Abstract
Changes in the hemostatic system and chronic hemostatic activation are frequently observed in patients with cancer, even in the absence of venous thromboembolism (VTE). VTE is a leading cause of death among patients with cancer and contributes to long-term mortality in patients with early as well as advanced-stage cancer. Mounting evidence suggests that components of the clotting cascade and associated vascular factors play an integral part in tumor progression, invasion, angiogenesis, and metastasis formation. Furthermore, there are intriguing in vitro and animal findings that anticoagulants, in particular the low molecular weight heparins (LMWHs), exert an antineoplastic effect through multiple mechanisms, including interference with tumor cell adhesion, invasion, metastasis formation, angiogenesis, and the immune system. Several relatively small randomized controlled clinical trials of anticoagulation as cancer therapy in patients without a VTE diagnosis have been completed. These comprise studies with LMWH, unfractionated heparin, and vitamin K antagonists, with overall encouraging but nonconclusive results and some limitations. Meta-analyses performed for the American Society of Clinical Oncology VTE Guidelines Committee and the Cochrane Collaboration suggest overall favorable effects of anticoagulation on survival of patients with cancer, mainly with LMWH. However, definitive clinical trials have been elusive and questions remain regarding the importance of tumor type and stage on treatment efficacy, the impact of fatal thromboembolic events, optimal anticoagulation therapy, and safety with differing chemotherapy regimens. Although the LMWHs and related agents hold promise for improving outcomes in patients with cancer, additional studies of their efficacy and safety in this setting are needed.
INTRODUCTION
Patients with cancer have a substantially increased risk of venous thromboembolism (VTE) compared with patients without cancer.1–8 Changes in the hemostatic system and evidence of chronic hemostatic activation, including disseminated intravascular coagulation, are frequently observed in patients with cancer, even in the absence of VTE.9–14 There are many links between the hemostatic system and tumor cells, including fibrin or fibrinogen surrounding the tumor tissue bed.15–18 It has been postulated that components of the clotting cascade and associated vascular factors play an integral part in tumor progression, invasion, angiogenesis, and metastasis formation.19–23 Furthermore, intriguing in vitro and animal findings suggest that anticoagulants, in particular the low molecular weight heparins (LMWH), exert an antineoplastic effect, most likely by interfering with metastasis formation.24–42
ASSOCIATION OF VTE WITH MORTALITY IN PATIENTS WITH CANCER
VTE is associated with significant morbidity, including hospitalization, reduced pulmonary function, and post-thrombotic syndrome. Compared with patients without cancer, patients with cancer experience increased VTE recurrences and bleeding complications on anticoagulation therapy.7,43 In addition, a venous thrombotic event may impact the chemotherapy schedule and potential future therapeutic choices. The most concerning sequela remains the increased risk of mortality with VTE. Several reports have shown that VTE is a leading cause of death among patients with cancer.44,45 Sorensen et al46 were among the first to define the worse prognosis and reduced survival of patients with cancer with VTE compared with control patients matched by age, sex, cancer type, and year of diagnosis. Chew et al47 evaluated retrospectively the impact of VTE among patients in the California Cancer Registry and found that after adjusting for stage, VTE patients with cancer continue to experience increased mortality. In a subsequent analysis restricted to patients with breast cancer, increased mortality again was observed even after adjusting for comorbid conditions. This appeared most pronounced in early-stage patients and the first few months after VTE diagnosis.48 Based on a prospectively accrued, and closely followed cancer cohort, including more than 4,000 patients with solid tumor and lymphoma starting a new chemotherapy regimen at 115 oncology practices throughout the United States, Kuderer et al49 determined that VTE is an independent risk factor for mortality during the initial months of chemotherapy in patients with cancer of all stages. This remained the case after adjusting for all major risk factors including age, sex, ethnicity, cancer type and stage, chemotherapy type and relative dose intensity, performance status, body mass index, comorbid conditions, and major laboratory abnormalities.49 Additional research is needed to clarify if excess death in VTE patients with cancer is mainly due to more aggressive disease versus due to the occurrence of fatal thromboembolic events.
PROTHROMBOTIC MECHANISMS DUE TO CANCER
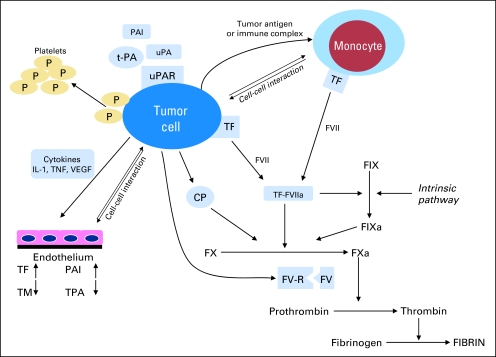
Evidence of hemostatic activation is a common finding in most patients with cancer.10,12,50,51 Patients with cancer exhibit numerous patient-, disease-, and treatment-related predisposing factors for venous thrombosis. There are many ways that the tumor may contribute to a hypercoagulable state, including acute phase reactions, hemodynamic changes, tissue necrosis, and aberrant protein metabolism.51,52 However, probably the most important factors contributing to the general prothrombotic state in patients with cancer derive from the tumor cells themselves. Their specific prothrombotic properties and their ability to further induce a hypercoagulable environment have been reviewed previously, in particular by Falanga et al51,52 (Fig 1), and will be addressed in further detail elsewhere in this special issue.
Fig 1.
The tumor cell promotes a hypercoagulable state and activates the hemostatic system, utilizing cell surface proteins such as tissue factor (TF), cancer procoagulant (CP), tissue plasminogen activator (t-PA), urokinase plasminogen activator (uPA), as well as plasminogen activator inhibitor 1 (PAI-1) and 2 (PAI-2). Interaction with other blood cells (eg, monocytes, platelets, endothelial cells) occurs (A) directly by cell-cell interaction; or (B) indirectly by cytokine release promoting prothrombotic endothelial changes. IL, interleukin; P, protein; F, factor; TM, thrombomodulin; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; R, receptor; FV-R, factor V receptor. Adapted from Falanga.5
Briefly, tissue factor (TF), cancer procoagulant (CP), and to a lesser extent tumor mucins are the best described tumor procoagulants. TF is a transmembrane protein that forms a macromolecular complex with factor VII to activate both factor IX and X.53 CP is mainly found in malignant tissue and is a 68kDa cysteine protease that activates factor X directly, independent of factor VII.54 Both TF and CP are expressed in large numbers in human and animal tumors including leukemic cells, particularly the promyelocytic leukemic subtype.54–62
Most components of the fibrinolytic system are found to be expressed in tumor cells. These include tissue plasminogen activator (t-PA), urokinase plasminogen activator (u-PA), as well as plasminogen activator inhibitor 1 (PAI-1) and 2 (PAI-2).63–65 In addition to their role in hemostasis, fibrinolytic factors participate in the process of tumor invasion necessary for metastasis formation and may contribute to the increased risk of bleeding.55,64,65 Tumor cells also release cytokines, such as tumor necrosis factor (TNF), interleukin-1(IL-1), and vascular endothelial growth factor (VEGF), which in turn promote prothrombotic endothelial changes and angiogenesis.18,66–69 IL-1β and TNF-α together with bacterial lipopolysaccharides (endotoxins) increase the endothelial expression of TF and PAI-1, while downregulating thrombomodulin (TM), with resulting decrease in protein C activation, one of the principal anticoagulant defense systems.18,66,67,69–73 Cytokine release and endotoxins can also rapidly increase t-PA, which tends to be followed by an even larger rise in PAI-1, resulting in an overall procoagulant state.68 At the same time, endothelial activation by IL-1β and TNF-α leads to increases in expression of endothelial adhesion molecules. These enable tumor-endothelial cell binding and likely facilitate tumor cell extravasation and invasion.74–77 In addition, tumor cells have the ability to interact either directly or through soluble mediators with other blood cells, especially monocytes, macrophages, and platelets, which mainly promote thrombosis through the clotting cascade or platelet activation.
