Abstract
Purpose
We conducted a prospective trial to evaluate late effects in pediatric patients with low-grade glioma (LGG) treated with conformal radiation therapy (CRT).
Patients and Methods
Between August 1997 and August 2006, 78 pediatric patients with LGG (mean age, 9.7 years; standard deviation, ±4.4 years) received 54 Gy of CRT with a 10-mm clinical target volume margin. Tumor locations were diencephalon (n = 58), cerebral hemisphere (n = 3), and cerebellum (n = 17). Baseline and serial evaluations were performed to identify deficits in cognition, endocrine function, and hearing. Deficits were correlated with clinical factors and radiation dose within specific normal tissue volumes.
Results
Cognitive effects of CRT through 5 years after CRT correlated with patient age, neurofibromatosis type 1 status, tumor location and volume, extent of resection, and radiation dose. The effect of age exceeded that of radiation dose; patients younger than 5 years experienced the greatest decline in cognition. Before CRT, growth hormone (GH) secretion abnormality was diagnosed in 24% of tested patients, and 12% had precocious puberty. The 10-year cumulative incidence of GH replacement was 48.9%; of thyroid hormone replacement, 64.0%; of glucocorticoid replacement, 19.2%; and of gonadotropin-releasing hormone analog therapy, 34.2%. The mean ± standard errors of the cumulative incidence of hearing loss at 10 years did not exceed 5.7% ± 3.3% at any frequency.
Conclusion
To our knowledge, this is the largest series of prospectively followed children with LGG to undergo irradiation. Adverse effects are limited and predictable for most patients; however, this study provides additional evidence that CRT should be delayed for young patients and identifies the potential benefits of reducing radiation dose to normal brain.
INTRODUCTION
Pediatric patients with low-grade glioma (LGG) are characterized by long-term survivorship and the potential for late effects from tumor and treatment. Radiotherapy is the most effective nonsurgical treatment for pediatric LGG, as 5-year progression-free survival (PFS) estimates exceed 80%.1a–4 Radiotherapy is known to cause a broad range of adverse effects that have the potential to impact numerous functional domains and quality of life. Cognitive dysfunction and endocrinopathy are considered the most prevalent sequelae of irradiation,5–7 whereas vasculopathy with stroke and malignant transformation are more severe but less common.8–13
Efforts to minimize sequelae, including the use of chemotherapy as the preferred initial treatment for younger patients, have been given priority in the design of treatment regimens for pediatric LGG.14–18 This approach has been influenced by concern about the adverse effects of irradiation on the basis of earlier experiences with conventional methods. With the advent of newer methods comes the opportunity to re-evaluate the effects of irradiation and influential clinical factors.
We designed a trial to prospectively study the central nervous system (CNS) sequelae of conformal radiation therapy (CRT) in pediatric patients with LGG and to estimate their incidence, time to onset, and severity. Our goals were to document the ability of CRT to preserve CNS function, to identify risk factors of treatment effects, and to provide opportunities to study dose-volume reduction strategies and treatment regimens.
PATIENTS AND METHODS
Patients
Between August 1997 and August 2006, seventy-eight pediatric patients (39 boys, 39 girls) diagnosed with LGG were enrolled on a phase II study of CRT at St Jude Children's Research Hospital. The mean age at CRT was 9.7 years (standard deviation, ±4.3 years; median age, 8.9 years; range, 2.2 to 19.8 years). Patients were characterized by tumor location (central or diencephalic/optic pathway, n = 58; cerebral hemisphere, n = 3; cerebellum, n = 17), prior treatment with chemotherapy (n = 25), number of surgical procedures (none, n = 13; one, n = 42; > one, n =, 23), extent of resection (no biopsy, n = 13; biopsy, n = 30; subtotal resection, n = 35), and tumor grade (WHO grade 1, n = 67; WHO grade 2, n = 11). Hydrocephalus was present at diagnosis in 31 patients, ventriculoperitoneal shunt was required for 29 patients, and 13 patients had documented neurofibromatosis type 1 (NF-1).
Radiation Therapy
The method of CRT has been described previously.19 Three patients were treated with intensity-modulated radiation therapy. A dose of 54 Gy was prescribed to the planning target volume in 1.8-Gy fractions during a period of 6 weeks. The planning target volume represented a 0.3- to 0.5-cm geometric expansion of the clinical target volume, which was an anatomically constrained 10-mm expansion of the gross-residual tumor and/or tumor bed. Cumulative and differential dose-volume curves were calculated for total brain, supratentorial brain, infratentorial brain, left and right temporal lobes, hypothalamus, and left and right cochleae. The mean dose was calculated for the same volumes. (Appendix Table A1, online only).
