Abstract
Purpose
For nearly two decades, multiple retrospective reports, small prospective studies, and meta-analyses have arrived at conflicting results regarding the value of timing surgical intervention for breast cancer on the basis of menstrual cycle phase. We present the results of a multi–cooperative group, prospective, observational trial of menstrual cycle phase and outcome after breast cancer surgery, led by the North Central Cancer Treatment Group (NCCTG) in collaboration with the National Surgical Adjuvant Breast and Bowel Project (NSABP) and the International Breast Cancer Study Group (IBCSG).
Patients and Methods
Premenopausal women age 18 to 55 years, who were interviewed for menstrual history and who were surgically treated for stages I to II breast cancer, had serum drawn within 1 day of surgery for estradiol, progesterone, and luteinizing hormone levels. Menstrual history and hormone levels were used to determine menstrual phase: luteal, follicular, and other. Disease-free survival (DFS) and overall survival (OS) rates were determined by Kaplan-Meier method and were compared by using the log-rank test and Cox proportional hazard modeling.
Results
Of 1,118 women initially enrolled, 834 women comprised the study cohort: 230 (28%) in luteal phase; 363 (44%) in follicular phase; and 241 grouped as other. During a median follow-up of 6.6 years, and in analysis that accounted for nodal disease, estrogen receptor status, adjuvant radiation therapy or chemotherapy, neither DFS nor OS differed with respect to menstrual phase. The 5-year DFS rates were 82.7%, 82.1%, and 79.2% for follicular, luteal, or other phases, respectively. Corresponding OS survival rates were 91.9%, 92.2%, and 91.8%, respectively.
Conclusion
When menstrual cycle phases were strictly defined, neither DFS nor OS differed between women who underwent surgery during the follicular phase versus the luteal phase. Nearly 30% of the patients did not meet criteria for either follicular- or luteal-phase categories.
INTRODUCTION
The concept of timing surgical intervention to treat breast cancer in premenopausal women based on the phase of the menstrual cycle originated with a preliminary communication by Hrushesky et al1 in 1989. These investigators had found a correlation between estrous stage at primary mammary cancer resection and risk of subsequent pulmonary metastases in mice.2 They then conducted a retrospective study of 44 premenopausal women, in whom disease recurrence and death were more common when surgery was performed during the perimenstrual interval (ie, menstrual cycle days 0 through 6 and 21 through 36) compared with midcycle (ie, menstrual cycle days 7 through 20). Subsequent studies did not support the same favorable window for surgical treatment during midcycle, as reported by Hrushesky et al.1
Follicular- Versus Luteal-Phase Theory
Subsequently, a series of retrospective studies of women who underwent primary breast cancer surgery from the 1970s to early 1990s used last menstrual period (LMP) to determine menstrual cycle phase at time of surgery. Although Badwe et al3 failed to corroborate the findings of Hrushesky et al, when Badwe et al divided the menstrual cycle into follicular (ie, days 3 through 12 after LMP) and luteal (ie, days 0 through 2 and 13 through 32) phases, 10-year overall survival (OS) was reduced in women who underwent operation during the follicular phase (54%) compared with the luteal phase (84%). These findings led the Guy's Hospital breast unit staff to schedule surgery at least 12 days after LMP, as they concluded that the disadvantage delay would be outweighed by the potential for long-term benefit. A similar observation was reported by Senie et al4 from Memorial Sloan-Kettering Cancer Center, in which there was a 10-year recurrence rate of 43% during the follicular phase as opposed to 29% during the luteal phase. In response to the Senie study, Current Opinion in Obstetrics and Gynecology stated, “Few papers produce immediate change in medical practice. This one should! The survival data for women undergoing breast surgery during the luteal phase of the menstrual cycle compared with those undergoing breast surgery during the follicular phase of the menstrual cycle are compelling.”5(p843)
Veronesi et al6 found disease-free survival (DFS) in women who underwent operation during follicular phase was significantly decreased relative to women who underwent operation during luteal phase, but the difference was only in women with node-positive disease: 63.3% versus 75.5%, respectively.
In contrast to these reports, numerous reports failed to demonstrate a survival difference according to the Hrushesky breakdown of menstrual cycle relative to breast cancer surgery.3,4,7,8 Other analyses, which defined the menstrual cycle as follicular (ie, days 1 through 14 after LMP) and luteal (ie, days ≥ 15 after LMP) phases, found no differences in recurrence or survival with respect to the timing of breast cancer surgery in premenopausal women.9–14 However, a meta-analysis of the 21 published studies before 1994 concluded that the effect of timing of surgery on survival was significant, and its odds reduction was 16% for treatment in the luteal phase.15
Within months of the meta-analysis report, Kroman et al14 reported the largest such study. When using data from 1,635 premenopausal women from 1977 to 1989 collected in the nationwide registry of the Danish Breast Cancer Cooperative Group, the 5-year OS rates were 79% and 80% for surgical intervention during the luteal and follicular phases, respectively.
The inaccuracies inherent in determination of menstrual phase on the basis of LMP combined with conflicting findings on the impact of surgical timing on survival outcome (Tables 1 and 2) led to the search for both retrospective cohorts with preoperative circulating hormone determinations and prospective studies to address the question of the most appropriate time for surgery. Ville10 gathered such a retrospective cohort and found no difference in DFS or OS with respect to menstrual phase at surgery as determined by circulating levels of estradiol (E2), progesterone (Pg), and luteinizing hormone (LH).
Table 1.