IMPACT OF HEMOSTATIC SYSTEM ON CANCER GROWTH AND PROGRESSION
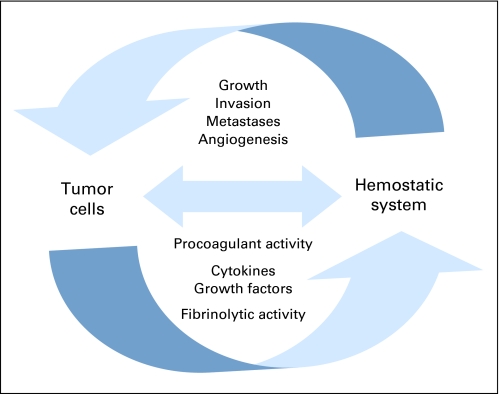
Patients presenting with idiopathic venous thrombosis are at increased risk of developing cancer in subsequent years.78–81 Thrombosis may be an early manifestation of an occult tumor. However, there is mounting evidence that the hemostatic system itself contributes to tumor cell survival and cancer progression. Several mechanisms have been implicated, in which various factors of the hemostatic system may aid in cancer cell survival, proliferation, invasion, metastasis formation, and tumor blood vessel formation (Fig 2).21,82,83
Fig 2.
Positive feedback loop between tumor and the hemostatic system: tumor can promote a procoagulant state. Components of the activated hemostatic system, in particular the platelet-fibrin–rich tumor microthrombi, also promote tumor growth, angiogenesis, invasion, and metastasis formation.
Fibrin deposits surrounding tumors and tumor cells may not only protect against immunologic attack,15–17 but also form a necessary matrix or support stroma for tumor tissue,21,22,84,85 and stimulate angiogenesis.86 Next to its dual role in fibrin formation and platelet activation, thrombin appears to contribute in concert with TF to tumor cell invasion and metastasis formation.87,88 Furthermore, thrombin is believed to act as an autocrine growth and proinflammatory stimulant through cleavage and activation of protease activated receptors (PARs), and can induce the expression of c-myc proto-oncogene and TF.21,89–96
In its role as primary initiator of hemostasis, TF results in thrombin and fibrin matrix formation. Furthermore, TF contributes to angiogenesis, tumor cell migration, and tumor progression by various pathways that include clotting-dependent and clotting-independent mechanisms and can result in direct or indirect induction of growth hormones, for example, vascular endothelial growth factor (VEGF).83,97,98 TF and VEGF closely colocalize and correlate with microvessel density, disease progression, and more advanced disease in breast and other cancers.97,99–102 The TF-VIIa complex directly induces tumor production of VEGF, other growth factors, and cytokines through signaling, mainly via PARs as well.103–106 TF expression and activity is an important determinant of tumor metastatic potential and TF inhibition decreases metastasis development in animal models.99,107–110 The TF-VIIa complex inhibits apoptosis and prolongs tumor cell survival through thrombin-independent cellular pathways, including signaling via p44/42 MAPK and Jak/STAT pathways.111,112
Platelets may contribute to tumor cell survival in the bloodstream and overall tumor growth. Tumor cells entering blood vessels quickly recruit platelets to form tumor-platelet aggregates and later microthrombi that also include fibrin and leukocytes.113,114 These tumor microthrombi help cancer cells survive the hostile environment in blood vessels and facilitate lodging at distant sites.113,114 Platelets also contribute to angiogenesis and potentially to tumor growth by secreting a variety of growth and stimulatory factors, including VEGF, platelet-derived growth factor, transforming growth factor, thrombin, and fibrinogen.113–115
P- and L-selectins play a critical role in cancer cell interaction with endothelial cells, platelets, and leukocytes and are important for adhesion and metastasis formation.30,35,82,116–118 Neutrophils and monocytes that are commonly found around tumor blood vessels contribute to metastasis formation by facilitating tumor cell extravasation.119,120 More importantly, knockout mice for P- or L-selectin demonstrate a marked attenuation of metastasis formation, and double-deficient mice have a near abrogation of metastases.30,82,116–118
ANTINEOPLASTIC EFFECTS OF ANTICOAGULANTS IN PRECLINICAL STUDIES
As early as the 1930s, heparins were reported to interfere with several vital steps of tumor progression and in particular metastasis formation in laboratory animals, including tumor growth, tumor cell motility, migration, invasion, metastases development, and cancer survival.26,121–127 Numerous studies confirmed that heparins prolong survival of laboratory animals after tumor cell inoculation.26–34,36 This antineoplastic effect of heparin likely involves multiple mechanisms, as has been investigated by numerous preclinical studies.
Antiangiogenic Effect of Heparins and Impact on Growth Factors
In addition to inhibiting or decreasing fibrin and thrombin formation and their secondary stimulatory effects,21,84,128 heparins have significant antiangiogenic properties.129–136 In vitro studies have shown that LMWHs in contrast to heparin can inhibit the activity of VEGF and basic fibroblast growth factor (bFGF), resulting in reduced endothelial cell growth.132,133,136 This phenomenon appears to be dependent on the specific molecular weight of heparins and was not observed with the pentasaccharide fondaparinux.132,133,136 Heparins also interfere with angiogenesis indirectly by reducing TF expression.137
Antiproliferative Effects of Heparins
In most preclinical studies, the antiproliferative effects of LMWH and unfractionated heparin (UFH) did not directly affect primary tumor growth,31,33,34 but rather interfered with metastasis formation.26,28–31,33–36 Heparins' main antiproliferative effect is on noncancer cells, such as endothelial cells, epithelial cells, fibroblasts, and vascular smooth muscles.133,138–140 Heparins also have been implicated in inducing differentiation and apoptosis in in vitro studies.32,37,141
Effects of Heparins on the Metastatic Process: Cancer Cell Adhesion and Migration
Tumor cell migration and vascular adhesion is an integral part of metastasis development. Heparins intervene in several steps of the metastatic process. For any cellular movement through tissue and endothelial cell layers, the extracellular matrix needs to be modulated and degraded. The cancer cell achieves this with the secretion of heparinases and other proteolytic enzymes. Heparins reduce tumor invasiveness by inhibiting heparinases and other extracellular matrix components.36,142,143 The binding and inhibition of P- and L-selectins by heparin is a key mechanism by which it interferes with crucial cancer cell interactions with endothelium, platelets, and leukocytes, vital for metastasis development as previously discussed.30,35,82,116–118,144
Effects of Heparins on the Immune System
Natural killer (NK) cells are key players in the destruction of circulating tumor cells before lodging at a distant site. The NK cell tumoricidal activity appears to be enhanced by both LMWH and UFH through increased activity of TNF and interferon in mice experiments.145 As mentioned previously, heparin also blocks crucial selectin binding for leukocyte-mediated tumor cell extravasation.82,117
Antineoplastic Effects of Warfarin
There is less evidence to suggest that vitamin K antagonists exert an antineoplastic effect. Nevertheless, some evidence indicates that warfarin may reduce metastasis formation in part by activating macrophages.146 Warfarin may also have an inhibitory effect on carcinoma-induced and bFGF-associated angiogenesis.100,147
CLINICAL TRIAL EVIDENCE OF ANTICOAGULATION IMPACT ON CANCER SURVIVAL
While heparins have been implicated in potentially enhancing the therapeutic effects of surgery and chemotherapy,38,148–153 this has never been conclusively confirmed. Evidence in support of the hypothesis that anticoagulation may improve cancer survival comes otherwise from two major types of clinical studies. The first category was of patients treated for established VTE with different anticoagulants to determine which agent was most effective in preventing recurrent thrombosis. In these trials, survival was either not assessed at all or evaluated as a secondary outcome. In the second category of studies, the primary goal was to study the impact of anticoagulants on cancer survival in patients without a diagnosis of VTE. The results of both types of studies support the hypothesis that the administration of anticoagulants may improve survival independent of thrombosis.
Clinical Trials of Anticoagulation As VTE Therapy in Patients With Cancer With VTE
Clinical trials of anticoagulation in patients with documented VTE also included patients with cancer. Subgroup analyses of patients with cancer in these trials have suggested that initial LMWH can improve survival compared with UFH. Prandoni et al154 compared initial treatment of patients with proximal deep vein thrombosis (DVT) with either LMWH or UFH. During the 6 months of follow-up, 44% of the patients with cancer in the UFH group died compared with 7% of patients on LMWH (P = .02). Meta-analyses of this and several subsequent randomized trials of symptomatic VTE demonstrated improved survival in patients with cancer on LMWH compared with UFH.155–158 Hettiarachchi et al157 compared the mortality rates of LMWH versus UFH treatment for DVT in patients with cancer across nine randomized controlled trials in general medical patients with cancer representing 18% of the trial population. Among patients with cancer receiving LMWH, fewer deaths were reported in the first 3 months than in those receiving UFH (odds ratio, 0.61; 95% CI, 0.40 to 0.93). However, it is surprising that such a difference would occur with only brief initial exposure differences to either LMWH or UFH, which were both subsequently followed by the same vitamin K antagonist. Given the post hoc analyses of cancer subgroups in these trials, their results remain hypothesis generating only.