Pre- and Post-Treatment Evaluations
The goal of this report was to describe cognitive function during the first 5 years after CRT and the incidence of hormone replacement therapy (HRT) and hearing loss during the first 10 years after CRT. Cognitive testing used an age-appropriate battery20–28a to assess global intellectual functioning, memory, social-emotional adjustment, academic skills, and adaptive functioning at baseline, at 6 months, and yearly through 5 years. Additional testing was mandated for the time periods of 7 to 8 years and 10 years after CRT. The incidence of HRT was determined from our database that records the initiation of HRT for each patient and accounts for those who were replacing hormones at the initiation of CRT. Our method of evaluation has been previously described.29 The first 50 patients were subjected to a battery of provocative tests before and after CRT. We restricted our reporting to the baseline assessment of growth hormone (GH) secretion of these patients. Standard audiology was performed at baseline and every 6 months after radiation therapy. The following frequencies were included: 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz. At year 5 of the study, when it was recognized that hearing loss was infrequent, the protocol was amended to reduce the frequency of evaluation to yearly. Patients were excluded from the assessment of hearing loss if they could not speak English. Data at specific frequencies were excluded from the analysis if there was a pre-existing hearing deficit. The definitions for hearing loss have been described.30 Clinical and imaging examinations were performed every 3 months for the first 2 years, every 6 months through 5 years, and then yearly through 10 years.
Statistical Analysis
The mixed model was used to estimate the longitudinal trends and changes of psychological and behavioral scores since CRT and to assess the effects of clinical and demographic variables on these trends and changes.31 The volumes of two radiation therapy (RT) dose intervals (0 to 30 Gy and 30 to 60 Gy) were calculated for each patient and were included in the mixed model as two covariate variables, by which the volume effects of low-dose and high-dose RT could be assessed simultaneously and more accurately. Although it was consistently shown that the volume effect of high-dose RT was greater than that of low-dose RT for any response variables, the ratio in effect of high and low doses could be substantially different for different response variables. The statistical model of cumulative incidence (CI) with competing risk also was used to estimate the needs of HRT and/or therapy for precocious puberty for patients after they had received CRT.32 In this model, the incidence time of a therapy was the first time that the therapy was given to the patient, and the competing risk for the incidence was any of local or distant failures, secondary malignancy, or death. All analyses in this study were carried out by using the SAS statistical package (SAS for Windows, version 9.1.3; SAS Institute, Cary, NC).
RESULTS
Cognitive Effects
Psychology scores were estimated by using linear mixed models through 5 years of follow-up. The parameter estimates for the battery of tests are listed in Table 1. Significant improvements were noted in the Child Behavior Checklist (CBCL) internalizing and behavioral problem scores and in the Woodcock Johnson—Revised visual auditory learning. Significant declines were noted for Wechsler Individual Achievement Test reading and spelling and for Vineland communication. Five years after CRT, only the decline in spelling scores was clinically significant. The average patient's score decreased from 98 to 90 points, but 90 still represented an average-range score (average score range, 85 to 115).
Table 1.
Models of Cognitive Effects After CRT for Pediatric Low-Grade Glioma
| Evaluation | No. of Patients Who Had at Least Two Measures | Score* |
|||
|---|---|---|---|---|---|
| Baseline | Change per Month | Month 60 | P† | ||
| IQ | 55 | 98.9642 | −0.0591 | 95.4182 | |
| Math | 55 | 96.9703 | −0.0435 | 94.3603 | |
| Reading | 56 | 98.9448 | −0.0989 | 93.0108 | .0039 |
| Spelling | 56 | 98.2341 | −0.1434 | 89.6301 | .0014 |
| Memory | 53 | 47.5523 | 0.0164 | 48.5363 | |
| Behavior problems‡ | 55 | 49.2340 | −0.0556 | 45.8980 | .0641 |
| Externalizing‡ | 58 | 43.9829 | −0.0099 | 43.3889 | |
| Internalizing‡ | 58 | 51.5753 | −0.0550 | 48.2753 | .0248 |
| Activities | 55 | 43.2365 | 0.0031 | 43.4225 | |
| School | 53 | 41.8430 | −0.0515 | 38.7530 | .0479 |
| Socialization | 56 | 44.5348 | −0.0084 | 44.0308 | |
| Communication | 57 | 94.6115 | −0.1308 | 86.7635 | .0041 |
| Composite | 57 | 94.4170 | −0.1026 | 88.2610 | .0433 |
| Daily living | 57 | 94.0500 | −0.0635 | 90.2400 | |
| Socialization | 57 | 98.7889 | −0.0559 | 95.4349 | |
| Visual auditory learning | 30 | 92.2834 | 0.1768 | 102.8914 | < .0001 |
NOTE. Instruments for each evaluation are as follows: IQ, Bayley second edition; Wechsler Preschool and Primary Scale of Intelligence revised; Wechsler Intelligence Test for Children third edition or Wechsler Adult Intelligence Scale revised, as appropriate for age; math, reading, and spelling: Wechsler Individual Achievement Test; memory: California Verbal Learning Test: Child Version; behavior problems, externalizing, internalizing, activities, school, socialization: Child Behavior Checklist; communication composite, daily living, socialization: Vineland Adaptive Behavior Scale; and visual auditory learning: Woodcock Johnson revised, visual auditory learning subtest.
