Abstract
Purpose
To examine consequences of deferred treatment (DT) as initial management of prostate cancer (PCa) in a contemporary, prospective cohort of American men diagnosed with PCa.
Participants and Methods
We evaluated deferred treatment for PCa in the Health Professionals Follow-up Study, a prospective study of 51,529 men. Cox proportional hazards models were used to calculate hazard ratios (HRs) for time to eventual treatment among men who deferred treatment for more than 1 year after diagnosis. HRs for time to metastasis or death as a result of PCa were compared between patients who deferred treatment and those who underwent immediate treatment within 1 year of diagnosis.
Results
From among 3,331 cohort participants diagnosed with PCa from 1986 to 2007, 342 (10.3%) initially deferred treatment. Of these, 174 (51%) remained untreated throughout follow-up (mean 7.7 years); the remainder were treated an average of 3.9 years after diagnosis. Factors associated with progression to treatment among DT patients included younger age, higher clinical stage, higher Gleason score, and higher prostate-specific antigen at diagnosis. We observed similar rates for development of metastases (n = 20 and n = 199; 7.2 v 8.1 per 1,000 person-years; P = .68) and death as a result of PCa (n = 8 and n = 80; 2.4 v 2.6 per 1,000 person-years; P = .99) for DT and immediate treatment, respectively.
Conclusion
In this nationwide cohort, more than half the men who opted for DT remained without treatment for 7.7 years after diagnosis. Older men and men with lesser cancer severity at diagnosis were more likely to remain untreated. PCa mortality did not differ between DT and active treatment patients.
INTRODUCTION
Prostate cancer (PCa) is the most common noncutaneous cancer in American men, with 186,320 new cases diagnosed in 2008, but it is less commonly lethal, with only 28,660 deaths.1 Although PCa carries a 3% lifetime risk of death for US males, it may be present in more than 40% of men older than age 50, according to autopsy data.2,3 One retrospective and two prospective long-term studies, before the advent of prostate-specific antigen (PSA) screening, suggested that localized PCa of low or intermediate Gleason score has a low rate of progression to symptomatic or fatal disease.4–6
These natural history data suggested that many cancers of the prostate may have an indolent clinical course and that a substantial proportion of patients with PCa may defer definitive intervention. Randomized trials have shown survival benefit associated with definitive primary treatment for intermediate- or high-risk cancers among younger men,7,8 but such treatment can impair quality of life.9–11 Consequently, several single-institution studies explored deferred treatment or active surveillance, with intention of treating patients if cancers showed a propensity for local progression during follow-up.12–14
Analyses of CaPSURE and Medicare cohorts suggest that such deferred treatment or active surveillance approaches are commonly used.15–18 However, there is a paucity of information from national cohorts regarding long-term outcomes of patients initially managed by deferred treatment or active surveillance. We investigated the Health Professionals Follow-up Study (HPFS) cohort to identify determinants of progression to treatment and to examine rates of clinical metastases and death as a result of PCa during the PSA era.
PARTICIPANTS AND METHODS
Study Participants
The HPFS is a prospective, ongoing nationwide cohort study, comprising 51,529 men who initially enrolled in 1986. Participants provided detailed information about medical history and risk factors for cancer, heart disease, and other diseases. The group is composed of 29,683 dentists, 4,185 pharmacists, 3,745 optometrists, 2,218 osteopathic physicians, 1,600 podiatrists, and 10,098 veterinarians. Among the study participants are 531 African Americans and 877 Asian Americans.
Every 2 years, questionnaires are mailed (response rate, 96%) inquiring about diseases and health-related topics, including whether PCa has been diagnosed. After a participant reports a diagnosis of PCa, medical records and pathology reports are sought to confirm the diagnosis and provide detailed information about the PCa diagnosis, pathology, treatments, PSA values, and treating physicians' contact information. Date of initial diagnosis and cancer severity measures are taken from the procured medical record. Information about PCa recurrence is also obtained. Treating physicians are contacted and asked to provide relevant medical records. Periodic follow-up questionnaires are sent to participants to ascertain treatment, PSA changes, and disease progression.