The CLOT study, a large randomized controlled trial of VTE treatment in patients with cancer, was conducted comparing LMWH (dalteparin) for 6 months to dalteparin for a brief initial period followed by a vitamin K antagonist.159 Whereas the primary outcome of this trial was recurrent thrombosis, survival was assessed as a secondary outcome and not found to be improved in the dalteparin arm compared with controls. A post hoc subgroup analysis, focusing only on the patients with nonmetastatic disease, reported the 12-month all-cause mortality at 20% in the dalteparin group compared with 35% in vitamin K antagonist treated patients (P = .04).160 However, in patients with metastatic cancer, no survival difference was observed between dalteparin (72%) and oral anticoagulant (69%) study arms (P = .46).
Clinical Trials of Anticoagulation As Cancer Therapy in Patients With Cancer Without VTE
Several randomized controlled trials have been undertaken to directly study the impact of anticoagulant therapy (LMWH, UFH, and warfarin) on overall survival in patients with cancer without VTE.
Small-Cell Lung Cancer Trials
In an early anticoagulation survival study, Zacharski et al161 randomly assigned patients with lung, colon, head and neck, and prostate cancer to standard treatment with or without the addition of warfarin for an average of 26 weeks. Significant improvements in time to tumor progression and in overall survival were reported among the 50 patients with small-cell lung cancer, while no difference in survival was observed in other tumor types. In another study, 328 patients with small-cell lung cancer receiving chemotherapy were randomly assigned to concurrent warfarin or not.162 A significant improvement in objective tumor response was observed with a trend toward improved overall survival. A subsequent study of warfarin in 347 patients with limited-stage small-cell lung cancer demonstrated no significant improvement in response rate, disease-free, or overall survival.163 A recent randomized controlled trial of LMWH in 84 patients with small-cell lung cancer demonstrated median progression-free survival of 10 months with LMWH compared with 6 months in chemotherapy control patients (P = .01) with improvements observed in patients with both limited and extensive disease.164 In a multicenter trial of 277 patients with small-cell lung cancer randomly assigned to chemotherapy alone or with UFH for 5 weeks,165 patients receiving UFH experienced improved response rates (P = .04), and median survival (P = .01) with greatest benefit in limited-stage disease. Whereas most studies of anticoagulants as cancer treatment have demonstrated improved survival in small-cell lung cancer, such studies have been limited by small sample size, heterogeneous cancer patient populations potentially leading to an imbalance of important prognostic factors between randomization arms, outdated chemotherapy, and limited data on thromboembolic and bleeding complications.
Trials in Other Cancer Types
The impact of LMWH on survival in other tumor types has also been studied in randomized trials. In a study of 385 patients with advanced malignancy receiving standard treatment with or without dalteparin or placebo for 1 year, Kakkar et al166 observed no significant differences in survival up to 3 years. However, in a post hoc landmark analysis of 102 patients still alive at 17 months, significant improvement in survival was found for patients receiving dalteparin. Although the improved outcome in patients with less advanced cancer is consistent with studies discussed above, these results must be interpreted with caution. In another LMWH trial, 302 patients with locally advanced or metastatic solid tumors were randomly assigned to nadroparin or placebo for 6 weeks.167 With a mean follow-up of 1 year, a significant improvement in overall survival was observed (relative risk, 0.75; 95% CI, 0.59 to 0.96; P = .02) among patients receiving nadroparin. Sideras et al168 could not confirm these findings in a trial with 144 advanced solid tumor patients randomly assigned to standard treatment with or without dalteparin, but results were limited by small sample size.
Meta-Analyses of Anticoagulation Therapy
Before 2004, there were several meta-analyses suggesting improved survival in patients with cancer on anticoagulation therapy in the absence of venous thrombosis.169–172 However, results of these studies were confounded by including nonrandomized studies or favorable post hoc subgroup analyses from trials in general medical patients. They also preceded the publication of several more recent trials,164,167,168,173–175 and reporting on bleeding events was limited.
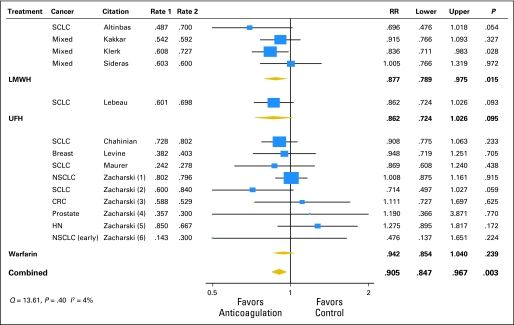
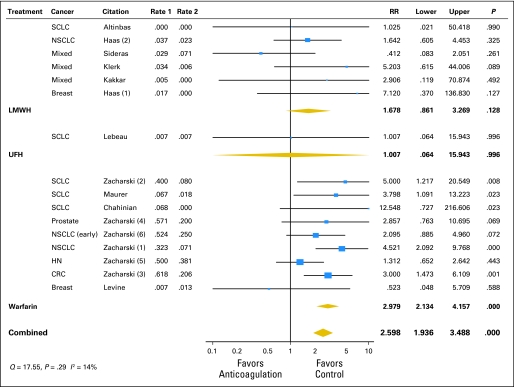
As part of the activities of the American Society of Clinical Oncology Venous Thromboembolism Guidelines Panel, Kuderer et al176 performed a meta-analysis and systematic review of all RCTs of the efficacy and safety of anticoagulant trials (LMWH, UFH, vitamin K antagonists) in the treatment of patients with cancer without venous thrombosis. Across the 11 eligible trials, anticoagulation significantly decreased overall 1-year mortality with a relative risk of 0.905 (95% CI, 0.85 to 0.97; P = .003; Fig 3). For LMWH, the relative risk for mortality was 0.88 (95% CI, 0.79 to 0.98; P = .015), compared with a nonsignificant effect of warfarin, resulting in an absolute risk reduction in mortality of 8% for LMWH. Major bleeding episodes occurred less frequently in patients who received LMWH compared with those who received warfarin (Fig 4), with an absolute risk increase of 1% in bleeding with LMWH therapy compared with 11.5% with warfarin. Although this difference in major bleeding between LMWH and warfarin could be in part a result of differences in study design and trial population, these results are consistent with previous findings.159,177–181 Overall, fatal bleeding was a rare event. Most trials did not report on thrombosis outcomes.176 These findings were confirmed in subsequent reviews, including the Cochrane Collaboration with their separate reports on heparins and vitamin K antagonists.182–184
Fig 3.
Meta-analysis of anticoagulation studies evaluating the impact on mortality in cancer patients without venous thrombosis: 1-year overall mortality by type of anticoagulation. SCLC, small-cell lung cancer; LMWH, low molecular weight heparin; UFH, unfractionated heparin; NSCLC, non–small-cell lung cancer; CRC, colorectal cancer; HN, head and neck cancer. Adapted from Kuderer et al.176
Fig 4.
Meta-analysis of anticoagulation studies evaluating the impact on mortality in cancer patients without venous thrombosis: major bleeding complications by type of anticoagulation. SCLC, small-cell lung cancer; NSCLC, non–small-cell lung cancer; HN, head and neck cancer; CRC, colorectal cancer. Adapted from Kuderer et al.176
CONCLUSIONS AND FUTURE RESEARCH
Mounting evidence suggests that components of the clotting cascade play an integral part in tumor progression, invasion, angiogenesis, and metastasis formation. Furthermore, there are intriguing experimental findings suggesting that especially LMWHs exert an antineoplastic effect through multiple mechanisms. When combined in meta-analyses, available randomized controlled trials of anticoagulation as cancer therapy in patients without VTE suggested favorable effects of LMWH on mortality of patients with cancer. However, definitive large clinical trials remain elusive. Only one relatively small trial with unfractionated heparin has been reported, not allowing any firm conclusions. Numerous additional questions remain regarding the importance of tumor type and stage on treatment efficacy, the impact of prevention of fatal venous and arterial thromboembolic events, optimal anticoagulation therapy, bleeding complications with differing chemotherapy regimens, and potential special patient monitoring needs.
