Abstract
Purpose
The aim of this study was to evaluate the clinical relevance of increases in quantitative polymerase chain reaction (QPCR) levels in patients with chronic myelogenous leukemia (CML) who are in complete cytogenetic response (CGCR) on therapy. Patients with Philadelphia chromosome (Ph)–positive CML receiving tyrosine kinase inhibitors (TKIs) are frequently monitored for response by QPCR studies for minimal molecular disease. The clinical significance of increasing levels of QPCR in patients in CGCR is uncertain.
Patients and Methods
One hundred sixteen patients in durable CGCR, and on imatinib therapy for at least 18 months, had increases in QPCR levels (documented at least twice consecutively) as defined by literature reports. These were further analyzed by the achievement of major molecular response (MMR) defined as QPCR ≤ 0.05%, as well as by the degree of increase in QPCR.
Results
Only 11 (9.5%) of 116 patients with increases in QPCR had CML progression; 10 of them were among 44 patients (23%) who either lost a MMR or never had a MMR, and had more than 1 log increase of QPCR.
Conclusion
Most patients with increases in QPCR remain in CGCR. Patients who lose a MMR or never achieve a MMR, and have more than 1 log increase of QPCR, should be monitored more closely, and may be evaluated for mutations of BCR-ABL kinase domain and considered for investigational therapeutic interventions.
INTRODUCTION
Therapy with imatinib mesylate, a selective BCR-ABL tyrosine kinase inhibitor (TKI), has resulted in a major change in the prognosis of patients with Philadelphia chromosome (Ph)–positive chronic myeloid leukemia (CML). In long-term follow-up studies, imatinib therapy was associated with a single best complete cytogenetic response (CGCR) rate of 87%, an estimated 5-year CGCR rate of 67% and a survival rate of 90%.1–4 Quantitative molecular studies, using polymerase chain reaction (PCR) analysis to quantify the amount of CML residual disease, particularly in CGCR, have shown a 3-log reduction of disease in approximately 40% to 60% of patients.5–8
The outstanding therapeutic results have shifted attention to predicting, by molecular studies, which patients in CGCR would have a high likelihood of relapse,8–12 and who might therefore benefit from escalating the imatinib dose13–14 or changing therapy to the more potent second generation TKIs like dasatinib, nilotinib, or bosutinib.15–17 Some studies have associated a two-fold, a 0.5-log, or a 1-log increase of molecular disease with a higher rate of detection of mutations and/or a higher relapse rate.7,9,18,19 This has resulted in recommendations of frequent monitoring of patient status by quantitative PCR (QPCR) molecular studies (eg, every 2 to 3 months).12,20 In the community practice, which uses different sources of samples (blood v marrow), different handling and shipping procedures of samples to reference laboratories, and which rely on commercial laboratories, frequent monitoring has at times resulted in confusion about the interpretation of results when variations of QPCR studies are observed. This has also led to therapeutic interventions which could have been unnecessary or even harmful to patients who were having excellent responses on imatinib. This is even more notable since 0.5-log variations of QPCR can be part of the testing variability, even in the best reference laboratories.21
To address this issue, we analyzed our institutional experience in patients with CML on imatinib therapy who had achieved a durable CGCR, to assess whether variations in QPCR levels, defined to be significant in some literature studies, benefit from therapeutic interventions.