From 51,529 men within the HPFS cohort, 3,662 men reported a diagnosis of PCa between 1986 and 2007 and have provided clinical information postdiagnosis. For this analysis, we excluded 52 participants with 1 year or less of follow-up and 279 patients with incomplete treatment information. The excluded patients had pathologic characteristics similar to those of the patients included in the analysis, but their mean age was 2.4 years older. The remaining 3,331 participants were actively followed for a median of 8.0 years (range, 1.1 to 21.7 years). Among these, 342 men (10.3%) elected DT, and 2,989 opted for active treatment. For this analysis, DT was defined as no treatment for at least 1 year after the date of PCa diagnosis, because similar time frames have been used in prior studies of DT.15–18 The primary end point was time to initiation of active treatment, whereas the secondary end point was time to metastasis or death as a result of PCa. This study of PCa outcomes for the HPFS is approved by the institutional review board of the Harvard School of Public Health.
As covariates in the analysis, we evaluated age at diagnosis, race, height, and body mass index at diagnosis. We also considered clinical and pathologic characteristics including clinical stage, Gleason score, treatment type, PSA at diagnosis, and modified D'Amico criteria. Modified D'Amico criteria were used to divide patients into three prognostic groups: low (PSA ≤ 10 ng/mL, Gleason score < 7, and clinical stage T1 or T2), medium (PSA 10.1 to 20 ng/mL or Gleason score 7, with clinical stage T1 or T2), and high (PSA > 20 ng/mL, Gleason score greater than 7, or clinical stage T3 or greater).19 We used these modified criteria (that do not distinguish between T2 substages) because we were unable to distinguish clinical T2a versus T2b versus T2c substages from each other reliably and because the definitions of these substages were changed twice by the American Joint Commission on Cancer during the period of cohort enrollment and follow-up.
Statistical Analyses
Those who deferred treatment for at least 1 year were compared across categories of demographic indicators, tumor characteristics, and clinical characteristics with those who received immediate treatment, using the Fisher's exact test. The t test and Wilcoxon test were used to compare means and medians across groups. P ≤ .05 was considered significant. Among DT patients, Cox proportional hazards models were used to analyze associations between demographic indicators, tumor characteristics, clinical characteristics, and time to eventual treatment. Among all patients, Cox proportional hazards models were used to assess DT decisions and other covariates in relation to PCa progression (development of clinical metastasis or cancer-specific death). We checked the proportionality assumption by introducing a cross-product term of a specific variable of interest by a function of time into the model and testing for its statistical significance. No significant violation of the proportionality assumption was found.
All Cox proportional hazards models were adjusted for age and time of diagnosis (before 1992 v after 1992). Multivariate models included diagnostic factors that were significant in the age-adjusted models but also included other important clinical factors that were not significant. Hazard ratios (HRs) and 95% CIs were calculated. We used the most extreme category with the largest number of events as the reference group. When evaluating PCa prognostic risk group, the lowest level of risk was used as the reference group. In the analysis of time to treatment, DT participants contributed person-time starting 1 year after diagnosis and were censored at the date of last follow-up. In the analysis of time to metastasis or death as a result of PCa, participants contributed person-time starting at diagnosis and were censored at the date of last follow-up. When conducting Cox proportional hazards regression analyses, we took into account possible competing risks of other deaths by applying the method of Andersen et al.20 We also compared the incidence rates of metastatic disease and death as a result of PCa for the 342 DT participants and the 2,989 participants who were initially treated for PCa, using Fisher's exact tests. All analyses were conducted using SAS software, version 9 (SAS Institute, Cary, NC).
RESULTS
Those who underwent active treatment had a mean follow-up time of 8.6 years (standard deviation [SD], 4.5 years; median, 8.1 years; range, 1.1 to 21.7 years) while DT patients had a mean follow-up time of 8.3 years (SD, 4.6 years; median, 7.8 years; range, 1.1 to 21.2 years). Of the 3,331 participants diagnosed with PCa in our analysis, 342 (10.3%) elected DT. Table 1 lists the baseline sociodemographic, clinical, and pathologic characteristics of subjects stratified by treatment or DT subgroups. Compared with those undergoing active treatment, DT patients at diagnosis had significantly lower Gleason score (P = .002), had lower clinical stage (P < .0001), had lower PSA at diagnosis (P = .04), were 4.8 years older at diagnosis (P < .0001), and were 0.4 inches shorter. No patients younger than age 50 elected DT compared with 15 such patients who pursued active treatment (P < .0001).