To better elucidate the clinical benefits of anticoagulation therapy in patients with cancer, ongoing and future trials should focus on homogenous patient populations with the same tumor type, extent of disease, and treatment regimen, modeled after other well-designed cancer trials. In addition, these trials should report on standard survival and treatment response outcomes as well as clinically relevant thromboembolic events and bleeding complications. Despite some remaining hurdles, LMWH and related agents hold promise for improving cancer outcomes.
Acknowledgment
We thank Nancy Thomasson for her invaluable administrative assistance.
Footnotes
Supported by Grants No. NIH 5T32 CA009307-30 from the National Cancer Institute (N.M.K.), 1R01HL095109-01 from the National Heart, Lung and Blood Institute (C.W.F.), U01 HL072289 and U54 HL77878; and U01 DD000014 from the Centers for Disease Control and Prevention (T.L.O.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Charles W. Francis, Eisai (C), Boehringer-Ingelheim (C) Stock Ownership: None Honoraria: Charles W. Francis, Eisai Research Funding: Thomas L. Ortel, Eisai; Charles W. Francis, Takeda Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Nicole M. Kuderer, Thomas L. Ortel, Charles W. Francis
Administrative support: Thomas L. Ortel, Charles W. Francis
Provision of study materials or patients: Nicole M. Kuderer
Collection and assembly of data: Nicole M. Kuderer, Charles W. Francis
Data analysis and interpretation: Nicole M. Kuderer, Thomas L. Ortel, Charles W. Francis
Manuscript writing: Nicole M. Kuderer, Thomas L. Ortel, Charles W. Francis
Final approval of manuscript: Nicole M. Kuderer, Thomas L. Ortel, Charles W. Francis
REFERENCES
- 1.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 2.White R, Alcalay A, Zhou H, et al. Venous thromboembolism in 68,142 patients with colorectal cancer. J Thromb Haemost. 2005;3(suppl) abstr OR056. [Google Scholar]
- 3.Green KB, Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10:499–530. doi: 10.1016/s0889-8588(05)70349-x. [DOI] [PubMed] [Google Scholar]
- 4.Rickles FR, Levine MN. Epidemiology of thrombosis in cancer. Acta Haematol. 2001;106:6–12. doi: 10.1159/000046583. [DOI] [PubMed] [Google Scholar]
- 5.Falanga A. Mechanisms of hypercoagulation in malignancy and during chemotherapy. Haemostasis. 1998;28(suppl 3):50–60. doi: 10.1159/000022405. [DOI] [PubMed] [Google Scholar]
- 6.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: Validation of a thrombosis risk score. J Thromb Haemost. 2004;2:2156–2161. doi: 10.1111/j.1538-7836.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 7.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: Determination of frequency and characteristics. Thromb Haemost. 2002;87:575–579. [PubMed] [Google Scholar]
- 8.Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285–291. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 9.von Tempelhoff GF, Dietrich M, Hommel G, et al. Blood coagulation during adjuvant epirubicin/cyclophosphamide chemotherapy in patients with primary operable breast cancer. J Clin Oncol. 1996;14:2560–2568. doi: 10.1200/JCO.1996.14.9.2560. [DOI] [PubMed] [Google Scholar]
- 10.von Tempelhoff GF, Dietrich M, Niemann F, et al. Blood coagulation and thrombosis in patients with ovarian malignancy. Thromb Haemost. 1997;77:456–461. [PubMed] [Google Scholar]
- 11.Goldenberg N, Kahn SR, Solymoss S. Markers of coagulation and angiogenesis in cancer-associated venous thromboembolism. J Clin Oncol. 2003;21:4194–4199. doi: 10.1200/JCO.2003.05.165. [DOI] [PubMed] [Google Scholar]
- 12.Rickles FR, Levine M, Edwards RL. Hemostatic alterations in cancer patients. Cancer Metastasis Rev. 1992;11:237–248. doi: 10.1007/BF01307180. [DOI] [PubMed] [Google Scholar]
- 13.Sallah S, Husain A, Sigounas V, et al. Plasma coagulation markers in patients with solid tumors and venous thromboembolic disease receiving oral anticoagulation therapy. Clin Cancer Res. 2004;10:7238–7243. doi: 10.1158/1078-0432.CCR-04-0445. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH, Bettigole RE, Robson E, et al. Fibrinogen kinetics in patients with neoplastic disease. Cancer. 1978;41:1113–1122. doi: 10.1002/1097-0142(197803)41:3<1113::aid-cncr2820410346>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak HF. Tumors: Wounds that do not heal: Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 16.Dvorak HF, Dvorak AM, Manseau EJ, et al. Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis, and fibroplasia in guinea pigs: Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. J Natl Cancer Inst. 1979;62:1459–1472. [PubMed] [Google Scholar]
- 17.Dvorak HF, Senger DR, Dvorak AM. Fibrin as a component of the tumor stroma: Origins and biological significance. Cancer Metastasis Rev. 1983;2:41–73. doi: 10.1007/BF00046905. [DOI] [PubMed] [Google Scholar]
- 18.Dvorak HF, Nagy JA, Berse B, et al. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann N Y Acad Sci. 1992;667:101–111. doi: 10.1111/j.1749-6632.1992.tb51603.x. [DOI] [PubMed] [Google Scholar]
- 19.Browder T, Folkman J, Pirie-Shepherd S. The hemostatic system as a regulator of angiogenesis. J Biol Chem. 2000;275:1521–1524. doi: 10.1074/jbc.275.3.1521. [DOI] [PubMed] [Google Scholar]
- 20.Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest. 2003;124:58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 21.Palumbo JS, Mullins ES, Degen JL. Genetic analysis of hemostatic factors and cancer. In: Khorana A, Francis C, editors. Cancer-Associated Thrombosis. New York, NY: Informa Healthcare; 2008. pp. 51–63. [Google Scholar]
- 22.Palumbo JS, Talmage KE, Liu H, et al. Plasminogen supports tumor growth through a fibrinogen-dependent mechanism linked to vascular patency. Blood. 2003;102:2819–2827. doi: 10.1182/blood-2003-03-0881. [DOI] [PubMed] [Google Scholar]
- 23.Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 24.Bobek V, Boubelik M, Kovarik J, et al. Inhibition of adhesion breast cancer cells by anticoagulant drugs and cimetidine. Neoplasma. 2003;50:148–151. [PubMed] [Google Scholar]
- 25.Bobek V, Kovarik J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother. 2004;58:213–219. doi: 10.1016/j.biopha.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Drago JR, Weed P, Fralisch A. The evaluation of heparin in control of metastasis of Nb rat androgen-insensitive prostate carcinoma. Anticancer Res. 1984;4:171–172. [PubMed] [Google Scholar]
- 27.Hejna M, Raderer M, Zielinski CC. Inhibition of metastases by anticoagulants. J Natl Cancer Inst. 1999;91:22–36. doi: 10.1093/jnci/91.1.22. [DOI] [PubMed] [Google Scholar]
- 28.Lee AE, Rogers LA, Longcroft JM, et al. Reduction of metastasis in a murine mammary tumour model by heparin and polyinosinic-polycytidylic acid. Clin Exp Metastasis. 1990;8:165–171. doi: 10.1007/BF00117789. [DOI] [PubMed] [Google Scholar]
- 29.Lee JK, Choi B, Sobel RA, et al. Inhibition of growth and angiogenesis of human neurofibrosarcoma by heparin and hydrocortisone. J Neurosurg. 1990;73:429–435. doi: 10.3171/jns.1990.73.3.0429. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig RJ, Boehme B, Podda M, et al. Endothelial P-selectin as a target of heparin action in experimental melanoma lung metastasis. Cancer Res. 2004;64:2743–2750. doi: 10.1158/0008-5472.can-03-1054. [DOI] [PubMed] [Google Scholar]
- 31.Maat B, Hilgard P. Anticoagulants and experimental metastases-evaluation of antimetastatic effects in different model systems. J Cancer Res Clin Oncol. 1981;101:275–283. doi: 10.1007/BF00410113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer T, Hart IR. Mechanisms of tumour metastasis. Eur J Cancer. 1998;34:214–221. doi: 10.1016/s0959-8049(97)10129-0. [DOI] [PubMed] [Google Scholar]