Abbreviations: CRT, conformal radiation therapy; IQ, intelligence quotient.
Score = baseline + ([change per month] × time).
P value for change per month.
Increasing scores represent worsening performance.
NF-1
Thirteen of the 78 patients included in this research were diagnosed with NF-1. They had significantly lower baseline scores for intelligence quotient (IQ; −11.49 points; P = .0468), reading (−13.09 points; P = .0139), spelling (−12.33 points; P = .0485), and communication (−12.78 points; P = .0200). A significant decline was observed in the CBCL activities scores for patients with NF-1 (−0.1222 points/mo; P = .0484). After 5 years, the average score of a patient with NF-1 would decrease from 44.2139 to 36.8819, whereas the average score of a patient without NF-1 would be unchanged at 44.2806 (average score range, 40 to 60).
Age at CRT
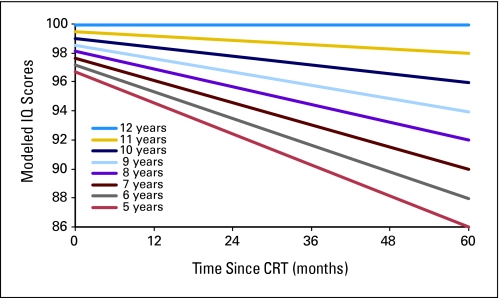
Age impacted baseline scores and change over time. Lower CBCL school scores were observed at baseline for younger patients (0.6616 points/year of age; P = .0400). Each year of increasing age reduced the decline in IQ by 0.0256 points per month (P = .0025; Fig 1). When other factors were excluded, a 10-point decline in IQ 5 years after RT would be expected for a child age 5 years at the time of irradiation. Each year of increasing age resulted in lower (ie, improved) CBCL externalizing (−0.0275 points/mo; P = .0039) and internalizing (−0.0181 points/mo; P = .0362) scores.
Fig 1.
Modeled intelligence quotient (IQ) scores after conformal radiation therapy (CRT) by age for pediatric low-grade glioma. Age is measured in years, and time is measured in months after the start of CRT.
Surgery
The impact of surgery on psychology scores was determined by comparing patients on the basis of extent of resection (ie, no biopsy, biopsy, or subtotal resection). Psychology scores before CRT were significantly better for patients who underwent biopsy only compared with subtotal resection: the affected measures included California Verbal Learning Test (CVLT) (P = .0212), CBCL activities (P = .0152), behavior problems (P = .0377), and externalizing scores (P = .0055). Extent of resection influenced change over time; the biopsy group had worse scores in visual auditory learning (−0.2392 points/mo; P = .0052) compared with the subtotal resection group.
Hydrocephalus, Shunt, Pre-CRT Chemotherapy, and the Planning Target Volume
Patients who did not have hydrocephalus had higher CVLT scores at baseline (+5.70 points; P = .0804). Those who did not have hydrocephalus had higher CBCL externalizing scores at baseline (+4.3698 points; P = .0411). Pre-CRT chemotherapy had no impact on baseline psychology scores, although those not treated with chemotherapy showed a trend toward higher scores on visual auditory learning (+10.71 points; P = .0983). The size of the planning target volume affected baseline values of reading (−0.0479 points/mL; P = .0320), communication (−0.0720 points/mL; P = .0016), and daily living scores (−0.0409 points/mL; P = .0628).
Radiation Dose and Volume
Models that correlated cognitive effect with radiation dose were generated by using dose-volume data from the supratentorial, infratentorial, and total brain volumes. Models could not be generated with temporal lobe data. The relative volumes that received doses between 0 to 30 Gy and 30 to 60 Gy were the covariates. In most models, the parameter estimate for the percent volume that received a dose (ie, dose-volume interval) of 30 to 60 Gy (V30-60Gy) was statistically significant; whereas the significance of the dose-volume interval of 0 to 30 Gy (V0-30Gy) was inconsistent. Because of its importance and ease of use, age was included in the models. Age had the greatest impact on baseline values and increased the statistical significance of the longitudinal estimates. Psychology measures and the P values of significant dose-volume parameters are listed in Table 2 to demonstrate the impact of high- and low-dose volumes across the range of measures.
