human leukocytes. Toxicology. 1993;82:221–242. doi: 10.1016/0300-483x(93)90066-2. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff SJ. Myeloperoxidase: Friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 8.Nakazato T, Sagawa M, Yamato K, et al. Myeloperoxidase is a key regulator of oxidative stress mediated apoptosis in myeloid leukemic cells. Clin Cancer Res. 2007;13:5436–5445. doi: 10.1158/1078-0432.CCR-07-0481. [DOI] [PubMed] [Google Scholar]
- 9.Michalides R, Griekspoor A, Balkenende A, et al. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Kumar AP, Piedrafita FJ, Reynolds WF. Peroxisome proliferator-activated receptor gamma ligands regulate myeloperoxidase expression in macrophages by an estrogen-dependent mechanism involving the −463GA promoter polymorphism. J Biol Chem. 2004;279:8300–8315. doi: 10.1074/jbc.M311625200. [DOI] [PubMed] [Google Scholar]
- 11.Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Curr Opin Hematol. 2000;7:53–58. doi: 10.1097/00062752-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Piedrafita FJ, Molander RB, Vansant G, et al. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem. 1996;271:14412–14420. doi: 10.1074/jbc.271.24.14412. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosone CB, Ahn J, Singh KK, et al. Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res. 2005;65:1105–1111. [PubMed] [Google Scholar]
- 14.Hutchins LF, Green SJ, Ravdin PM, et al. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: Treatment results of Intergroup Protocol INT-0102. J Clin Oncol. 2005;23:8313–8321. doi: 10.1200/JCO.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 15.Rae JM, Cordero KE, Scheys JO, et al. Genotyping for polymorphic drug metabolizing enzymes from paraffin-embedded and immunohistochemically stained tumor samples. Pharmacogenetics. 2003;13:501–507. doi: 10.1097/00008571-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 16.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100:229–235. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 17.Van Schooten FJ, Boots AW, Knaapen AM, et al. Myeloperoxidase (MPO) −463G->A reduces MPO activity and DNA adduct levels in bronchoalveolar lavages of smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:828–833. [PubMed] [Google Scholar]
- 18.Wu X, Schabath MB, Spitz MR. Myeloperoxidase promoter region polymorphism and lung cancer risk. Methods Mol Med. 2003;75:121–133. doi: 10.1385/1-59259-324-0:121. [DOI] [PubMed] [Google Scholar]
- 19.Ahn J, Gammon MD, Santella RM, et al. Myeloperoxidase (MPO) genotype, fruit and vegetable consumption, and breast cancer risk. Cancer Res. 2004;64:7634–7639. doi: 10.1158/0008-5472.CAN-04-1843. [DOI] [PubMed] [Google Scholar]
- 20.Lin SC, Chou YC, Wu MH, et al. Genetic variants of myeloperoxidase and catechol-O-methyltransferase and breast cancer risk. Eur J Cancer Prev. 2005;14:257–261. doi: 10.1097/00008469-200506000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Gu J, Wu TT, et al. Genetic variations in radiation and chemotherapy drug action pathways predict clinical outcomes in esophageal cancer. J Clin Oncol. 2006;24:3789–3798. doi: 10.1200/JCO.2005.03.6640. [DOI] [PubMed] [Google Scholar]
- 22.Bewick MA, Conlon MS, Lafrenie RM. Polymorphisms in manganese superoxide dismutase, myeloperoxidase and glutathione-S-transferase and survival after treatment for metastatic breast cancer. Breast Cancer Res Treat. 2008;111:93–101. doi: 10.1007/s10549-007-9764-8. [DOI] [PubMed] [Google Scholar]
- 23.Mallet WG, Mosebrook DR, Trush MA. Activation of (+−)-trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene to diolepoxides by human polymorphonuclear leukocytes or myeloperoxidase. Carcinogenesis. 1991;12:521–524. doi: 10.1093/carcin/12.3.521. [DOI] [PubMed] [Google Scholar]
- 24.Petruska JM, Mosebrook DR, Jakab GJ, et al. Myeloperoxidase-enhanced formation of (+−)-trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene-DNA adducts in lung tissue in vitro: A role of pulmonary inflammation in the bioactivation of a procarcinogen. Carcinogenesis. 1992;13:1075–1081. doi: 10.1093/carcin/13.7.1075. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie SJ, Baker MS, Buffinton GD, et al. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatsumi T, Fliss H. Hypochlorous acid and chloramines increase endothelial permeability: Possible involvement of cellular zinc. Am J Physiol. 1994;267:H1597–H1607. doi: 10.1152/ajpheart.1994.267.4.H1597. [DOI] [PubMed] [Google Scholar]
- 27.Vissers MC, Carr AC, Chapman AL. Comparison of human red cell lysis by hypochlorous and hypobromous acids: Insights into the mechanism of lysis. Biochem J. 1998;330:131–138. doi: 10.1042/bj3300131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds WF, Rhees J, Maciejewski D, et al. Myeloperoxidase polymorphism is associated with gender specific risk for Alzheimer's disease. Exp Neurol. 1999;155:31–41. doi: 10.1006/exnr.1998.6977. [DOI] [PubMed] [Google Scholar]
- 29.Norris J, Fan D, Aleman C, et al. Identification of a new subclass of Alu DNA repeats which can function as estrogen receptor-dependent transcriptional enhancers. J Biol Chem. 1995;270:22777–22782. doi: 10.1074/jbc.270.39.22777. [DOI] [PubMed] [Google Scholar]