Table 1.
Baseline Clinical and Pathologic Characteristics of Prostate Cancer Patients Diagnosed in the HPFS Cohort From July 1986 to May 2007
| Characteristic | Total (N = 3,331)* | Deferred Treatment (n = 342) | Any Treatment (n = 2,989)* | P† |
|---|---|---|---|---|
| Mean follow-up time, years | 8.6 | 8.3 | 8.6 | .24 |
| Age at diagnosis, years | ||||
| Mean | 68.4 | 72.7 | 67.9 | < .0001 |
| < 50 | 0.5 | 0 | 0.5 | < .0001 |
| 50-59 | 12.8 | 5.9 | 13.6 | |
| 60-69 | 44.6 | 27.8 | 46.5 | |
| 70-79 | 37.8 | 52.1 | 36.1 | |
| 80+ | 4.4 | 14.3 | 3.3 | |
| Race | ||||
| White | 96.7 | 95.7 | 96.9 | .25 |
| Black | 0.9 | 1.8 | 0.8 | |
| Asian | 1.1 | 1.2 | 1.1 | |
| Other | 1.3 | 1.2 | 1.3 | |
| Mean height, inches | 70.1 | 69.7 | 70.1 | .006 |
| BMI | ||||
| < 21 | 4.0 | 4.7 | 3.9 | .25 |
| 21 to < 25 | 39.5 | 42.9 | 39.1 | |
| 25 to < 30 | 46.0 | 44.5 | 46.2 | |
| 30+ | 10.5 | 7.9 | 10.8 | |
| Stage | ||||
| T1 | 51.7 | 63.2 | 50.5 | < .0001 |
| T2 | 41.4 | 34.1 | 42.2 | |
| T3 | 5.3 | 2.3 | 5.6 | |
| T4 | 0 | 0 | 0 | |
| N1/M1 | 1.6 | 0.3 | 1.7 | |
| PSA at diagnosis, ng/mL | ||||
| Median | 7.0 | 6.7 | 7.0 | .10 |
| < 4 | 11.8 | 16.5 | 11.3 | .04 |
| 4 to < 10 | 58.8 | 53.7 | 59.3 | |
| 10-20 | 20.0 | 21.7 | 19.8 | |
| > 20 | 9.4 | 8.1 | 9.6 | |
| Biopsy Gleason score | ||||
| < 6 | 21.2 | 28.9 | 20.4 | .002 |
| 6 | 45.6 | 46.9 | 45.5 | |
| 7 | 25.0 | 17.2 | 25.8 | |
| 8+ | 8.2 | 7.0 | 8.3 |
NOTE. Tabulated values are percentages unless otherwise indicated. The analysis does not include 279 participants whose treatment information was unavailable to determine initial cancer case status (eg, deferred treatment v active treatment).
Abbreviations: HPFS, Health Professionals Follow-up Study; BMI, body mass index; PSA, prostate-specific antigen.
Of the 2,989 participants who received immediate treatment, 1,599 had a radical prostatectomy, 549 had external radiotherapy monotherapy, 239 had brachytherapy monotherapy, 368 had androgen deprivation therapy plus external radiotherapy or brachytherapy, 181 had androgen deprivation monotherapy, and 53 had other types of treatment.
Deferred treatment v any treatment.
Of the men electing DT, 174 (51%) remained untreated throughout follow-up (mean follow-up of those who remained untreated, 7.7 years; range, 1.1 to 21.2 years), and the remaining 168 (49%) were treated an average of 3.9 years postdiagnosis. Table 2 lists the mean and median time from PCa diagnosis to treatment for the various treatment subgroups as well as the mean and median follow-up since PCa diagnosis for patients who remained untreated throughout follow-up.
Table 2.