- 33.Milas L, Hunter N, Basic I. Treatment with cortisone plus heparin or hexuronyl hexoaminoglycan sulfates of murine tumors and their lung deposits. Clin Exp Metastasis. 1985;3:247–255. doi: 10.1007/BF01585080. [DOI] [PubMed] [Google Scholar]
- 34.Sciumbata T, Caretto P, Pirovano P, et al. Treatment with modified heparins inhibits experimental metastasis formation and leads, in some animals, to long-term survival. Invasion Metastasis. 1996;16:132–143. [PubMed] [Google Scholar]
- 35.Stevenson JL, Choi SH, Varki A. Differential metastasis inhibition by clinically relevant levels of heparins–correlation with selectin inhibition, not antithrombotic activity. Clin Cancer Res. 2005;11:7003–7011. doi: 10.1158/1078-0432.CCR-05-1131. [DOI] [PubMed] [Google Scholar]
- 36.Vlodavsky I, Mohsen M, Lider O, et al. Inhibition of tumor metastasis by heparanase inhibiting species of heparin. Invasion Metastasis. 1994;14:290–302. [PubMed] [Google Scholar]
- 37.Zacharski LR, Ornstein DL. Heparin and cancer. Thromb Haemost. 1998;80:10–23. [PubMed] [Google Scholar]
- 38.Zacharski LR, Ornstein DL, Mamourian AC. Low-molecular-weight heparin and cancer. Semin Thromb Hemost. 2000;26(suppl 1):69–77. doi: 10.1055/s-2000-9499. [DOI] [PubMed] [Google Scholar]
- 39.Kudrijashov BA, Kalishevskaya TM, Kolomina SM. Blood anticoagulating system and malignant tumours. Nature. 1969;222:548–550. doi: 10.1038/222548a0. [DOI] [PubMed] [Google Scholar]
- 40.Smorenburg SM, Van Noorden CJ. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol Rev. 2001;53:93–105. [PubMed] [Google Scholar]
- 41.Smorenburg SM, Vink R, te Lintelo M, et al. In vivo treatment of rats with unfractionated heparin (UFH) or low molecular weight heparin (LMWH) does not affect experimentally induced colon carcinoma metastasis. Clin Exp Metastasis. 1999;17:451–456. doi: 10.1023/a:1006648429914. [DOI] [PubMed] [Google Scholar]
- 42.Van Den Brenk HA, Burch WM, Kelly H, et al. Venous diversion trapping and growth of blood-borne cancer cells en route to the lungs. Br J Cancer. 1975;31:46–61. doi: 10.1038/bjc.1975.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prandoni P. Cancer and thromboembolic disease: How important is the risk of thrombosis? Cancer Treat Rev. 2002;28:133–136. doi: 10.1016/s0305-7372(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 44.Ambrus JL, Ambrus CM, Mink IB, et al. Causes of death in cancer patients. J Med. 1975;6:61–64. [PubMed] [Google Scholar]
- 45.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 47.Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 48.Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 49.Kuderer NM, Culakova E, Francis CW, et al. Venous thromboembolism represents a major risk factor for early mortality in patients receiving cancer chemotherapy. J Clin Oncol. 2008;26:506s. abstr 9521. [Google Scholar]
- 50.Iberti TJ, Miller M, Abalos A, et al. Abnormal coagulation profile in brain tumor patients during surgery. Neurosurgery. 1994;34:389–394. doi: 10.1227/00006123-199403000-00001. discussion 394-395, 1994. [DOI] [PubMed] [Google Scholar]
- 51.Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patient. Semin Thromb Hemost. 1999;25:173–182. doi: 10.1055/s-2007-994919. [DOI] [PubMed] [Google Scholar]
- 52.Falanga A. Tumor cell prothrombotic properties. Haemostasis. 2001;31(suppl 1):1–4. [PubMed] [Google Scholar]
- 53.Andoh K, Kubota T, Takada M, et al. Tissue factor activity in leukemia cells: Special reference to disseminated intravascular coagulation. Cancer. 1987;59:748–754. doi: 10.1002/1097-0142(19870215)59:4<748::aid-cncr2820590414>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 54.Falanga A, Gordon SG. Isolation and characterization of cancer procoagulant: A cysteine proteinase from malignant tissue. Biochemistry. 1985;24:5558–5567. doi: 10.1021/bi00341a041. [DOI] [PubMed] [Google Scholar]
- 55.Barbui T, Finazzi G, Falanga A. The impact of all-trans-retinoic acid on the coagulopathy of acute promyelocytic leukemia. Blood. 1998;91:3093–3102. [PubMed] [Google Scholar]
- 56.Donati MB, Semeraro N. Cancer cell procoagulants and their pharmacological modulation. Haemostasis. 1984;14:422–429. doi: 10.1159/000215100. [DOI] [PubMed] [Google Scholar]
- 57.Edwards RL, Silver J, Rickles FR. Human tumor procoagulants: Registry of the Subcommittee on Haemostasis and Malignancy of the Scientific and Standardization Committee, International Society on Thrombosis and Haemostasis. Thromb Haemost. 1993;69:205–213. [PubMed] [Google Scholar]
- 58.Falanga A, Alessio MG, Donati MB, et al. A new procoagulant in acute leukemia. Blood. 1988;71:870–875. [PubMed] [Google Scholar]
- 59.Falanga A, Consonni R, Marchetti M, et al. Cancer procoagulant and tissue factor are differently modulated by all-trans-retinoic acid in acute promyelocytic leukemia cells. Blood. 1998;92:143–151. [PubMed] [Google Scholar]
- 60.Falanga A, Iacoviello L, Evangelista V, et al. Loss of blast cell procoagulant activity and improvement of hemostatic variables in patients with acute promyelocytic leukemia administered all-trans-retinoic acid. Blood. 1995;86:1072–1081. [PubMed] [Google Scholar]
- 61.Koyama T, Hirosawa S, Kawamata N, et al. All-trans retinoic acid upregulates thrombomodulin and downregulates tissue-factor expression in acute promyelocytic leukemia cells: Distinct expression of thrombomodulin and tissue factor in human leukemic cells. Blood. 1994;84:3001–3009. [PubMed] [Google Scholar]
- 62.Saito T, Koyama T, Nagata K, et al. Anticoagulant effects of retinoic acids on leukemia cells. Blood. 1996;87:657–665. [PubMed] [Google Scholar]
- 63.Carroll VA, Binder BR. The role of the plasminogen activation system in cancer. Semin Thromb Hemost. 1999;25:183–197. doi: 10.1055/s-2007-994920. [DOI] [PubMed] [Google Scholar]
- 64.Kwaan HC. The plasminogen-plasmin system in malignancy. Cancer Metastasis Rev. 1992;11:291–311. doi: 10.1007/BF01307184. [DOI] [PubMed] [Google Scholar]
- 65.Kwaan HC, Keer HN. Fibrinolysis and cancer. Semin Thromb Hemost. 1990;16:230–235. doi: 10.1055/s-2007-1002674. [DOI] [PubMed] [Google Scholar]
- 66.Falanga A, Marchetti M, Giovanelli S, et al. All-trans-retinoic acid counteracts endothelial cell procoagulant activity induced by a human promyelocytic leukemia-derived cell line (NB4) Blood. 1996;87:613–617. [PubMed] [Google Scholar]
- 67.Gianni M, Norio P, Terao M, et al. Effects of dexamethasone on pro-inflammatory cytokine expression, cell growth and maturation during granulocytic differentiation of acute promyelocytic leukemia cells. Eur Cytokine Netw. 1995;6:157–165. [PubMed] [Google Scholar]
- 68.van Hinsbergh VW, Bauer KA, Kooistra T, et al. Progress of fibrinolysis during tumor necrosis factor infusions in humans. Concomitant increase in tissue-type plasminogen activator, plasminogen activator inhibitor type-1, and fibrin(ogen) degradation products. Blood. 1990;76:2284–2289. [PubMed] [Google Scholar]
- 69.Brown LF, Detmar M, Claffey K, et al. Vascular permeability factor/vascular endothelial growth factor: A multifunctional angiogenic cytokine. EXS. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- 70.Bevilacqua MP, Pober JS, Majeau GR, et al. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: Characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986;83:4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colucci M, Balconi G, Lorenzet R, et al. Cultured human endothelial cells generate tissue factor in response to endotoxin. J Clin Invest. 1983;71:1893–1896. doi: 10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dittman WA, Majerus PW. Structure and function of thrombomodulin: A natural anticoagulant. Blood. 1990;75:329–336. [PubMed] [Google Scholar]