Table 2.
Significant Parameter Estimates to Model Decline in Psychology Test Scores With Radiation Dose, Age, and Time After Conformal Radiation Therapy for Pediatric Low-Grade Glioma
| Dose-Volume Interval by Psychology Test |
P by Volume of Brain |
||
|---|---|---|---|
| Total Brain | Supratentorial | Infratentorial | |
| IQ | |||
| V0-30Gy | .0193 | .0091 | .0032 |
| V30-60Gy | .0106 | .0105 | .0089 |
| Math | |||
| V0-30Gy | |||
| V30-60Gy | .0705 | .0165 | |
| Reading | |||
| V0-30Gy | |||
| V30-60Gy | .0013 | .0025 | .0106 |
| Spelling | |||
| V0-30Gy | |||
| V30-60Gy | .0389 | .0350 | |
| CBCL externalizing | |||
| V0-30Gy | .0043 | .0053 | .0061 |
| V30-60Gy | .0386 | ||
| Vineland communication | |||
| V0-30Gy | |||
| V30-60Gy | .0481* | ||
| Visual auditory learning† | |||
| V0-30Gy | .0001 | .0001 | .0009 |
| V30-60Gy | |||
NOTE. Instruments for each evaluation are as follows: IQ, Bayley second edition; Wechsler Preschool and Primary Scale of Intelligence revised; Wechsler Intelligence Test for Children third edition or Wechsler Adult Intelligence Scale revised, as appropriate for age; math, reading, and spelling: Wechsler Individual Achievement Test; externalizing behavior: Child Behavior Checklist; communication: Vineland Adaptive Behavior scale; and visual auditory learning: Woodcock Johnson—Revised Visual Auditory Learning subtest.
Abbreviations: V0-30Gy, percent volume between 0 to 30 Gy; V30-60Gy, percent volume between 30 to 60 Gy; IQ, intelligence quotient; CBCL, Child Behavior Checklist.
Model did not include age.
Increasing V0-30Gy resulted in improved scores.
Models of IQ and CBCL Externalizing Scores on the Basis of Radiation Dosimetry
Models that included IQ and the CBCL externalizing score had statistically significant parameter estimates for both of the dose-volume intervals, V0-30Gy and V30-60Gy.
The equation for IQ is represented by the following expression:
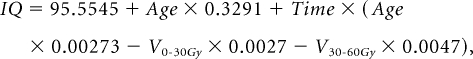 |
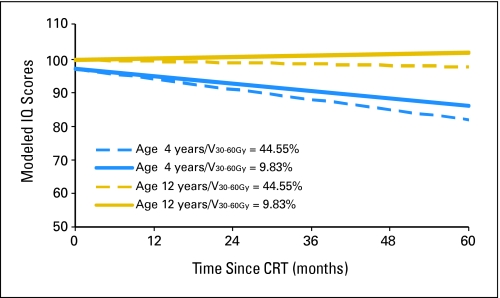
in which age at CRT was measured in years and time was measured in months after the start of CRT. V0-30Gy and V30-60Gy were, respectively, the percent volume of the supratentorial brain that received a dose of 0 to 30 Gy and of 30 to 60 Gy. Figure 2 shows modeled differences in IQ during the first 5 years after CRT on the basis of age and radiation dosimetry. The ages of 4 and 12 years were chosen along with V0-30Gy = 55.45% and V30-60Gy = 44.55% and V0-30Gy = 90.17% and V30-60Gy = 9.83%. These values were chosen because they represented V30-60Gy values of one standard deviation (17.36%) above and below the mean of 27.19%, respectively.
Fig 2.
Modeled intelligence quotient (IQ) scores after conformal radiation therapy (CRT) by age and supratentorial brain dose-volume intervals for pediatric low-grade glioma. Age is measured in years, and time is measured in months after CRT. The dose-volume intervals V0-30Gy and V30-60Gy represent the percent volume of the supratentorial brain that received dose within the specified interval.