Eventual Treatments and Average Time to Treatment Among HPFS Patients Initially Managed With Deferred Treatment
| Treatment Type | No. of Patients | Average Time to Treatment or Most Recent Follow-Up (years) Since PCa Diagnosis* |
|
|---|---|---|---|
| Mean | Median | ||
| Radical prostatectomy | 40 | 2.7 | 1.7 |
| XRT only | 35 | 3.5 | 2.5 |
| Brachytherapy only | 22 | 3.7 | 2.3 |
| ADT with radiation therapy | 37 | 5.9 | 3.3 |
| ADT only | 21 | 4.1 | 3.7 |
| Other therapy | 13 | 3.3 | 2.3 |
| Continuing without treatment | 174 | 7.7 | 6.8 |
| Total | 342 | ||
Abbreviations: HPFS, Health Professionals Follow-up Study; PCa, prostate cancer; XRT, external radiation therapy; ADT, androgen deprivation therapy.
For each treatment type, the end points were observed and the median follow-up was the median of the time from PCa diagnosis to the date of initiating treatment. For patients continuing without treatment, the end points were not observed and the median follow-up was the median of the time from PCa diagnosis to the date of last follow-up.
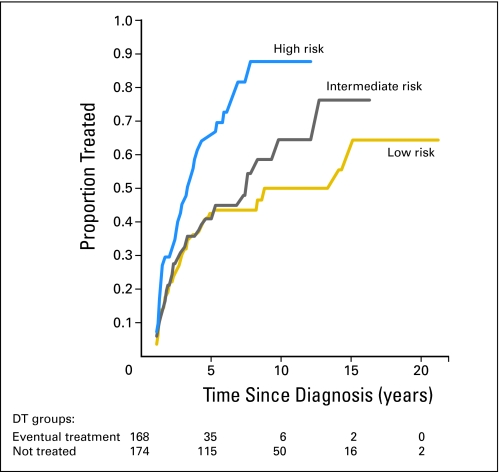
We sought to identify factors (among several demographic, pathologic, and clinical characteristics) associated with progression from DT to active treatment. For each factor, the hazard rate of the reference level for progressing from DT to active treatment was compared with those of other levels. Significant predictors of eventual active treatment after 1 year included younger age at diagnosis (P = .003), higher clinical stage (P = .005), higher PSA (P = .01), higher Gleason score (P = .01), or worse prognostic risk group category (the risk grouping combines PSA, stage, and Gleason score; P < .0001). On multivariate analysis, age at diagnosis, clinical stage, PSA at diagnosis, and Gleason score remained significant predictors of eventual active treatment (HRs are listed in Table 3). PCa prognostic risk group was evaluated separately, and the high-risk group was a significant predictor of eventual active treatment (HR of active treatment in the high-risk group relative to referent low-risk group, 2.33; 95% CI, 1.51 to 3.60). Figure 1 compares these risk strata using a cumulative incidence plot. Among initial DT cases, 38% of the low-risk group, 39% of the intermediate-risk group, and 60% of the high-risk group were treated within 5 years.
Table 3.
Association of Baseline Clinical and Pathologic Characteristics of Deferred Treatment Group With Progression to Treatment After 1 Year
| Characteristic | Age-Adjusted Hazard Ratio* | 95% CI | Multivariate Hazard Ratio† | 95% CI |
|---|---|---|---|---|
| Age at diagnosis, years | ||||
| < 60 | 2.10 | 1.15 to 3.84 | 2.15 | 1.16 to 3.97 |
| 60-69 | 1.71 | 1.23 to 2.38 | 1.80 | 1.27 to 2.53 |
| 70+ | 1.00 | 1.00 | ||
| Race | ||||
| White | 1.00 | 1.00 | ||
| Non-white | 1.07 | 0.50 to 2.28 | 1.11 | 0.51 to 2.39 |
| BMI | ||||
| < 25 | 1.07 | 0.78 to 1.45 | 1.08 | 0.79 to 1.48 |
| 25+ | 1.00 | 1.00 | ||
| Stage | ||||
| T1 | 1.00 | 1.00 | ||
| T2+ | 1.49 | 1.08 to 2.07 | 1.52 | 1.09 to 2.13 |
| PSA at diagnosis, ng/mL | ||||
| < 4 | 0.29 | 0.15 to 0.56 | 0.33 | 0.17 to 0.65 |
| 4 to < 10 | 0.51 | 0.31 to 0.85 | 0.57 | 0.34 to 0.96 |
| 10-20 | 0.55 | 0.31 to 0.97 | 0.61 | 0.34 to 1.08 |
| > 20 | 1.00 | 1.00 | ||
| Gleason score | ||||
| < 6 | 0.46 | 0.29 to 0.74 | 0.50 | 0.31 to 0.80 |
| 6 | 0.64 | 0.42 to 0.96 | 0.69 | 0.45 to 1.05 |
| > 6 | 1.00 | 1.00 |
Abbreviations: BMI, body mass index; PSA, prostate-specific antigen.