- 73.Moore KL, Esmon CT, Esmon NL. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989;73:159–165. [PubMed] [Google Scholar]
- 74.Honn KV, Tang DG, Chen YQ. Platelets and cancer metastasis: More than an epiphenomenon. Semin Thromb Hemost. 1992;18:392–415. doi: 10.1055/s-2007-1002578. [DOI] [PubMed] [Google Scholar]
- 75.Giavazzi R, Foppolo M, Dossi R, et al. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest. 1993;92:3038–3044. doi: 10.1172/JCI116928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 77.Lauri D, Needham L, Martin-Padura I, et al. Tumor cell adhesion to endothelial cells: Endothelial leukocyte adhesion molecule-1 as an inducible adhesive receptor specific for colon carcinoma cells. J Natl Cancer Inst. 1991;83:1321–1324. doi: 10.1093/jnci/83.18.1321. [DOI] [PubMed] [Google Scholar]
- 78.Prandoni P, Lensing AW, Buller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med. 1992;327:1128–1133. doi: 10.1056/NEJM199210153271604. [DOI] [PubMed] [Google Scholar]
- 79.Nierodzik M, Karpatkin S. Hypercoagulability preceding cancer. Does hypercoagulability awaken dormant tumor cells in the host? J Thromb Haemost. 2005;3:577–580. doi: 10.1111/j.1538-7836.2005.01174.x. [DOI] [PubMed] [Google Scholar]
- 80.Baron JA, Gridley G, Weiderpass E, et al. Venous thromboembolism and cancer. Lancet. 1998;351:1077–1080. doi: 10.1016/S0140-6736(97)10018-6. [DOI] [PubMed] [Google Scholar]
- 81.Sorensen HT, Mellemkjaer L, Steffensen FH, et al. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med. 1998;338:1169–1173. doi: 10.1056/NEJM199804233381701. [DOI] [PubMed] [Google Scholar]
- 82.Borsig L, Stevenson JL, Varki A. Heparin in cancer: Role of selectin interactions. In: Khorana A, Francis C, editors. Cancer-Associated Thrombosis. New York, NY: Informa Healthcare; 2008. pp. 97–113. [Google Scholar]
- 83.Taubman M. New York, NY: Informa Healthcare; 2008. Tissue Factor in Cancer Angiogenesis and Coagulopathy. [Google Scholar]
- 84.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 85.Clarke N. Intracellular location of tissue thromboplastin and possible relation to fibrin deposits in human neoplasms. Nature. 1965;205:608–610. doi: 10.1038/205608a0. [DOI] [PubMed] [Google Scholar]
- 86.Olander JV, Bremer ME, Marasa JC, et al. Fibrin-enhanced endothelial cell organization. J Cell Physiol. 1985;125:1–9. doi: 10.1002/jcp.1041250102. [DOI] [PubMed] [Google Scholar]
- 87.Palumbo JS, Talmage KE, Massari JV, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Booden MA, Eckert LB, Der CJ, et al. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol Cell Biol. 2004;24:1990–1999. doi: 10.1128/MCB.24.5.1990-1999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li JJ, Huang YQ, Basch R, et al. Thrombin induces the release of angiopoietin-1 from platelets. Thromb Haemost. 2001;85:204–206. [PubMed] [Google Scholar]
- 90.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–362. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Zain J, Huang YQ, Feng X, et al. Concentration-dependent dual effect of thrombin on impaired growth/apoptosis or mitogenesis in tumor cells. Blood. 2000;95:3133–3138. [PubMed] [Google Scholar]
- 92.Carney DH, Stiernberg J, Fenton JW., Jr Initiation of proliferative events by human alpha-thrombin requires both receptor binding and enzymic activity. J Cell Biochem. 1984;26:181–195. doi: 10.1002/jcb.240260306. [DOI] [PubMed] [Google Scholar]
- 93.Paris S, Pouyssegur J. Growth factors activate the Na+/H+ antiporter in quiescent fibroblasts by increasing its affinity for intracellular H+ J Biol Chem. 1984;259:10989–10994. [PubMed] [Google Scholar]
- 94.Galdal KS, Lyberg T, Evensen SA, et al. Thrombin induces thromboplastin synthesis in cultured vascular endothelial cells. Thromb Haemost. 1985;54:373–376. [PubMed] [Google Scholar]
- 95.Raben DM, Cunningham DD. Effects of EGF and thrombin on inositol-containing phospholipids of cultured fibroblasts: Stimulation of phosphatidylinositol synthesis by thrombin but not EGF. J Cell Physiol. 1985;125:582–590. doi: 10.1002/jcp.1041250330. [DOI] [PubMed] [Google Scholar]
- 96.Van Obberghen-Schilling E, Chambard JC, Paris S, et al. Alpha-thrombin-induced early mitogenic signalling events and G0 to S-phase transition of fibroblasts require continual external stimulation. Embo J. 1985;4:2927–2932. doi: 10.1002/j.1460-2075.1985.tb04025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Deng Y, Luther T, et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abe K, Shoji M, Chen J, et al. Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. Proc Natl Acad Sci U S A. 1999;96:8663–8668. doi: 10.1073/pnas.96.15.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bromberg ME, Sundaram R, Homer RJ, et al. Role of tissue factor in metastasis: Functions of the cytoplasmic and extracellular domains of the molecule. Thromb Haemost. 1999;82:88–92. [PubMed] [Google Scholar]
- 100.Shoji M, Hancock WW, Abe K, et al. Activation of coagulation and angiogenesis in cancer: Immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol. 1998;152:399–411. [PMC free article] [PubMed] [Google Scholar]
- 101.Ueno T, Toi M, Koike M, et al. Tissue factor expression in breast cancer tissues: Its correlation with prognosis and plasma concentration. Br J Cancer. 2000;83:164–170. doi: 10.1054/bjoc.2000.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khorana AA, Ahrendt SA, Ryan CK, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–2875. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 103.Guba M, Yezhelyev M, Eichhorn ME, et al. Rapamycin induces tumor-specific thrombosis via tissue factor in the presence of VEGF. Blood. 2005;105:4463–4469. doi: 10.1182/blood-2004-09-3540. [DOI] [PubMed] [Google Scholar]
- 104.Versteeg HH, Hoedemaeker I, Diks SH, et al. Factor VIIa/tissue factor-induced signaling via activation of Src-like kinases, phosphatidylinositol 3-kinase, and Rac. J Biol Chem. 2000;275:28750–28756. doi: 10.1074/jbc.M907635199. [DOI] [PubMed] [Google Scholar]
- 105.Albrektsen T, Sorensen BB, Hjorto GM, et al. Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J Thromb Haemost. 2007;5:1588–1597. doi: 10.1111/j.1538-7836.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hjortoe GM, Petersen LC, Albrektsen T, et al. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood. 2004;103:3029–3037. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mueller BM, Reisfeld RA, Edgington TS, et al. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci U S A. 1992;89:11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mueller BM, Ruf W. Requirement for binding of catalytically active factor VIIa in tissue factor-dependent experimental metastasis. J Clin Invest. 1998;101:1372–1378. doi: 10.1172/JCI930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bromberg ME, Konigsberg WH, Madison JF, et al. Tissue factor promotes melanoma metastasis by a pathway independent of blood coagulation. Proc Natl Acad Sci U S A. 1995;92:8205–8209. doi: 10.1073/pnas.92.18.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nobeyama Y, Okochi-Takada E, Furuta J, et al. Silencing of tissue factor pathway inhibitor-2 gene in malignant melanomas. Int J Cancer. 2007;121:301–307. doi: 10.1002/ijc.22637. [DOI] [PubMed] [Google Scholar]
- 111.Jiang X, Guo YL, Bromberg ME. Formation of tissue factor-factor VIIa-factor Xa complex prevents apoptosis in human breast cancer cells. Thromb Haemost. 2006;96:196–201. [PubMed] [Google Scholar]
- 112.Versteeg HH, Spek CA, Slofstra SH, et al. FVIIa: TF induces cell survival via G12/G13-dependent Jak/STAT activation and BclXL production. Circ Res. 2004;94:1032–1040. doi: 10.1161/01.RES.0000125625.18597.AD. [DOI] [PubMed] [Google Scholar]