Luteal-Phase Survival Advantage
| Study | Total No. of Patients | Menstrual Cycle Determination | % DFS |
||
|---|---|---|---|---|---|
| Luteal | Follicular | Special Circumstances | |||
| Badwe3 | 249 | LMP | 84 | 54 | Node-positive; ER status, no difference |
| Senie4 | 283 | LMP | 71 | 57 | |
| Veronesi6 | 1,175 | LMP | 75.5 | 63.3 | Node-positive only |
| Goldhirsch13 | 1,033 | LMP | 58 | 53 | Node-positive only* |
| Badwe16 | 150 | LMP | 68 | 38 | |
| Badwe17 | 93 | Hormone | Hazard ratio, 2.1; follicular (node-positive only) | ||
| Saad18 | 96 | LMP | 72 | 40 | |
| Holli19 | 267 | ||||
| Mohr20 | 289 | ||||
| Cooper21 | 112 | LMP | 75 | 45 | ER-positive only |
Abbreviations: DFS, disease-free survival; LMP, last menstrual period; ER, estrogen receptor.
% DFS with ER-negative group: luteal, 59; follicular, 42.
Table 2.
Studies That Showed No Survival Advantage on the Basis of Phase of Menstrual Cycle
| Study | Total No. of Patients | Follow-Up (years) | Menstrual Cycle Determination |
|---|---|---|---|
| Powles7 | 81 | 11 | LMP |
| Gelber8 | 245 | 9 | LMP |
| Ville10 | 165 | Hormonal | |
| Low12 | 125 | ||
| Kroman14 | 1,635 | 10 | LMP |
| Pujol22 | 360 | ≤ 7 | LMP, hormonal |
| Thorpe23 | 412 | 3 | Prospective: LMP, hormonal |
| Nathan24 | 132 | ≤ 11 | LMP |
| Ville25 | 279 | 5 | LMP |
| Rageth26 | 217 | 5.1 | LMP |
| Donegan27 | 97 | ≤ 10 | LMP |
| Corder28 | 157 | LMP | |
| Gnant29 | 385 | 5 | LMP |
| Wobbes30 | 89 | 4.1 | Hormonal |
| Milella31 | 248 | 5 | LMP |
Abbreviation: LMP, last menstrual period.
The first prospective study enrolled 360 women who underwent a one-stage surgical procedure,22 but relapse-free survival (RFS) and OS did not differ by the menstrual cycle phase of surgical intervention. Recently, Thorpe et al23 reported the 3-year overall survival and DFS rates of a multicenter, prospective study that incorporated both LMP and hormonal data in 256 patients. The timing of surgery in relation to menstrual cycle phase had no significant impact on 3-year survival.
We present here the results of a multi–cooperative group, prospective, observational trial of menstrual cycle phase and outcome after surgery for early-stage breast cancer, led by the North Central Cancer Treatment Group (NCCTG) in collaboration with the National Surgical Adjuvant Breast and Bowel Project (NSABP) and the International Breast Cancer Study Group (IBCSG). Menstrual cycle phase was defined by hormone levels and patient menstrual history obtained within 1 day of surgery. Enrollment was limited to women with regular menstrual cycles.
PATIENTS AND METHODS
This prospective, observational, phase III clinical trial was designed to document the proportion of women for whom the menstrual phase at primary breast surgical intervention could be determined by circulating hormone levels and menstrual history and to assess whether DFS differs with respect to menstrual cycle phase (follicular v luteal) at the time of primary surgery.
Eligibility
This trial enrolled premenopausal women age 18 to 55 years who had regular menstrual cycles of 21- to 35-days duration and pathologic stages I to II breast cancer, in whom all gross disease—including ductal carcinoma in situ—was surgically removed either in a one-stage or two-stage procedure. Surgical treatment consisted of an open biopsy followed by a mastectomy or breast-conserving surgery with or without sentinel node biopsy and/or axillary nodal dissection. Fine needle aspirates and core or stereotactic needle biopsies were allowed before the definitive procedure. Chemotherapy and/or radiotherapy were allowed in accordance with internationally accepted criteria, as per investigator's discretion. Eligibility required serum be drawn within 1 calendar day of the lumpectomy/mastectomy for women who underwent a one-stage procedure and within 1 calendar day of each stage for women who underwent a two-stage procedure. Exclusion criteria were as follows: oral contraceptive use, lactation within the past 3 months, galactorrhea, neoadjuvant therapy, previous breast cancer, and history of any cancer (except squamous or basal cell skin carcinoma) in which the patient was not disease-free for at least 10 years. This trial was performed after approval by local institutional review boards in accordance with assurances filed with and approved by the US Department of Health and Human Services. Written informed consent was provided by each patient before entry on study.
Patients were observed every 6 months for the first year postregistration and annually for the next 2 to 10 years postregistration for adjuvant therapy information, disease recurrence, and death.
Patients were interviewed at the time of the primary cancer surgery to determine the menstrual history. Blood sampling occurred within 1 day of surgery, and serum samples were shipped frozen to a central laboratory (Mayo Medical Laboratory, Rochester, MN) for E2, Pg, and LH determinations. Serum hormone levels, menstrual cycle length, and day of last menses were used to determine the menstrual phase at which surgery occurred.
Menstrual phase categories were defined as follows: normal luteal phase, serum Pg level ≥ 5 ng/mL (with menstrual cycle interval of 21 to 35 days)32; normal follicular phase, serum Pg level ≤ 3 ng/mL before cycle day 21 (with menstrual cycle interval 21 to 35 days)32; anovulation, serum Pg level less than 3 ng/mL after cycle day 21; possible luteal phase, serum Pg between 3 and 5 ng/mL; persistent corpus luteum, serum Pg level greater than 3 ng/mL before cycle day 5; and oligo-ovulation, serum Pg level greater than 3 ng/mL after cycle day 35. For purposes of statistical analyses, primary comparison was between groups of normal luteal phase and normal follicular phase; groups of anovulation through oligo-ovulation were combined into the category of other.