PATIENTS AND METHODS
All patients with newly diagnosed Ph-positive CML in chronic phase treated on imatinib studies were analyzed. These patients were treated on protocols requiring cytogenetic (or fluorescent in situ hybridization) and QPCR analyses every 3 months in the first 2 years, and every 6 months thereafter. The focus of this analysis was patients who might yield the best information on the value of molecular monitoring of minimal residual disease—that is patients who were in a stable CGCR. Thus, the subjects of more detailed analysis were patients in durable CGCR (0% Ph-positive metaphases documented continuously for at least 6 months), on imatinib therapy for at least 18 months, who demonstrated increases of QPCR to levels reported to be significant in the literature, and in whom such increases were documented consecutively at least twice on repeat QPCR testing. We also analyzed patients by: imatinib dose; whether they had maintained a major molecular response, lost it, or never achieved it; and whether the increase in QPCR was more than 2-fold to ≤ 0.5 log, between 0.5 and 1 log, more than 1 log up to 2 logs, or more than 2 logs. A major molecular response (MMR) was defined as a BCR-ABL/ABL transcript level of 0.05% or lower. This was based on our institutional equivalent of a 3-log reduction of CML disease from the average pretreatment baseline of our patients with CML in chronic phase. Patients were then analyzed according to the imatinib dose intervention. A cytogenetic relapse was defined as any presence of Ph-positive metaphases by routine cytogenetic analysis.
Progression-free survival was measured from the time a significant increase in QPCR was documented at least twice consecutively until disease progression, defined as death, development of accelerated or blastic phases, or eventual loss of cytogenetic or hematologic response. Survival was calculated from the time of the documented increase in QPCR until death from any cause.
RESULTS
Two hundred fifty-eight patients with newly diagnosed CML in chronic phase treated with imatinib were evaluated. Fifty patients received initially standard-dose imatinib, 400 mg daily, and 208 patients received high-dose imatinib, 400 mg twice daily. A total of 188 patients were documented to show an increase in QPCR levels on imatinib therapy; 72 patients had an increase in QPCR documented only once and were therefore excluded from subsequent analyses. Thus, 116 patients had documented increases in QPCR at least on two consecutive occasions. These included 21 patients who had increases in QPCR on standard-dose imatinib regimens (ie, 400 mg daily), and 95 patients who had increases in QPCR on high-dose imatinib regimens (ie, 400 mg twice daily). Patients were grouped by the initial imatinib dose, not the actual dose they were receiving at the time of the documentation of increases in QPCR.
Outcome of Patients With Increases in QPCR But Without Loss of MMR
Twenty-eight patients had a two-fold or greater increase in QPCR but never lost a MMR (ie, QPCR always ≤ 0.05%). These included three patients on standard-dose imatinib and 25 patients on imatinib 800 mg daily. None had dose modifications. All 28 patients remained in CGCR with a median follow-up time of 37 months (range, 1 to 62 months) from the time of increase in QPCR (one died of heart attack and congestive heart failure in CGCR).
Outcome of Patients With increases in QPCR and Loss of MMR
Twelve patients had an increase in QPCR and loss of a MMR (ie, increase in QPCR from ≤ 0.05% to > 0.05%) on standard-dose imatinib (Table 1). One patient had an increase in QPCR by two-fold to 1 log, seven patients had an increase between 1 log to 2 logs, and four patients had an increase by more than 2 logs. Of these 12 patients, 10 had no change of therapy; all remained in CGCR with a median follow-up time of 44 months (range, 18 to 57 months) from the time of QPCR increase. One of the 12 patients had an imatinib dose escalation to 800 mg daily after the increase in QPCR after treatment interruption resulting in cytogenetic relapse; he reachieved a CGCR (Table 1). The second patient had an increase in QPCR followed by cytogenetic relapse; with imatinib dose escalation, he achieved a transient CGCR but eventually had a cytogenetic relapse.
Table 1.
Outcome of Patients in CGCR on Imatinib Starting Dose of 400 mg Daily With Significant Increases in QPCR
| QPCR Level by QPCR Log Increase | No. of Patients | Imatinib Dose Escalation | CG Relapse | CML Progression |
|---|---|---|---|---|
| Loss of MMR | ||||
| > 0.5-1 | 1 | 0 | 0 | 0 |
| > 1-2 | 7 | 0 | 1 | 0 |
| > 2 | 4 | 2 | 2 | 1 |
| Not in MMR | ||||
| < 1 | 3 | 0 | 0 | 0 |
| > 1 | 3 | 0 | 3 | 2 |
Abbreviations: MMR, major molecular response; CGCR, cytogenetic complete response; CML, chronic myelogenous leukemia; QPCR, quantitative polymerase chain reaction.