- 113.Amirkhosravi A, Amaya M, Siddiqui F, et al. Blockade of GpIIb/IIIa inhibits the release of vascular endothelial growth factor (VEGF) from tumor cell-activated platelets and experimental metastasis. Platelets. 1999;10:285–292. doi: 10.1080/09537109975915. [DOI] [PubMed] [Google Scholar]
- 114.Amirkhosravi A, Mousa SA, Amaya M, et al. Antimetastatic effect of tinzaparin, a low-molecular-weight heparin. J Thromb Haemost. 2003;1:1972–1976. doi: 10.1046/j.1538-7836.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 115.Mohle R, Green D, Moore MA, et al. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borsig L, Wong R, Feramisco J, et al. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Borsig L, Wong R, Hynes RO, et al. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci U S A. 2002;99:2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laubli H, Stevenson JL, Varki A, et al. L-selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res. 2006;66:1536–1542. doi: 10.1158/0008-5472.CAN-05-3121. [DOI] [PubMed] [Google Scholar]
- 119.Wu QD, Wang JH, Condron C, et al. Human neutrophils facilitate tumor cell transendothelial migration. Am J Physiol Cell Physiol. 2001;280:C814–C822. doi: 10.1152/ajpcell.2001.280.4.C814. [DOI] [PubMed] [Google Scholar]
- 120.Bevilacqua MP, Nelson RM. Endothelial-leukocyte adhesion molecules in inflammation and metastasis. Thromb Haemost. 1993;70:152–154. [PubMed] [Google Scholar]
- 121.Goerner A. The influence of anti-clotting agents on transplantation and growth of tumor tissue. J Lab Clin Med. 1930;16:369–372. [Google Scholar]
- 122.Hagmar B. Effect of heparin, epsilon-aminocaproic acid and coumarin on tumour growth and spontaneous metastasis formation. Pathol Eur. 1968;3:622–630. [PubMed] [Google Scholar]
- 123.Hagmar B, Norrby K. Evidence for effects of heparin on cell surfaces influencing experimental metastases. Int J Cancer. 1970;5:72–84. doi: 10.1002/ijc.2910050110. [DOI] [PubMed] [Google Scholar]
- 124.Hilgard P, Beyerle L, Hohage R, et al. The effect of heparin on the initial phase of metastasis formation. Eur J Cancer. 1972;8:347–352. doi: 10.1016/0014-2964(72)90031-x. [DOI] [PubMed] [Google Scholar]
- 125.Koike A. Mechanism of blood-borne metastases. I: Some factors affecting lodgment and growth of tumor cells in the lungs. Cancer. 1964;17:450–460. doi: 10.1002/1097-0142(196404)17:4<450::aid-cncr2820170406>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 126. Reference deleted.
- 127.Owen CA., Jr Anticoagulant treatment of rats with Walker 256 carcinosarcoma. J Cancer Res Clin Oncol. 1982;104:191–193. doi: 10.1007/BF00402067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Collen A, Smorenburg SM, Peters E, et al. Unfractionated and low molecular weight heparin affect fibrin structure and angiogenesis in vitro. Cancer Res. 2000;60:6196–6200. [PubMed] [Google Scholar]
- 129.Folkman J. Tumor angiogenesis and tissue factor. Nat Med. 1996;2:167–168. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- 130.Folkman J, Langer R, Linhardt RJ, et al. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983;221:719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- 131.Folkman J, Weisz PB, Joullie MM, et al. Control of angiogenesis with synthetic heparin substitutes. Science. 1989;243:1490–1493. doi: 10.1126/science.2467380. [DOI] [PubMed] [Google Scholar]
- 132.Jayson GC, Gallagher JT. Heparin oligosaccharides: Inhibitors of the biological activity of bFGF on Caco-2 cells. Br J Cancer. 1997;75:9–16. doi: 10.1038/bjc.1997.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khorana AA, Sahni A, Altland OD, et al. Heparin inhibition of endothelial cell proliferation and organization is dependent on molecular weight. Arterioscler Thromb Vasc Biol. 2003;23:2110–2115. doi: 10.1161/01.ATV.0000090671.56682.D7. [DOI] [PubMed] [Google Scholar]
- 134.Norrby K. Heparin and angiogenesis: A low-molecular-weight fraction inhibits and a high-molecular-weight fraction stimulates angiogenesis systemically. Haemostasis. 1993;23(suppl 1):141–149. doi: 10.1159/000216923. [DOI] [PubMed] [Google Scholar]
- 135.Norrby K, Ostergaard P. Basic-fibroblast-growth-factor-mediated de novo angiogenesis is more effectively suppressed by low-molecular-weight than by high-molecular-weight heparin. Int J Microcirc Clin Exp. 1996;16:8–15. doi: 10.1159/000179145. [DOI] [PubMed] [Google Scholar]
- 136.Soker S, Goldstaub D, Svahn CM, et al. Variations in the size and sulfation of heparin modulate the effect of heparin on the binding of VEGF165 to its receptors. Biochem Biophys Res Commun. 1994;203:1339–1347. doi: 10.1006/bbrc.1994.2329. [DOI] [PubMed] [Google Scholar]
- 137.Pepe G, Giusti B, Attanasio M, et al. Tissue factor and plasminogen activator inhibitor type 2 expression in human stimulated monocytes is inhibited by heparin. Semin Thromb Hemost. 1997;23:135–141. doi: 10.1055/s-2007-996081. [DOI] [PubMed] [Google Scholar]
- 138.Au YP, Kenagy RD, Clowes MM, et al. Mechanisms of inhibition by heparin of vascular smooth muscle cell proliferation and migration. Haemostasis. 1993;23(suppl 1):177–182. doi: 10.1159/000216926. [DOI] [PubMed] [Google Scholar]
- 139.Bennett MR, Evan GI, Newby AC. Deregulated expression of the c-myc oncogene abolishes inhibition of proliferation of rat vascular smooth muscle cells by serum reduction, interferon-gamma, heparin, and cyclic nucleotide analogues and induces apoptosis. Circ Res. 1994;74:525–536. doi: 10.1161/01.res.74.3.525. [DOI] [PubMed] [Google Scholar]
- 140.Tiozzo R, Cingi MR, Pietrangelo A, et al. Effect of heparin-like compounds on the in vitro proliferation and protein synthesis of various cell types. Arzneimittelforschung. 1989;39:15–20. [PubMed] [Google Scholar]
- 141.Li HL, Ye KH, Zhang HW, et al. Effect of heparin on apoptosis in human nasopharyngeal carcinoma CNE2 cells. Cell Res. 2001;11:311–315. doi: 10.1038/sj.cr.7290101. [DOI] [PubMed] [Google Scholar]
- 142.Lapierre F, Holme K, Lam L, et al. Chemical modifications of heparin that diminish its anticoagulant but preserve its heparanase-inhibitory, angiostatic, anti-tumor and anti-metastatic properties. Glycobiology. 1996;6:355–366. doi: 10.1093/glycob/6.3.355. [DOI] [PubMed] [Google Scholar]
- 143.Engelberg H. Actions of heparin that may affect the malignant process. Cancer. 1999;85:257–272. doi: 10.1002/(sici)1097-0142(19990115)85:2<257::aid-cncr1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 144.Koenig A, Norgard-Sumnicht K, Linhardt R, et al. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins: Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sylvester DM, Liu SY, Meadows GG. Augmentation of antimetastatic activity of interferon and tumor necrosis factor by heparin. Immunopharmacol Immunotoxicol. 1990;12:161–180. doi: 10.3109/08923979009019667. [DOI] [PubMed] [Google Scholar]
- 146.Maat B. Selective macrophage inhibition abolishes warfarin-induced reduction of metastasis. Br J Cancer. 1980;41:313–316. doi: 10.1038/bjc.1980.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Colman RW, Jameson BA, Lin Y, et al. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543–550. [PubMed] [Google Scholar]
- 148.Fielding LP, Hittinger R, Grace RH, et al. Randomised controlled trial of adjuvant chemotherapy by portal-vein perfusion after curative resection for colorectal adenocarcinoma. Lancet. 1992;340:502–506. doi: 10.1016/0140-6736(92)91708-g. [DOI] [PubMed] [Google Scholar]
- 149.Kingston RD, Fielding JW, Palmer MK. Peri-operative heparin: A possible adjuvant to surgery in colo-rectal cancer? Int J Colorectal Dis. 1993;8:111–115. doi: 10.1007/BF00299339. [DOI] [PubMed] [Google Scholar]