Adjusted for age and time of diagnosis.
Adjusted for age, time of diagnosis, clinical stage, PSA at diagnosis, and Gleason score.
Fig 1.
Cumulative incidence of treatment initiation among deferred treatment (DT) patients by prostate cancer risk group. Prostate cancer risk was stratified using a modification of the D'Amico risk criteria: low (prostate-specific antigen [PSA] ≤ 10 ng/mL, Gleason score < 7, and clinical stage T1 or T2), intermediate (PSA 10.1 to 20 ng/mL or Gleason score 7, with clinical stage T1 or T2), and high (PSA > 20 ng/mL, Gleason score > 7, or clinical stage T3 or greater. Ninety-four participants could not be classified by risk group and are not included in this figure.
We also examined deferred treatment versus immediate treatment for the joint outcome of PCa metastases or mortality (eg, lethal disease), in multivariate hazard models adjusted for age, clinical stage, PSA at diagnosis, Gleason score, and time of diagnosis (Table 4). As expected, in these models, clinical stage, PSA, and Gleason score were strongly predictive of the hazard of metastasis or death related to PCa. Furthermore, in a similar model, prognostic risk category was strongly predictive of lethal disease (intermediate-risk HR, 2.88; 95% CI, 1.83 to 4.54; high-risk HR, 5.76; 95% CI, 3.76 to 8.84). DT compared with immediate treatment was not associated with any significant increase in PCa mortality or metastasis, independent of these diagnostic clinical variables (multivariate HR, 1.03; 95% CI, 0.61 to 1.75).
Table 4.
Association of Deferred Treatment Decision and Baseline Clinical and Pathologic Characteristics of HPFS Patients With Incident Clinical Metastasis or Death As a Result of Prostate Cancer
| Baseline Characteristic | No. of Patients | No. of Events | Age-Adjusted Hazard Ratio* | 95% CI | Multivariate Hazard Ratio† | 95% CI |
|---|---|---|---|---|---|---|
| Treated within 12 months | 2,940 | 199 | 1.00 | 1.00 | ||
| Deferred treatment | 341 | 20 | 0.82 | 0.49 to 1.37 | 1.03 | 0.61 to 1.75 |
| Age at diagnosis, years | ||||||
| 40 to < 60 | 434 | 25 | 0.77 | 0.48 to 1.23 | 0.89 | 0.55 to 1.43 |
| 60 to < 70 | 1,467 | 95 | 0.84 | 0.61 to 1.15 | 0.89 | 0.65 to 1.23 |
| 70+ | 1,380 | 99 | 1.00 | 1.00 | ||
| Stage at diagnosis | ||||||
| T1 | 1,630 | 65 | 1.00 | 1.00 | ||
| T2 | 1,306 | 116 | 1.73 | 1.24 to 2.44 | 1.71 | 1.21 to 2.42 |
| T3 | 168 | 31 | 3.70 | 2.32 to 5.92 | 2.24 | 1.38 to 3.66 |
| PSA at diagnosis, ng/mL | ||||||
| 0 to < 4 | 370 | 6 | 0.06 | 0.02 to 0.24 | 0.07 | 0.02 to 0.28 |
| 4 to < 10 | 1,853 | 91 | 0.50 | 0.37 to 0.69 | 0.58 | 0.43 to 0.80 |
| 10+ | 898 | 104 | 1.00 | 1.00 | ||
| Gleason score at diagnosis | ||||||
| 2-5 | 607 | 36 | 0.30 | 0.19 to 0.47 | 0.38 | 0.24 to 0.59 |
| 6 | 1,316 | 48 | 0.34 | 0.23 to 0.50 | 0.43 | 0.29 to 0.64 |
| > 6 | 934 | 97 | 1.00 | 1.00 |
NOTE. Fifty participants who had metastases at initial diagnosis were excluded from this calculation. For this time-to-event analysis, the starting point was the date of diagnosis. The end point was the date of metastases or death as a result of prostate cancer (PCa), or the end of follow-up, whichever came first. An event occurred if a PCa patient developed metastases or died as a result of PCa before the end of follow-up. A censoring occurred if a PCa patient's disease did not metastasize or the patient did not die as a result of PCa until the end of follow-up.