Thirty-six patients lost a MMR on imatinib 800 mg daily (Table 2). Of these patients, 11 had a 0.5- to 1-log increase; all 11 patients continued on imatinib without dose escalation (five at 800 mg, five at 600 mg, one at 400 mg daily) and remained in CGCR with a median follow-up time of 30 months (range, 10 to 47 months) from the time of increase in QPCR (Table 2).
Table 2.
Outcome of Patients in CGCR on Imatinib Starting Dose of 800 mg Daily With Significant Increases in QPCR
| PCR Increase at Imatinib Dose (mg) | No. of Patients | Imatinib Dose Escalation | CG Relapse | CML Progression |
|---|---|---|---|---|
| Loss of MMR, % | ||||
| > 0.5-1 | ||||
| 800 | 5 | — | 0 | 0 |
| 600 | 5 | 0 | 0 | 0 |
| 400 | 1 | 0 | 0 | 0 |
| > 1 to 2 | ||||
| 800 | 11 | — | 3 (2 transient loss of CGCR) | 1 |
| 600 | 4 | 0 | 1 | 1 |
| 400 | 3 | 0 | 0 | 1 (died; cause non-CML) |
| > 2 | ||||
| 800 | 2 | — | 1 | 1 |
| 600 | 1 | 0 | 0 | 0 |
| 400 | 4 | 2 no | 1 | 1 |
| 2 yes | 2 | 1 | ||
| Not in MMR, % | ||||
| < 0.5 | ||||
| 800 | 7 | — | 0 | 0 |
| 600 | 4 | 0 | 1 | 1 |
| > 0.5-1 | ||||
| 800 | 10 | — | 0 | 0 |
| 600 | 4 | 0 | 0 | 1 (died; cause non-CML) |
| 400 | 4 | 3 | 0 | 3 |
| > 1 to 2 | ||||
| 800 | 4 | — | 2 | 1 (died; cause non-CML) |
| 300 | 1 | 1 (from 300 mg to 400 mg) | 1 | 0 |
Abbreviations: CGCR, cytogenetic complete response; QPCR, quantitative polymerase chain reaction; PCR, polymerase chain reaction; CG, cytogenetic; CML, chronic myelogenous leukemia; MMR, major molecular response.
Eighteen had a loss of MMR and an increase in QPCR of higher than 1 to 2 logs (11 on 800 mg, four at 600 mg, three at 400 mg); all but three remained in CGCR (although two had transient loss of CGCR; Table 2). In the latter three patients, the progression was associated with an imatinib dose reduction to 400 mg daily in one, and to noncompliance (missing approximately 50% of the imatinib doses) in the second; the third patient died in CGCR from complications of surgery for bowel obstruction.
Seven patients had a loss of MMR and an increase in QPCR of higher than 2 logs (Table 2). Among five patients who had no imatinib dose escalation (two at 800 mg, one at 600 mg, two at 400 mg), two had CML progression. The other two patients had the higher than 2-log increase in QPCR at 400 mg: one had a cytogenetic relapse but recaptured a CGCR after imatinib dose escalation to 800 mg daily; and one had CML progression despite an imatinib dose escalation to 600 mg daily.
Outcome of Patients With Increases in QPCR Without Ever Achieving MMR
Six patients never achieved a MMR on standard-dose imatinib and had an increase in QPCR by two-fold to 1 log in three patients and by more than 1 log in three patients. All six patients continued at the same dose: two patients progressed and one patient had transient cytogenetic relapse and reachieved a CGCR (Table 1).
Thirty-four patients had an increase in QPCR without ever achieving a MMR on high-dose imatinib (Table 2).
Among 11 patients who had a two-fold to lower than 0.5-log increase, the increase occurred at the same imatinib dose in seven patients, (all remain in CGCR), and at a reduced imatinib dose (600 mg daily) in four patients (one had disease progression; Table 2).