Statistical Design and Analysis
The primary end point of this trial was DFS, which was defined as the time from registration to the recurrence of tumor at any local, regional, or distant location; the detection of a second primary cancer; or death as a result of any cause without documentation of recurrence. Ipsilateral breast tumor recurrences after breast-conserving surgery were considered an event. A secondary end point was OS, which was defined as time from registration to death as a result of any cause.
This study was designed with the assumption that 75% of the patients would have surgery during the follicular phase, the enrollment period would be 5 years, and the follow-up period after the close of enrollment would be 3 years. With a sample size of 804 women, a two-sided α = .05 log-rank test would have a power of 82% to detect a 10% difference in the 5-year DFS rate from 67.5% to 77.5% when the follicular group has the poorer DFS, or a power of 86% to detect a 10% difference in the 5-year DFS rate from 67.5% to 77.5% when the luteal group has the poorer DFS. The expected number of events was 218.
For each menstrual cycle group, the distributions of DFS and OS were estimated by using the Kaplan-Meier method. A log-rank test and univariate Cox proportional hazard modeling were used to assess whether the distributions of DFS or OS differed with respect to menstrual cycle phase at surgery or other patient/disease characteristics. For each end point, multivariate Cox modeling was used to obtain a subset of patient/disease characteristics that provided an adequate fit to the data. Residual plots were examined to assess model adequacy. Then, a likelihood ratio test was used to assess whether menstrual cycle phase at surgery made a significant contribution to this model. Interaction between estrogen receptor status (ER) and menstrual cycle phase at surgery was assessed.
RESULTS
Study Cohort
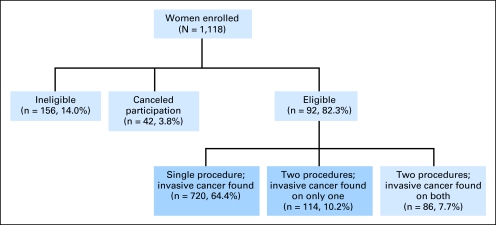
From July 1996 through December 2001, 1,118 (Fig 1) women were enrolled onto this trial by the NSABP (69.3%), NCCTG (16.5%), and IBCSG (14.2%). One hundred fifty-six women (14.0%) were declared ineligible because of pathology findings that disease was not stages I to II (n = 58), blood was not drawn per protocol (n = 46), residual disease remained after final surgery (n = 18), length of menstrual cycle was not 21 to 35 days or was not known (n = 15), chemotherapy or hormonal therapy was administered before first or second operative procedure (n = 15), oral contraceptive use occurred within 3 months of study entry (n = 3), and prior hysterectomy had been performed (n = 1). Seven women (0.6%) signed a consent form but refused to participate before the first or second operative procedure. An additional 35 patients (3.1%) were administratively canceled from additional participation because their blood specimens were improperly drawn, lost in transit, grossly hemolyzed, or thawed during shipment. Of the remaining 920 women, 720 women underwent a single surgical procedure in which cancer was detected, and 114 women underwent two surgical procedures in which cancer was identified at only one procedure. These 834 women comprised our study cohort.
Fig 1.
Distribution of patients according to eligibility and single- v two-stage operations.
Menstrual Phase
At the time of the surgical procedure, 230 women (28%) were classified as normal luteal phase; 363 women (44%) were classified as normal follicular phase, and the remaining 241 patients were grouped as other. Of those in the other group, 142 women (17%) were anovulatory, 71 women (9%) had a questionable luteal phase, 15 women (2%) were oligo-ovulatory, eight women (1%) had a persistent corpus luteum, and five women (1%) had inconsistent or contradictory information so that they could not be classified. Patient, disease, and treatment characteristics were well balanced between groups on the basis of menstrual cycle phase (Table 3).
Table 3.
Patient Demographic and Disease and Treatment Clinical Characteristics
| Characteristic | % of Patients by Menstrual Phase |
||
|---|---|---|---|
| Luteal (n = 230) | Follicular (n = 363) | Other (n = 241) | |
| Age at surgery, years | |||
| Median | 42 | 42 | 42 |
| Range | 28-52 | 22-54 | 23-53 |
| Ethnicity | |||
| White | 82.2 | 82.6 | 84.7 |
| African American | 9.1 | 8.3 | 9.5 |
| Asian | 2.6 | 4.1 | 1.2 |
| Hispanic | 2.6 | 3.3 | 3.3 |
| Native Hawaiian/Pacific Islander | 0.4 | 0.6 | 0 |
| Other | 0.9 | 0.8 | 1.2 |
| Not provided | 2.2 | 0.8 | 0 |
| Surgical procedure | |||
| One-step | 87.0 | 85.4 | 87.1 |
| Two-step | 13.0 | 14.6 | 12.9 |
| Estrogen receptor status | |||
| Positive | 72.6 | 67.8 | 70.5 |
| Borderline | 0.9 | 0.6 | 0.4 |
| Negative | 25.7 | 31.4 | 28.2 |
| Not done | 0.9 | 0.3 | 0.8 |
| Histology | |||
| Ductal | 90.9 | 88.2 | 90.5 |
| Lobular | 4.4 | 6.3 | 5.4 |
| Other | 5.7 | 5.5 | 4.1 |
| No. of positive nodes | |||
| Not evaluated | 0 | 1.4 | 0.8 |
| 0 | 62.2 | 58.9 | 58.9 |
| 1-3 | 28.7 | 30.3 | 30.3 |
| 4-9 | 6.1 | 7.2 | 7.1 |
| ≥ 10 | 3.0 | 2.2 | 2.9 |
| T stage | |||
| 1 | 62.6 | 61.7 | 55.2 |
| 2 | 36.5 | 37.7 | 43.6 |
| 3 | 0.9 | 0.6 | 1.3 |
| Adjuvant therapy | |||
| Chemotherapy | 73.0 | 73.3 | 78.8 |
| Radiation therapy | 63.0 | 69.2 | 69.7 |
| Hormonal therapy | 63.0 | 55.7 | 59.3 |
Clinical Outcomes
There have been 177 patients who developed recurrent disease or a second primary or who died without documentation of recurrence. First events included the following: local recurrence (n = 54), distant metastasis (n = 76), contralateral breast disease (n = 16), other second primary (n = 17), multiple disease event (n = 7), and death without disease recurrence (n = 7; Table 4). The estimated 5-year DFS rate was 81.5% (95% CI, 78.8% to 84.3%). There were 87 deaths, and reported causes of death included local/distant disease (n = 72), second primary disease (n = 5), other causes (n = 6), and unknown causes (n = 4). The median length of follow-up among the 747 patients known to be alive was 6.6 years (range, 1 day to 10.0 years). The estimated 5-year OS rate was 92.0% (95% CI, 90.1% to 93.9%).