Abbreviations: HPFS, Health Professionals Follow-Up Study; PSA, prostate-specific antigen.
Adjusted for age and time of diagnosis.
Adjusted for age, time of diagnosis, clinical stage, PSA at diagnosis, and Gleason score.
We observed similar rates for development of clinical metastases (n = 20 and n = 199; 7.2 events v 8.1 events per 1,000 person-years; P = .68) and death related to PCa (n = 8 and n = 80; 2.4 deaths v 2.6 deaths per 1,000 person-years; P = .99) for DT and immediate treatment groups, respectively. Among patients who began in the DT group, three of 139 low-risk patients and four of 109 intermediate- or high-risk patients died as a result of PCa during follow-up, whereas seven of 139 low-risk patients and eight of 108 intermediate- or high-risk patients (excluding one patient with metastasis at diagnosis) developed clinical metastases. Among patients who were initially treated, nine of 1,252 low-risk patients and 62 of 1,366 intermediate- or high-risk patients died as a result of PCa during follow-up, whereas 33 of 1,252 low-risk patients and 138 of 1,317 intermediate- or high-risk patients (excluding 49 patients with metastases at diagnosis) developed clinical metastases.
DISCUSSION
In our contemporary, nationwide cohort of 3,331 health professionals with PCa, 342 (10.3%) deferred treatment. Men opting for DT had significantly lower Gleason score, lower clinical stage, lower PSA, and older age at diagnosis than men undergoing active treatment. Fifty-one percent of DT patients remained untreated for the duration of follow-up. Significant predictors of progression from DT to active treatment after 1 year included younger age, higher clinical stage, higher PSA, higher Gleason score, or worse prognostic risk group category. With multivariate analysis, age at diagnosis, clinical stage, PSA at diagnosis, and Gleason score remained significant predictors of eventual active treatment. No statistically significant difference was observed between DT and early active treatment groups with respect to development of metastatic disease or death as a result of PCa. While these results suggest that DT may be a safe option for carefully selected men, these findings must be interpreted cautiously because statistical power was limited due to the small sample size.
Before the advent of PSA screening, some prospective and retrospective studies suggested that localized PCa having a low or intermediate Gleason score has a low rate of progression to symptomatic or fatal disease. Albertsen et al5 reviewed 767 prospectively followed patients (median observation, 24 years) showing low likelihood of cancer death. Johansson et al6 prospectively followed 223 men with localized cancer (mean, 21 years), noting minimal survival benefit from intervention within 10 to 15 years but significant decrease in progression-free, metastasis-free, and cancer-specific survival with intervention delay at 15 to 20 years of follow-up. Chodak et al4 retrospectively reviewed 828 watchful waiting cases and calculated an 87% disease-specific survival 10 years after diagnosis for men with grade 1 or 2 tumors. While these and similar data sets are historically important in understanding the natural history of untreated cancer, their patients were diagnosed before the PSA era and are thus less easily generalized to contemporary urology practices in which PSA testing plays an integral role in PCa management. Moreover, these data reflect conservative management wherein primary treatment was not included in the management schema for many patients; in contrast, contemporary approaches to deferred treatment embrace primary therapy as an appropriate option to be exercised in the setting of progressive biology or increased histopathologic aggressiveness during follow-up monitoring.
Several experiences with DT during the PSA era have previously been published, although these are typically from single-institution referral centers. Zietman et al12 retrospectively reviewed 199 men managed by watchful waiting (WW), showing 74% likelihood of progression to treatment or all-cause mortality within 7 years. Carter et al13 followed 81 men with localized PCa (median, 23 months; range, 12 to 58 months), with 31% developing disease progression. Patel et al21 followed 88 patients with localized cancer for a median of 44 months (range, 7 to 172 months), reporting that 38% eventually pursued active treatment. Klotz14 followed 299 patients with localized cancer (median, 55 months) with 40% opting for active treatment. Dall'Era et al22 followed 321 men with localized disease (median, 3.6 years; range, 1 to 17 years), noting that 24% received secondary treatment at a median of 3 years. Our study includes patients drawn from nationwide community urology practices, and therefore may be more generalizable than single-institution studies. Our study also extends the duration of follow-up among contemporary cohorts of deferred treatment in the post-PSA era, with half the subjects followed for more than 7 years compared with earlier post-PSA era studies that were generally limited to less than 5 years of follow-up.