Among 18 patients who had 0.5- to 1-log increase in QPCR, 10 had no change of imatinib dose; all of them remain in CGCR with a median follow-up time of 42 months (range, 29 to 54 months) from the increase in QPCR (Table 2). Eight patients had an increase in QPCR after imatinib dose reductions to 400 to 600 mg daily (Table 2); all eight remain in CGCR (one died in CGCR from non-CML causes) after a median follow-up time of 33 months (range, 17 to 43 months) from the increase in QPCR; three of patients had a dose escalation of imatinib (Table 2).
A third group of five patients had an increase in QPCR by more than 1 log but ≤ 2 log; four of them were on imatinib 800 mg daily; two progressed and one died of a non-CML cause (car accident); and one was at a reduced dose of imatinib 300 mg daily and reachieved a CGCR with dose escalation to 400 mg daily (Table 2).
The outcome of patients in the two imatinib dose cohorts is summarized in Table 3. The four patients who died from non-CML causes are censored at the time of death in the subsequent analysis. Overall, 11 (9.5%) of 116 patients with an increase in QPCR documented at least twice had CML progression. Ten of 11 patients (23%) Ten (23%) of 11 patients were among a group of 44 patients who had both lost a MMR and had a higher than 1 log increase in QPCR, or were not in MMR and had higher than 1 log increase in QPCR. The estimated 4-year progression-free survival rate (dated from time of increase in QPCR) was better among the 72 patients without an increase in QPCR versus the 44 patients with an increase in QPCR (4-year estimated rate, 98% v 72%; P < .01). However, the estimated 4-year survival rates were similar (98% v 97%), because nine of the 11 patients were later successfully treated with the second-generation tyrosine kinase inhibitors (five with dasatinib and four with bosutinib).
Table 3.
Outcome of Patients in CGCR by Increases in QPCR
| QPCR Log Increase | No. of Patients | Imatinib Dose Escalation | CML Progression | Follow-Up From QPCR Increase (months) |
|
|---|---|---|---|---|---|
| Median | Range | ||||
| Persistent MMR | |||||
| Any | 28 | 0 | 0 | 36 | 3-62 |
| Loss of MMR | |||||
| > 0.5-1 | 12 | 0 | 0 | 34 | 14-59 |
| > 1-2 | 25 | 0 | 2 | 31 | 6-52 |
| > 2 | 11 | 4 | 4 | 45 | 20-57 |
| Not in MMR | |||||
| < 1 | 32 | 3 | 1 | 35 | 10-70 |
| > 1 | 8 | 1 | 4 | 25 | 12-56 |
Abbreviations: CGCR, cytogenetic complete response; QPCR, quantitative polymerase chain reaction; CML, chronic myelogenous leukemia; MMR, major molecular response.
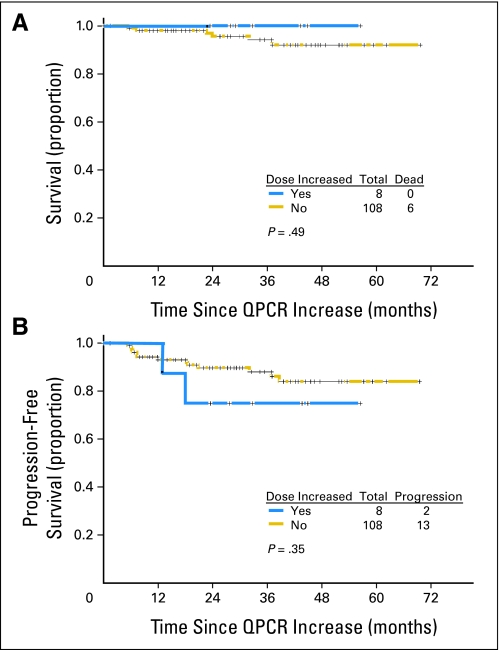
Survival and progression-free survival (including four non-CML deaths) of the total study group by whether the imatinib dose was not changed or escalated for an increase in QPCR are shown in Figure 1.