Table 4.
Clinical Outcomes According to Menstrual Phase
| Outcome | No. of Patients by Menstrual Phase |
||
|---|---|---|---|
| Luteal (n = 230) | Follicular (n = 363) | Other (n = 241) | |
| First event* | 52 | 73 | 52 |
| Locoregional recurrence | 12 | 26 | 16 |
| Distant metastases | 22 | 28 | 26 |
| Contralateral breast disease | 10 | 5 | 1 |
| Other second primary disease | 5 | 4 | 8 |
| Multiple disease events | 1 | 6 | 0 |
| Death without disease recurrence | 2 | 4 | 1 |
Percent of patients with first event by menstrual phase as follows: luteal, 23.6; follicular, 20.1; other, 21.6.
Menstrual Phase at Surgery and Clinical Outcome
DFS or OS in all patients did not differ with respect to menstrual phase at surgery (follicular v luteal v indeterminate: log-rank P = .639 and .456, respectively.) The estimated 5-year DFS rates were 82.7% (95% CI, 78.7% to 86.8%), 82.1% (95% CI, 77.1% to 87.5%), and 79.2% (95% CI, 73.9% to 84.7%) among women who had surgery during the follicular, luteal, or indeterminate phases, respectively.
The estimated 5-year OS rates were 91.9% (95% CI, 89.1% to 94.8%), 92.2% (95% CI, 88.7% to 95.9%), and 91.8% (95% CI, 88.2% to 95.5%) among women who had surgery during the follicular, luteal, or indeterminate phases, respectively.
Outcomes Among Women Who Underwent Surgery During the Follicular or Luteal Phase
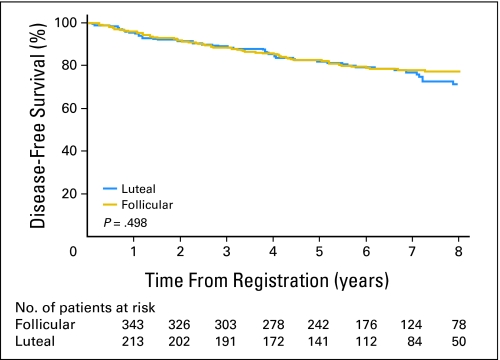
DFS time by univariate analysis did not differ between women who underwent surgery during the follicular phase versus the luteal phase (hazard ratio [HR], 0.88; 95% CI, 0.62 to 1.26; log-rank P = .498; Fig 2). After analysis accounted for nodal disease, ER status, adjuvant radiation therapy, and adjuvant chemotherapy, menstrual phase at surgery was not associated with DFS (n = 587; adjusted HR [HRadj]; [follicular v luteal], 0.83; 95% CI, 0.58 to 1.19; P = .319). The HRadj appeared to differ according to ER status (interaction Padj = .027; for ER-positive cohort: n = 416; HRadj [follicular v luteal], 1.16; 95% CI, 0.72 to 1.87; for ER-negative cohort: n = 171; HRadj [follicular v luteal], 0.532; 95% CI, 0.31 to 0.93).
Fig 2.
Disease-free survival of patients who underwent operation during follicular and luteal menstrual phases.
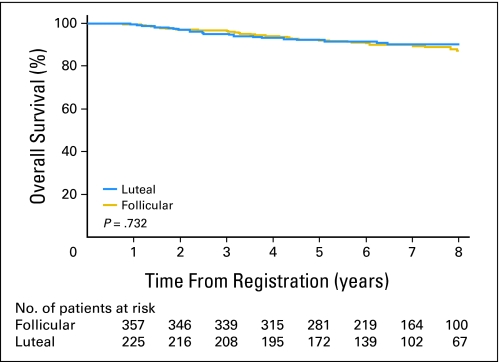
Similarly, survival time by univariate analysis was similar between women who underwent surgery in the follicular phase and those whose surgery was in the luteal phase (HR [follicular v luteal], 1.10; 95% CI, 0.64 to 1.88; log-rank P = .732; Fig 3). Moreover, OS did not differ with respect to menstrual phase at surgery after analysis was adjusted for nodal disease and ER tumor status (HRadj [follicular v luteal], 1.00; 95% CI, 0.58 to 1.71; P = .985).
Fig 3.
Overall survival of patients who underwent operation during follicular and luteal menstrual phases.
The interaction between ER status and menstrual phase was not significant for OS (interaction Padj = .232; for ER-positive cohort, n = 417; HRadj [follicular v luteal], 1.46; 95% CI, 0.68 to 3.16; for ER-negative cohort, n = 173; HRadj [follicular v luteal], 0.780; 95% CI, 0.36 to 1.68).