A combination of the known morbidity of PCa therapy, increasing information regarding treatment-related quality of life, and successful utilization of active surveillance in the aforementioned single-institution studies gradually led to a growing acceptance of DT as a management strategy for appropriately selected PCa patients.14,23 Miller et al18 utilized Surveillance, Epidemiology and End Results (SEER) data to delineate the incidence of initial curative therapy for men with low-risk PCa defined by age and grade criteria, observing that only 55% of these men underwent initial curative therapy between 2000 and 2002, while 45% underwent expectant management or androgen deprivation therapy. Although Koppie et al15 noted that 18.5% of low-risk CaPSURE patients defined by D'Amico criteria pursued WW as primary management in an early analysis of this cohort, this population compared with the SEER population was somewhat younger, had a lower proportion of nonwhite patients, and may differ in other ways.
CaPSURE is the only community-based, PSA-era, multiregional US cohort that has previously described aspects of WW as initial management. Koppie et al15 characterized WW CaPSURE patients as more likely to be older than age 74, white race, organ-confined disease, and Gleason score ≤ 7, with a 55% likelihood of treatment initiation within 5 years of diagnosis. Arredondo et al17 evaluated health-related quality of life among WW patients and found no statistically significant trends over time except reduction in sexual function score. Differences exist between the current cohort and the CaPSURE database. Whereas CaPSURE uses data acquired from patients in participating urology practices, this study is patient-centered and perhaps less susceptible to urologist selection bias toward patient treatment.
This study has some limitations. First, although the database provides information on a nationwide cohort of men, there was no uniform management regimen for health care providers. It is possible, however, that the lack of centralization in management may allow these findings to be generalized more readily than those from single-institution referral center studies. Second, the cohort of patients undergoing DT includes both WW patients (with no intention of future treatment) and active surveillance patients (with potential future treatment) and, as a result, may not distinguish differential impacts of WW versus active surveillance intent. Third, some patients with aggressive PCa have died between the inception of HPFS and the initiation of PCa recurrence follow-up in 2000. Such patients were not included in this study, allowing a possible skew toward patients with less aggressive disease (possibly bringing the DT group closer in line with contemporary DT cohorts). To address this limitation, our comparison of metastases and mortality between those opting for immediate treatment and those opting for deferred management used multivariate models, adjusted for timing of initial diagnosis and controlled for cancer severity. We found the relative risk to be similar between the patient groups, but the numbers of metastases and deaths as a result of PCa were low, potentially underpowering this analysis.
DT or WW was successfully used in this contemporary, American nationwide cohort, wherein more than half the men who opted for DT remained free of treatment for 7.7 years after diagnosis. Older men and men with lower cancer severity measures were more likely to remain untreated. Rates of PCa metastases or death among those men with low-risk cancer who opted for DT were similar to the rates of those who were initially treated, suggesting that appropriately selected patients may safely defer treatment for many years.
Acknowledgment
We thank the participants and staff of the Prostate Cancer Follow-up Study Survivor's Cohort for their valuable contributions.
Footnotes
Supported by Grants No. T32CA09001 (M.J.S.), R01CA95662 and U01CA113913 (M.G.S.), and P01CA55075 (J.M.C.) from the National Institutes of Health.