Fig 1.
(A) Survival and (B) progression-free survival by imatinib dose escalation. QPCR, quantitative polymerase chain reaction.
DISCUSSION
This study analyzed the clinical relevance of significant increases in QPCR in a large group of patients in stable CGCR on imatinib therapy for longer than 18 months. It surprisingly showed that increases in QPCR in CGCR have clinical relevance only in particular subsets. Only 11 (9.5%) of 116 patients in stable CGCR with increases in QPCR had CML progression. The patients at most risk, who should probably be monitored more frequently, are patients who show a loss of a MMR, or who are never in MMR, and in whom a higher than 1 log increase of BCR-ABL/ABL transcripts is observed. Ten patients with CML progression (23%) were among 44 patients fitting these criteria (Table 3). The survival of patients by whether the imatinib dose was not changed or was escalated were similar (Fig 1). This brings the issue of whether earlier interventions applied to the total group (in order to potentially benefit 9.5% or 23% of patients) was better than later interventions at the time of cytogenetic relapse.
The results of this analysis suggest that the current spreading practice of frequent QPCR monitoring (eg, every 2 to 3 months), and therapeutic interventions in patients in CGCR, are not necessary in most patients. In our practice, we monitor patients in stable CGCR (usually beyond the second year) with simultaneous QPCR and cytogenetic or fluorescent in situ hybridization testing every 6 months (to be reassured of concordance of results). Outside the context of research studies, perhaps a conservative approach of no therapeutic intervention would be most appropriate in patients in durable CGCR. Increases in QPCR may be due to several factors including inherent variabilities in QPCR measurements due to techniques, source of sample (blood v marrow), and intervariability between different laboratories. Thus, increases in QPCR in patients in CGCR should be simply followed more frequently (eg, every 3 months rather than 6 months) to monitor relevant trends.
A limitation of this analysis is the possibility that variations or increases in QPCR may be related to patient noncompliance. Studies evaluating compliance of patients with CML on therapy suggested suboptimal compliance in at least 20% to 40% of patients.22,23 Factors associated with poor compliance included older age, longer duration of CML, and a longer time period on imatinib therapy.24 All patients in this study were treated on protocols requiring visits and evaluations every 3 months in the first 2 years, and every 6 months thereafter. During these visits, patients were questioned about compliance to therapy, pill counts were conducted, and diaries were collected. Thus, compliance to therapy, which is an issue with any long-term oral therapy, is expected to be better among this study group than in community practice. This further highlights the issue of using an increase in QPCR in a patient with CML who is in durable CGCR on imatinib to change therapy, when such QPCR variations in community practice may be due, in addition to the technical considerations discussed earlier, to poor compliance of patients to imatinib therapy.
In summary, this study suggests that for patients with CML who are in durable CGCR on imatinib therapy, monitoring by QPCR is appropriate every 6 months. More frequent monitoring (eg, every 3 months) may be reasonable in patients who lose a MMR or never achieve a MMR, and who show higher than 1 log increase of CML burden (expected progression rate of 23%), although the benefit of earlier therapeutic intervention (v intervention at cytogenetic relapse) is not proven. Such patients may benefit from mutational analysis studies and may be good candidates for programs evaluating continuation of the same imatinib dose schedule versus escalating the imatinib dose or changing to second generation TKIs. Outside such investigations, therapeutic interventions, particularly a change to a second generation TKI or consideration of an allogeneic stem-cell transplant, are questionable in patients in CGCR.