DISCUSSION
In this prospective study of 834 premenopausal women with early-stage breast cancer who had biochemical definition of menstrual cycle phase, neither DFS nor OS differed between women who underwent surgery during the follicular phase compared with the luteal phase after a median follow-up of 6.6 years. Moreover, even after analysis was adjusted for nodal status and ER tumor status, menstrual phase at the time of surgery was not significantly associated with either DFS or OS. Menstrual phase for each patient was defined by the combination of menstrual history obtained at the time of surgery and hormonal values obtained from blood that had been drawn within 1 calendar day of the surgical intervention. Nevertheless, 29.3% of the patients did not meet criteria for either normal follicular- or luteal-phase categories. Notably, as a group, these patients classified in the other category did not differ with respect to DFS or OS from the patients in the normal follicular or luteal phases.
Although patients in this study with ER-negative tumors had better outcomes if their operations were performed in the follicular phase, Goldhirsch et al13 found precisely the opposite—a significantly improved outcome (ie, DFS) for operations during the luteal phase in this subpopulation. This provides additional support that conclusions should be based on the overall findings.
The controversy regarding a possible link between menstrual cycle phase at surgery for breast cancer and outcome has existed for almost 20 years,1 and the large majority of data are derived from retrospective studies with menstrual cycle phases defined by chart documentation of the LMP. This study is the largest prospective trial to address this question, in which menstrual cycle phase—follicular or luteal—was defined biochemically by hormone levels obtained within 1 day of surgery. Thorpe et al23 reported 3-year survival data for 256 patients who had hormonal characterization of follicular versus luteal phase at primary breast cancer surgery and saw no difference in outcome with menstrual cycle phase. In contrast to the criteria in this study for categorization of patients into luteal and follicular phases, the Thorpe study used an independent expert to assign the patients. Pujol et al conducted a similar study in 350 women and found no difference in outcome22 when biochemically determined cycle phase was compared with what would have been assigned on the basis of chart review. Notably, 52% of participants would have been misclassified on the basis of chart dates alone.
Some explanation must be considered to account for the multitude of studies, and even three meta-analyses,15,33,34 that concluded that patients who underwent operation during the luteal phase had improved survival, with odds reduction of 12% to 16%. Virtually all criticisms of prior retrospective studies have focused on two problems: different definitions of the favorable, luteal phase, timeframe or the potential inaccuracies in determination of menstrual cycle phase on the basis of LMP, as obtained from review of a patient's clinical records. As is apparent from this study, in which nearly 30% of patients could not be assigned to either menstrual phase despite hormonal levels, misclassification of such a large segment of a study population would certainly lead to discrepancies in findings.
One limitation of this study design was its lack of random assignment. However, at the time of study initiation, there were insufficient data to justify delaying surgical intervention for the sole purpose of randomly assigning patients to surgery on the basis of menstrual cycle phase. Even without random assignment, patient registration onto the study resulted in a fairly even distribution between follicular- versus luteal-phase surgical intervention, given our stringent criteria of normal follicle and luteal phases. Another possible limitation was the allowance of fine-needle aspiration or core needle biopsy for diagnostic purposes. We recognize that perturbation of the tumor occurs with these diagnostic procedures. However, such minimal intervention has not been recognized to alter prognosis and has become common practice (if not standard of care) in present-day breast cancer management. Finally, this study did not dictate the use of adjuvant therapy after surgery. It would have been highly unlikely that we could secure the needed investigator and patient agreement to standardize the approach used for all participants in this trial. Because we captured relevant adjuvant treatment information on all participating patients, we were able to determine that there were no imbalances in the patient group treatments in this regard.
In conclusion, this large, prospective study, which used biochemical definition of menstrual cycle phase, does not confirm a relationship between DFS or OS and timing of surgery on the basis of the menstrual cycle phase of premenopausal women with early-stage breast cancer. It emphasizes the crucial need for such studies to use stringent menstrual information with appropriate hormone determinations to classify the menstrual cycle phase of premenopausal surgical candidates.
Appendix
Proposed mechanisms.
As perplexing as the contradictory data regarding outcome are, several potential biologic mechanisms have been proposed to explain the putative adverse effect from breast cancer surgery performed during the follicular phase. Estrogen unopposed by progesterone during the follicular phase may affect adhesion molecules or growth factors in ways that facilitate tumor shedding or growth of shed metastases (Kontos M, Fentiman I: Breast J 12:518-525, 2006).3 Alternatively, progesterone production during the luteal phase could exert a protective or an inhibitory effect. Other authors have implicated alterations in the immune system, particularly natural-killer cell activity during the follicular phase that could impact on the metastatic cascade (Goldfarb Y, Ben-Eliyahu S: Breast Dis 26:99-114, 2006-2007).1
Menstrual cycle hormones.
During the menstrual cycle, there are sizable fluctuations in estrogen (E2), progesterone (Pg), follicle-stimulating hormone, and luteinizing hormone (LH) levels. The follicular phase begins with the first day of menses and ends with ovulation, during which time follicle-stimulating hormone stimulates follicle development and causes an increase in circulating E2 levels as the dominant follicle grows in size. A midcycle luteinizing hormone surge at the time of follicle maturation induces ovulation and subsequently converts the mature follicle to a corpus luteum, which produces both E2 and Pg in the luteal phase. Without pregnancy, E2 and Pg production by the corpus luteum wane, and the resulting withdrawal of steroid exposure to the uterine endometrium leads to menstruation. Therefore, the normal length of the follicular phase varies from 14- to 21-days duration, whereas the luteal phase remains more constant at 14-days duration.