- 150.Kohanna FH, Sweeney J, Hussey S, et al. Effect of perioperative low-dose heparin administration on the course of colon cancer. Surgery. 1983;93:433–438. [PubMed] [Google Scholar]
- 151.Nitti D, Wils J, Sahmoud T, et al. Final results of a phase III clinical trial on adjuvant intraportal infusion with heparin and 5-fluorouracil (5-FU) in resectable colon cancer (EORTC GITCCG 1983-1987): European Organisation for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. Eur J Cancer. 1997;33:1209–1215. doi: 10.1016/s0959-8049(97)00052-x. [DOI] [PubMed] [Google Scholar]
- 152.Torngren S, Rieger A. The influence of heparin and curable resection on the survival of colorectal cancer. Acta Chir Scand. 1983;149:427–429. [PubMed] [Google Scholar]
- 153.Wereldsma JC, Bruggink ED, Meijer WS, et al. Adjuvant portal liver infusion in colorectal cancer with 5-fluorouracil/heparin versus urokinase versus control: Results of a prospective randomized clinical trial (colorectal adenocarcinoma trial I) Cancer. 1990;65:425–432. doi: 10.1002/1097-0142(19900201)65:3<425::aid-cncr2820650309>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 154.Prandoni P, Lensing AW, Buller HR, et al. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet. 1992;339:441–445. doi: 10.1016/0140-6736(92)91054-c. [DOI] [PubMed] [Google Scholar]
- 155.Dolovich LR, Ginsberg JS, Douketis JD, et al. A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism: Examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Arch Intern Med. 2000;160:181–188. doi: 10.1001/archinte.160.2.181. [DOI] [PubMed] [Google Scholar]
- 156.Gould MK, Dembitzer AD, Doyle RL, et al. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med. 1999;130:800–809. doi: 10.7326/0003-4819-130-10-199905180-00003. [DOI] [PubMed] [Google Scholar]
- 157.Hettiarachchi RJ, Smorenburg SM, Ginsberg J, et al. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb Haemost. 1999;82:947–952. [PubMed] [Google Scholar]
- 158.Siragusa S, Cosmi B, Piovella F, et al. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: Results of a meta-analysis. Am J Med. 1996;100:269–277. doi: 10.1016/S0002-9343(97)89484-3. [DOI] [PubMed] [Google Scholar]
- 159.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 160.Lee AY, Rickles FR, Julian JA, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 161.Zacharski LR, Henderson WG, Rickles FR, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate: Final report of VA Cooperative Study #75. Cancer. 1984;53:2046–2052. doi: 10.1002/1097-0142(19840515)53:10<2046::aid-cncr2820531007>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 162.Chahinian AP, Propert KJ, Ware JH, et al. A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small-cell lung cancer by the Cancer and Leukemia Group B. J Clin Oncol. 1989;7:993–1002. doi: 10.1200/JCO.1989.7.8.993. [DOI] [PubMed] [Google Scholar]
- 163.Maurer LH, Herndon JE, Jr, Hollis DR, et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: A Cancer and Leukemia Group B study. J Clin Oncol. 1997;15:3378–3387. doi: 10.1200/JCO.1997.15.11.3378. [DOI] [PubMed] [Google Scholar]
- 164.Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266–1271. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 165.Lebeau B, Chastang C, Brechot JM, et al. Subcutaneous heparin treatment increases survival in small cell lung cancer: “Petites Cellules” Group. Cancer. 1994;74:38–45. doi: 10.1002/1097-0142(19940701)74:1<38::aid-cncr2820740108>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 166.Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The fragmin advanced malignancy outcome study (FAMOUS) J Clin Oncol. 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 167.Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 168.Sideras K, Schaefer PL, Okuno SH, et al. Low-molecular-weight heparin in patients with advanced cancer: A phase 3 clinical trial. Mayo Clin Proc. 2006;81:758–767. doi: 10.4065/81.6.758. [DOI] [PubMed] [Google Scholar]
- 169.Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: A meta-analysis of randomised clinical trials. Thromb Haemost. 2000;83:14–19. [PubMed] [Google Scholar]
- 170.Smorenburg SM, Hettiarachchi RJ, Vink R, et al. The effects of unfractionated heparin on survival in patients with malignancy–a systematic review. Thromb Haemost. 1999;82:1600–1604. [PubMed] [Google Scholar]
- 171.Smorenburg SM, Vink R, Otten HM, et al. The effects of vitamin K-antagonists on survival of patients with malignancy: A systematic analysis. Thromb Haemost. 2001;86:1586–1587. [PubMed] [Google Scholar]
- 172.Conti S, Guercini F, Iorio A. Low-molecular-weight heparin and cancer survival: Review of the literature and pooled analysis of 1,726 patients treated for at least three months. Pathophysiol Haemost Thromb. 2003;33:197–201. doi: 10.1159/000081508. [DOI] [PubMed] [Google Scholar]
- 173.Haas SK, Kakkar AK, Kemkes-Matthes B, et al. Topic 1 - Prevention of venous thromboembolism with low-molecular-weight heparin in patients with metastatic breast or lung cancer: Results of the TOPIC studies. J Thromb Haemost. 2005;3(suppl):1. abstr OR059. [Google Scholar]
- 174.Haas SK, Kakkar AK, Kemkes-Matthes B, et al. Topic 2 - Prevention of venous thromboembolism with low-molecular-weight heparin in patients with metastatic breast or lung cancer: Results of the TOPIC studies. J Thromb Haemost. 2005;3(suppl):1. abstr OR059. [Google Scholar]
- 175.Kakkar AK, Levine MN. Thrombosis and cancer: Implications beyond Trousseau. J Thromb Haemost. 2004;2:1261–1262. doi: 10.1111/j.1538-7836.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 176.Kuderer NM, Khorana AA, Lyman GH, et al. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: Impact on survival and bleeding complications. Cancer. 2007;110:1149–1161. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 177.Iorio A, Guercini F, Pini M. Low-molecular-weight heparin for the long-term treatment of symptomatic venous thromboembolism: Meta-analysis of the randomized comparisons with oral anticoagulants. J Thromb Haemost. 2003;1:1906–1913. doi: 10.1046/j.1538-7836.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 178.Lee A, Levine M. Treatment of venous thromboembolism in cancer patients. Cancer Control. 2005;12(suppl 1):17–21. doi: 10.1177/1073274805012003S04. [DOI] [PubMed] [Google Scholar]
- 179.Lee AY, Levine MN. Venous thromboembolism and cancer: Risks and outcomes. Circulation. 2003;107:I17–I21. doi: 10.1161/01.CIR.0000078466.72504.AC. [DOI] [PubMed] [Google Scholar]
- 180.Levine MN. Managing thromboembolic disease in the cancer patient: Efficacy and safety of antithrombotic treatment options in patients with cancer. Cancer Treat Rev. 2002;28:145–149. doi: 10.1016/s0305-7372(02)00042-7. [DOI] [PubMed] [Google Scholar]
- 181.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch Intern Med. 2002;162:1729–1735. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 182.Akl EA, Kamath G, Kim SY, et al. Oral anticoagulation may prolong survival of a subgroup of patients with cancer: A Cochrane systematic review. J Exp Clin Cancer Res. 2007;26:175–184. [PubMed] [Google Scholar]
- 183.Akl EA, van Doormaal FF, Barba M, et al. Parenteral anticoagulation for prolonging survival in patients with cancer who have no other indication for anticoagulation. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD006652. CD006652. [DOI] [PubMed] [Google Scholar]
- 184.Lazo-Langner A, Goss GD, Spaans JN, et al. The effect of low-molecular-weight heparin on cancer survival: A systematic review and meta-analysis of randomized trials. J Thromb Haemost. 2007;5:729–737. doi: 10.1111/j.1538-7836.2007.02427.x. [DOI] [PubMed] [Google Scholar]