W.V.S. and S.A.K. share lead authorship. M.G.S. and J.M.C. share senior authorship.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
See accompanying editorial on page 4935
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Martin G. Sanda, GTx (C), Amgen (C) Stock Ownership: None Honoraria: Martin G. Sanda, Eli Lilly Research Funding: Martin G. Sanda, Beckman Coulter Expert Testimony: Martin G. Sanda, CRICO/RMF (C) Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: William V. Shappley III, Stacey A. Kenfield, Julie L. Kasperzyk, Meir J. Stampfer, Martin G. Sanda, June M. Chan
Financial support: Meir J. Stampfer, Martin G. Sanda
Administrative support: Meir J. Stampfer, Martin G. Sanda
Provision of study materials or patients: Meir J. Stampfer
Collection and assembly of data: William V. Shappley III, Stacey A. Kenfield, Julie L. Kasperzyk, June M. Chan
Data analysis and interpretation: William V. Shappley III, Stacey A. Kenfield, Julie L. Kasperzyk, Weiliang Qiu, Martin G. Sanda, June M. Chan
Manuscript writing: William V. Shappley III, Stacey A. Kenfield, Julie L. Kasperzyk, Weiliang Qiu, Martin G. Sanda, June M. Chan
Final approval of manuscript: William V. Shappley III, Stacey A. Kenfield, Julie L. Kasperzyk, Weiliang Qiu, Meir J. Stampfer, Martin G. Sanda, June M. Chan
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2008: Estimated New Cancer Cases and Deaths by Sex, US, 2008. http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
- 2.Sakr WA, Grignon DJ, Crissman JD, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: An autopsy study of 249 cases. In Vivo. 1994;8:439–443. [PubMed] [Google Scholar]
- 3.Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Chodak GW, Thisted RA, Gerber GS, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. 1994;330:242–248. doi: 10.1056/NEJM199401273300403. [DOI] [PubMed] [Google Scholar]
- 5.Albertsen PC, Fryback DG, Storer BE, et al. Long-term survival among men with conservatively treated localized prostate cancer. JAMA. 1995;274:626–631. [PubMed] [Google Scholar]
- 6.Johansson JE, Holmberg L, Johansson S, et al. Fifteen-year survival in prostate cancer: A prospective, population-based study in Sweden. JAMA. 1997;277:467–471. [PubMed] [Google Scholar]
- 7.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 8.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 9.Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239–2247. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 10.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the prostate cancer outcomes study. J Natl Cancer Inst. 2000;92:1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 11.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 12.Zietman AL, Thakral H, Wilson L, et al. Conservative management of prostate cancer in the prostate specific antigen era: The incidence and time course of subsequent therapy. J Urol. 2001;166:1702–1706. [PubMed] [Google Scholar]
- 13.Carter HB, Walsh PC, Landis P, et al. Expectant management of nonpalpable prostate cancer with curative intent: Preliminary results. J Urol. 2002;167:1231–1234. [PubMed] [Google Scholar]
- 14.Klotz L. Active surveillance with selective delayed intervention: Using natural history to guide treatment in good risk prostate cancer. J Urol. 2004;172:S48–S50. doi: 10.1097/01.ju.0000141712.79986.77. [DOI] [PubMed] [Google Scholar]
- 15.Koppie TM, Grossfeld GD, Miller D, et al. Patterns of treatment of patients with prostate cancer initially managed with surveillance: Results from the CaPSURE database. Cancer of the Prostate Strategic Urological Research Endeavor. J Urol. 2000;164:81–88. [PubMed] [Google Scholar]
- 16.Scherr D, Swindle PW, Scardino PT. National Comprehensive Cancer Network. National Comprehensive Cancer Network guidelines for the management of prostate cancer. Urology. 2003;61:14–24. doi: 10.1016/s0090-4295(02)02395-6. [DOI] [PubMed] [Google Scholar]
- 17.Arredondo SA, Downs TM, Lubeck DP, et al. Watchful waiting and health related quality of life for patients with localized prostate cancer: Data from CaPSURE. J Urol. 2004;172:1830–1834. doi: 10.1097/01.ju.0000140758.04424.77. [DOI] [PubMed] [Google Scholar]
- 18.Miller DC, Gruber SB, Hollenbeck BK, et al. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98:1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 19.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 20.Andersen PK, Borgan O, Gill RD, et al. New York, NY: Springer; 1993. Statistical Models Based on Counting Processes; p. 495. [Google Scholar]
- 21.Patel MI, DeConcini DT, Lopez-Corona E, et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171:1520–1524. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 22.Dall'Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 23.el-Geneidy M, Garzotto M, Panagiotou I, et al. Delayed therapy with curative intent in a contemporary prostate cancer watchful-waiting cohort. BJU Int. 2004;93:510–515. doi: 10.1111/j.1464-410x.2003.04669.x. [DOI] [PubMed] [Google Scholar]