Online-only participant lists.
The following are North Central Cancer Treatment Group participating institutions: Alan Blair Cancer Center, Regina, SK; Alexander Cancer Care Center, Ann Arbor, MI; Carle Clinic Association, Urbana, IL; CentraCare Clinic, St Cloud, MN; Creighton Cancer Center, Omaha, NE; Duluth Clinic, Duluth, MN; Francisian Skemp Healthcare, LaCrosse, WI; Geisinger Clinic, Danville, PA; Illinois Cancer Care, Peoria, IL; Mayo Clinic: Minnesota, Florida, and Arizona; Mercy Cancer Center, Mason City, IA; MeritCare Hospital Community Clinical Oncology Program (CCOP), Fargo, ND; Ochsner Clinic, New Orleans, LA; Rapid City Regional Hospital, Rapid City, SD; Siouxland Hematology/Oncology Associates, Sioux City, IA; Toledo Community Hospital, Toledo, OH.
The following are National Surgical Adjuvant Breast and Bowel Project participating institutions: Akron City Hospital, Akron, OH; Aultman Hospital, Canton, OH; Baptist Medical Centers, Birmingham, AL; Bishop Clarkson Memorial Hospital, Omaha, NE; Boston Medical Center, Boston, MA; British Columbia Cancer Agency, Vancouver, Canada; Camden-Clark Memorial Hospital, Parkersburg, WV; Cancer Institute of New Jersey, New Brunswick, NJ; CCOP, Alton Ochsner Medical Foundation, New Orleans, LA; CCOP, Atlanta Regional, Atlanta, GA; CCOP, Benaroya Research Institute at Virginia Mason, Seattle, WA; CCOP, Columbus, OH; CCOP, Dayton, OH; CCOP, Evanston Northwestern Healthcare/Kellogg Cancer Center, IL; CCOP, Grand Rapids Clinical Oncology Program, Grand Rapids, MI; CCOP, Hematology-Oncology Associates of CNY, Syracuse, NY; CCOP, Kalamazoo, MI; CCOP, Kansas City, MO; CCOP, Main Line Health, Wynnewood, PA; CCOP, Metro-Minnesota, Minneapolis, MN; CCOP, Mt. Sinai Medical Center, Miami Beach, FL; CCOP, Northwest, Tacoma, WA; CCOP, Oklahoma, Tulsa, OK; CCOP, Scott and White Memorial Hospital, Temple, TX; CCOP, Southeast Cancer Control Consortium, Winston-Salem, NC; CCOP, Upstate Carolina, Spartanburg, SC; City of Hope National Medical Center, Duarte, CA; Clarian Health Partners, Inc., Indianapolis, IN; Cross Cancer Institute, Edmonton, Canada; Eastern Maine Medical Center, Bangor, ME; Eisenhower Army Medical Center, Fort Gordon, GA; Franklin Square Hospital Center, Baltimore, MD; Genesee Surgical Associates, PC, Rochester, NY; George Washington University Medical Center, Washington, DC; Georgetown University, Washington, DC; Hennepin County Medical Center, Minneapolis, MN; Henry Ford Health System, Detroit, MI; Jewish General Hospital, Montreal, Canada; Joe Arrington Cancer Research & Treatment Center, Lubbock, TX; Kent County Memorial Hospital, Warwick, RI; Kimmel Cancer Center at Jefferson, Philadelphia, PA; Lahey Clinic Medical Center, Burlington, MA; Lehigh Valley Hospital, Allentown, PA; MBCCOP, Gulf Coast, Mobile, AL; MBCCOP, Louisiana State University Health Sciences Center, New Orleans, LA; Mercy Regional Cancer, Redding, CA; Michigan State University, E. Lansing, MI; Mount Sinai Hospital, New York, NY; National Naval Medical Center, Bethesda, MD; Nebraska Methodist Hospital, Omaha, NE; New York Oncology Hematology PC, Albany, NY; Northern New Jersey, Hackensack, NJ; Ocala Oncology Center, Ocala, FL; Odette Cancer Centre, Toronto, Ontario, Canada; Oncology Alliance, Milwaukee, WI; Oregon Health and Science University, Portland, OR; Puget Sound Oncology Consortium, Seattle, WA; Rockford Health Physicians, Rockford, IL; Rush University Medical Center, Chicago, IL; South Pointe Hospital/CCHS, Warrensville Heights, OH; St Luke's Hospital, Bethlehem, PA; St Michael's Hospital, Toronto, Canada; Sutter Health Cancer Research Group-Eastern Division, Sacramento, CA; Tulane University Hospital and Clinic, New Orleans, LA; University of Arkansas for Medical Sciences, Little Rock, AR; University of Cincinnati, OH; University of Colorado Cancer Center, Denver, CO; University of Connecticut, Farmington, CT; University of Kansas Medical Center, Kansas City, KS; University of Montreal Hospital Group, Montreal, PQ; University of Pittsburgh, PA; University of Texas Southwestern Medical Center at Dallas, TX; University of Vermont, Burlington, VT; York Hospital, York, PA.
Online-only acknowledgment.