Footnotes
Supported by the Betty Foster Research Grant, and grant No. CML PO1 2 CA 49639-20 from the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Elias Jabbour, Bristol Myers Squibb, Novartis Research Funding: Hagop M. Kantarjian, Novartis Pharmaceuticals; Daniel Jones, Novartis Pharmaceuticals; Susan O'Brien, Novartis Pharmaceuticals; Jorge Cortes, Novartis Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Hagop M. Kantarjian
Provision of study materials or patients: Hagop M. Kantarjian, Jianqin Shan, Daniel Jones, Susan O'Brien, Mary Beth Rios, Elias Jabbour, Jorge Cortes
Collection and assembly of data: Hagop M. Kantarjian, Jianqin Shan, Mary Beth Rios
Data analysis and interpretation: Hagop M. Kantarjian, Jianqin Shan, Daniel Jones, Susan O'Brien, Mary Beth Rios, Elias Jabbour, Jorge Cortes
Manuscript writing: Hagop M. Kantarjian, Daniel Jones, Susan O'Brien, Elias Jabbour, Jorge Cortes
Final approval of manuscript: Hagop M. Kantarjian, Daniel Jones, Susan O'Brien, Elias Jabbour, Jorge Cortes
REFERENCES
- 1.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-α–based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Cortes J, O'Brien S, et al. Imatinib mesylate therapy in newly diagnosed patients with Philadelphia chromosome-positive chronic myelogenous leukemia: High incidence of early complete and major cytogenetic responses. Blood. 2003;101:97–100. doi: 10.1182/blood-2002-02-0545. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571: Gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160–166. [PubMed] [Google Scholar]
- 6.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 7.Branford S, Seymour J, Grigg A, et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res. 2007;13:7080–7086. doi: 10.1158/1078-0432.CCR-07-0844. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, O'Brien S, Shan J, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia – need for new response definitions? Cancer. 2008;112:837–845. doi: 10.1002/cncr.23238. [DOI] [PubMed] [Google Scholar]
- 9.Press R, Galderisi C, Yang R, et al. A half-log increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin Cancer Res. 2007;13:6136–6144. doi: 10.1158/1078-0432.CCR-07-1112. [DOI] [PubMed] [Google Scholar]
- 10.Branford S, Rudzki Z, Parkinson I, et al. Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR-ABL kinase domain mutations. Blood. 2004;104:2926–2932. doi: 10.1182/blood-2004-03-1134. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Knight K, Lucas C, et al. The role of serial BCR-ABL transcript monitoring in predicting the emergence of BCR-ABL kinase mutations in imatinib-treated patients with chronic myeloid leukemia. Haematologica. 2006;91:235–239. [PubMed] [Google Scholar]
- 12.Hughes TP, Deininger MW, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors—review and recommendations for “harmonizing” current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantarjian H, Talpaz M, O'Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J, Giles F, O'Brien S, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-α. Blood. 2003;102:83–86. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 15.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 17.Bruemmendorf T, Cervantes F, Kim D, et al. Bosutinib is safe and active in patients (pts) with chronic phase (CP) chronic myeloid leukemia (CML) with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. J Clin Oncol. 2008;26(suppl):372s. abstr 7001. [Google Scholar]
- 18.Press R, Love Z, Tronnes A, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107:4250–4256. doi: 10.1182/blood-2005-11-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 20.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, Schiffer C, Jones D, et al. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: Practical advice on the use and interpretation of monitoring methods. Blood. 2008;111:1774–1780. doi: 10.1182/blood-2007-09-110189. [DOI] [PubMed] [Google Scholar]
- 22.Michallet M, Maloisel F, Chaleteix C, et al. Management of chronic myeloid leukemia in clinical practice in France; results of the French cohort of the unmet needs in CML (UNIC) study. Haematologica. 2008:93. abstr 0545. [Google Scholar]
- 23.Steegmann JL, Michallet M, Morra E, et al. Use of imatinib in chronic phase CML in clinical practice: The UNIC study. Haematologica. 2008:93. abstr 0126. [Google Scholar]
- 24.Abraham I, Noens L, De Bock R, et al. Nonadherence with imatinib treatment in chronic myeloid leukemia is a function of disease, health knowledge, and social factors – results from the Adagio study. Haematologica. 93:2008. abstr 0551. [Google Scholar]