Trial N9431 International Breast Cancer Study Group Participating Groups and Centers: Australian New Zealand Breast Cancer Trials Group—Calvary Mater Newcastle, New South Wales (J.F. Forbes); Mater Hospital and Mater Adult Hospital Brisbane, Queensland (C. Pyke); Austin Health, Victoria (L. Castles); Maroondah Hospital, Victoria (J. Chirgwin); Auckland City Hospital, New Zealand (W.O. Jones); Waikato Hospital, New Zealand (I. Campbell); Italy—Istituto Europeo di Oncologia, Milano (A. Goldhirsch, F. Nolè); Centro di Riferimento Oncologico, Aviano (A. Veronesi, D. Crivellari); Chilean Cooperative Group for Oncologic Research (GOCCHI), Chile—J. Camacho; Clinica Las Condes, Santiago (J.C. Acevedo, A. Leon); Hospital Militar, Santiago (R. Schwartz); Hospital Clínico Universidad Católica, Santiago (M. Camus); Hospital Salvador, Santiago (C. Barriga); Fundación Arturo López Pérez, Santiago (M. Fritis); Hospital San Borja Arriarán, Santiago (J. Letzkus); Clínica Alemana, Santiago (E. Cunil); Hospital Naval Almirante Neff, Viña del Mar (A. Cubillos); Swiss Group for Clinical Cancer Research (SAKK) Switzerland—Kantonsspital, St Gallen (B. Thürlimann, T. Ruhstaller).
Footnotes
Supported in part by Grants No. CA25224 (North Central Cancer Treatment Group) and CA075364 (International Breast Cancer Study Group) from the National Institute of Health and by Public Health Service Grants No. U10-CA-12027, U10-CA-37377, U10-CA-69651, and U10-CA-69974 (all National Surgical Adjuvant Breast and Bowel Project).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Clive S. Grant, James N. Ingle, Vera J. Suman, Daniel A. Dumesic, D. Lawrence Wickerham, Richard D. Gelber, Lynn C. Hartmann
Financial support: Clive S. Grant, James N. Ingle, D. Lawrence Wickerham, Richard D. Gelber, Lynn C. Hartmann
Administrative support: Clive S. Grant, James N. Ingle, D. Lawrence Wickerham, Richard D. Gelber, Lynn C. Hartmann
Provision of study materials or patients: Clive S. Grant, James N. Ingle, Daniel A. Dumesic, D. Lawrence Wickerham, Richard D. Gelber, Patrick J. Flynn, Lorna M. Weir, Mattia Intra, Wayne O. Jones, Lynn C. Hartmann
Collection and assembly of data: Clive S. Grant, James N. Ingle, Vera J. Suman, Daniel A. Dumesic
Data analysis and interpretation: Clive S. Grant, James N. Ingle, Vera J. Suman, Daniel A. Dumesic, D. Lawrence Wickerham, Richard D. Gelber, Patrick J. Flynn, Lorna M. Weir, Mattia Intra, Wayne O. Jones, Edith A. Perez, Lynn C. Hartmann
Manuscript writing: Clive S. Grant, James N. Ingle, Vera J. Suman, Daniel A. Dumesic, D. Lawrence Wickerham, Richard D. Gelber, Lynn C. Hartmann
Final approval of manuscript: Clive S. Grant, James N. Ingle, Vera J. Suman, Daniel A. Dumesic, D. Lawrence Wickerham, Richard D. Gelber, Patrick J. Flynn, Lorna M. Weir, Mattia Intra, Wayne O. Jones, Edith A. Perez, Lynn C. Hartmann
REFERENCES
- 1.Hrushesky WJM, Bluming AZ, Gruber SA, et al. Menstrual influence on surgical cure of breast cancer. Lancet. 1989;2:949–952. doi: 10.1016/s0140-6736(89)90956-2. [DOI] [PubMed] [Google Scholar]
- 2.Ratajczak HV, Sothern RB, Hrushesky WJM. Estrous influence on surgical cure of a mouse breast cancer. J Exp Med. 1988;168:73–83. doi: 10.1084/jem.168.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badwe RA, Gregory WM, Chaudary MA, et al. Timing of surgery during menstrual cycle and survival of premenopausal women with operable breast cancer. Lancet. 1991;337:1261–1264. doi: 10.1016/0140-6736(91)92927-t. [DOI] [PubMed] [Google Scholar]
- 4.Senie RT, Rosen PP, Rhodes P, et al. Timing of breast cancer excision during the menstrual cycle influences duration of disease-free survival. Ann Intern Med. 1991;115:337–342. doi: 10.7326/0003-4819-115-5-337. [DOI] [PubMed] [Google Scholar]
- 5.Bates GW, Boone WR. The female reproductive cycle: New variations on an old theme. Curr Opin Obstet Gynecol. 1991;3:838–843. [PubMed] [Google Scholar]
- 6.Veronesi U, Luini A, Mariani L, et al. Effect of menstrual phase on surgical treatment of breast cancer. Lancet. 1994;343:1545–1547. doi: 10.1016/s0140-6736(94)92942-4. [DOI] [PubMed] [Google Scholar]
- 7.Powles TJ, Jones AL, Ashley S, et al. Menstrual effect on surgical cure of breast cancer. Lancet. 1989;2:1343–1344. [PubMed] [Google Scholar]
- 8.Gelber RD, Goldhirsch A. Menstrual effect on surgical cure of breast cancer. Lancet. 1989;2:1344. [Google Scholar]
- 9.Powles TJ, Ashley SE, Nash AG, et al. Timing of surgery in breast cancer. Lancet. 1991;337:1604. [Google Scholar]
- 10.Ville Y, Briere M, Lasry S, et al. Timing of surgery in breast cancer. Lancet. 1991;337:1603–1605. [PubMed] [Google Scholar]
- 11.Sainsbury R. Timing of surgery for breast cancer in relation to the menstrual cycle and survival of premenopausal women. Br J Surg. 1993;80:670. doi: 10.1002/bjs.1800800536. [DOI] [PubMed] [Google Scholar]
- 12.Low SC, Galea MH, Blamey RW. Timing breast cancer surgery. Lancet. 1991;338:691–693. [PubMed] [Google Scholar]
- 13.Goldhirsch A, Gelber RD, Castiglione M, et al. Menstrual cycle and timing of breast surgery in premenopausal node-positive breast cancer: Results of the international breast cancer study group (IBCSG) trial VI. Ann Oncol. 1997;8:751–756. doi: 10.1023/a:1008220301866. [DOI] [PubMed] [Google Scholar]
- 14.Kroman N, Højgaard A, Andersen KW, et al. Timing of surgery in relation to menstrual cycle does not predict the prognosis in primary breast cancer. Eur J Surg Oncol. 1994;20:430–435. [PubMed] [Google Scholar]
- 15.Fentiman IS, Gregory WM, Richards MA. Effect of menstrual phase on surgical treatment of breast cancer. Lancet. 1994;344:402. [PubMed] [Google Scholar]
- 16.Badwe RA, Richards MA, Fentiman IS, et al. Surgical procedures, menstrual cycle phase, and prognosis in operable breast cancer. Lancet. 1991;338:815–816. doi: 10.1016/0140-6736(91)90695-l. [DOI] [PubMed] [Google Scholar]
- 17.Badwe R, Wang DY, Gregory WM, et al. Serum progesterone at the time of surgery and survival in women with premenopausal operable breast cancer. Eur J Cancer. 1994;30A:445–448. doi: 10.1016/0959-8049(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 18.Saad Z, Bramwell V, Duff J, et al. Timing of surgery in relation to the menstrual cycle in premenopausal women with operable breast cancer. Br J Surg. 1994;81:217–220. doi: 10.1002/bjs.1800810219. [DOI] [PubMed] [Google Scholar]
- 19.Holli K, Isola J, Hakama M. Prognostic effect of timing of operation in relation to menstrual phase of breast cancer patient: Fact or fallacy. Br J Cancer. 1995;71:124–127. doi: 10.1038/bjc.1995.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohr P, Wang DY, Gregory WM, et al. Serum progesterone and prognosis in operable breast cancer. Br J Cancer. 1996;73:1552–1555. doi: 10.1038/bjc.1996.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper LS, Gillett CE, Patel NK, et al. Survival of premenopausal breast carcinoma patients in relation to menstrual cycle timing of surgery and estrogen receptor/progesterone receptor status of the primary tumor. Cancer. 1999;86:2053–2058. doi: 10.1002/(sici)1097-0142(19991115)86:10<2053::aid-cncr24>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Pujol P, Daures JP, Brouillet JP, et al. A prospective prognostic study of the hormonal milieu at the time of surgery in premenopausal breast carcinoma. Cancer. 2001;91:1854–1861. [PubMed] [Google Scholar]
- 23.Thorpe H, Brown SR, Sainsbury JR, et al. Timing of breast cancer surgery in relation to menstrual cycle phase: No effect on 3-year prognosis—The ITS Study. Br J Cancer. 2008;98:39–44. doi: 10.1038/sj.bjc.6604120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan B, Bates T, Anbazhagan R, et al. Timing of surgery for breast cancer in relation to the menstrual cycle and survival of premenopausal women. Br J Surg. 1993;80:43. doi: 10.1002/bjs.1800800115. [DOI] [PubMed] [Google Scholar]
- 25.Ville Y, Lasry S, Spyratos F, et al. Menstrual status and breast cancer surgery. Breast Cancer Res Treat. 1990;16:119–121. doi: 10.1007/BF01809296. [DOI] [PubMed] [Google Scholar]
- 26.Rageth JC, Wyss P, Unger C, et al. Timing of breast cancer surgery within the menstrual cycle: Influence on lymph-node involvement, receptor, postoperative metastatic spread and local recurrence. Ann Oncol. 1991;2:269–272. doi: 10.1093/oxfordjournals.annonc.a057935. [DOI] [PubMed] [Google Scholar]
- 27.Donegan WL, Shah D. Prognosis of patients with breast cancer related to the timing of operation. Arch Surg. 1993;128:309–313. doi: 10.1001/archsurg.1993.01420150065012. [DOI] [PubMed] [Google Scholar]
- 28.Corder A, Cross M, Julious SA, et al. The timing of breast cancer surgery within the menstrual cycle. Postgrad Med J. 1994;70:281–284. doi: 10.1136/pgmj.70.822.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gnant MF, Seifert M, Jakesz R, et al. Breast cancer and timing of surgery during menstrual cycle: A 5-year analysis of 385 pre-menopausal women. Int J Cancer. 1992;52:707–712. doi: 10.1002/ijc.2910520507. [DOI] [PubMed] [Google Scholar]
- 30.Wobbes T, Thomas CM, Segers MF, et al. The phase of the menstrual cycle has no influence on the disease-free survival of patients with mammary carcinoma. Br J Cancer. 1994;69:599–600. doi: 10.1038/bjc.1994.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milella M, Nisticò C, Ferraresi V, et al. Breast cancer and timing of surgery during menstrual cycle: A 5-year analysis of 248 premenopausal patients. Breast Cancer Res Treat. 1999;55:259–266. doi: 10.1023/a:1006276120841. [DOI] [PubMed] [Google Scholar]
- 32.Israel R, Mishell DR, Jr, Stone SC, et al. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol. 1972;112:1043. doi: 10.1016/0002-9378(72)90178-0. [DOI] [PubMed] [Google Scholar]
- 33.Badwe R, Bhansali M, Vaidya J. Unopposed estrogen and survival of breast cancer. Breast. 1998;7:66–71. [Google Scholar]
- 34.Lemon H, Rodriguez-Sierra J. Timing of breast cancer surgery during the luteal menstrual phase may improve prognosis. Nebr Med J. 1996;81:110–115. [PubMed] [Google Scholar]