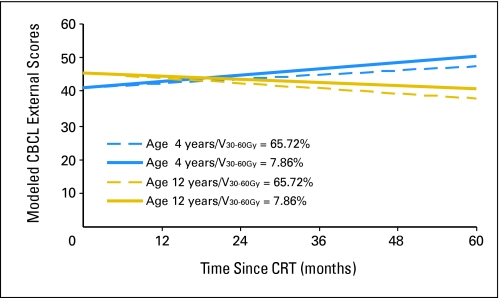
The equation for CBCL externalizing score is represented by the following expression:
in which age at CRT was measured in years and time was measured in months after the start of CRT. V0-30Gy and V30-60Gy were, respectively, the percent volume of the infratentorial brain that received a dose between 0 to 30 Gy and 30 to 60 Gy. Appendix Figure A1 (online only) shows modeled differences in IQ during the first 5 years after CRT on the basis of age and radiation dosimetry. The ages of 4 and 12 years were chosen along with V0-30Gy = 34.28% and V30-60Gy = 65.72% and V0-30Gy = 92.14% and V30-60Gy = 7.86%. These values were chosen because they represented V30-60Gy values of one standard deviation (28.93%) above and below the mean of 36.79%, respectively.
Simplified models of dose and effect were developed by using mean dose. The mean dose to specific brain volumes was found to impact baseline and longitudinal reading and math scores. Age was included in these models. Reading and math scores were estimated by equations listed in Table 3.
Table 3.
Models of Academic Achievement at the Time of Conformal Radiation Therapy to Specific Volumes of the Brain
| Test and Volume | Score |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α0 | α1 | α1P | α2 | α2P | α3 | α3P | β0 | β0P | β1 | β1P | |
| Reading* | |||||||||||
| Total brain | 124.215 | −.7334 | .1293 | −.0086 | .0088 | .1862 | .1275 | −.0001 | .0161 | ||
| Supratentorial brain | 115.522 | −.5972 | .2284 | −.0053 | .0740 | .1271 | .22895 | −.0001 | .0252 | ||
| Math* | |||||||||||
| Infratentorial brain | 93.057 | 1.5866 | .0778 | .0024 | .5233 | −.0008 | .0241 | .1072 | .1805 | −.0001 | .0442 |
NOTE. Score = α0 + age × α1 + dose × α2 + dose × age × α3 + time × (β0 + dose × β1).
Wechsler Individual Achievement Test.
Endocrinopathy
Pre-CRT HRT was noted and included thyroid hormone (n = 6), glucocorticoid (n = 3), desmopressin acetate (DDAVP) (n = 2), and gonadotropin-releasing hormone (GnRH) analog (n = 6). Provocative testing performed before CRT in 42 patients revealed that 43% had peak GH values less than 10 ng/mL and that 24% had peak GH values less than 7 ng/mL. The number of patients (of 50 patients total) on HRT at baseline, at 12 months, and at 24 months, respectively, were 14, 21, and 28 for thyroid hormone replacement; seven, nine, and 11 for glucocorticoid replacement; and six, eight, and 11 for GnRH analog. One patient required sex hormone replacement within 24 months of irradiation.
The CI (reported as mean ± SE) of HRT and treatment of precocious puberty was determined for the first 50 consecutive patients in the series. Treatment failure and HRT before CRT were considered competing risks. The 5- and 10-year CIs, respectively, (and the 10-year CI for 43 patients who had a mean hypothalamus dose ≥ 40 Gy) of growth hormone replacement were 46.0% ± 7.2% and 48.9% ± 7.4% (54.7%); of thyroid hormone replacement, 61.4% ± 7.5% and 64.0% ± 7.5% (69.1%); of glucocorticoid replacement, 19.2% ± 5.8% and 19.2% ± 5.8% (20.0%); of DDAVP replacement, 2.1% ± 2.1% and 5.2% ± 3.8% (6.2%); of sex hormone replacement, 8.0% ± 3.9% and 14.1% ± 5.0% (16.4%); and of GnRH analog therapy, 31.8% ± 7.1% and 34.2% ± 7.3% (35.3%). Two of the patients treated with GnRH analog therapy did not have documented precocious puberty but were treated at 38.5 and 49.5 months after CRT to increase time for growth promotion. (Fig 3).
Fig 3.
CI of hormone replacement therapy and therapy for precocious puberty after conformal radiation therapy (CRT) for pediatric low-grade glioma. CI, cumulative incidence; GnRH, gonadotropin-releasing hormone; DDAVP, desmopressin acetate.
Provocative testing performed before CRT in 42 corticosteroid-naïve patients revealed that 43% had peak GH values less than 10 ng/mL and that 24% had peak GH values less than 7 ng/mL. When the first 50 patients were considered, the number of patients on HRT at baseline, at 12 months, and at 24 months were 14, 21, and 28 for thyroid hormone replacement; seven, nine, and 11 for glucocorticoid replacement; and six, eight, and 11 for GnRH analog. One patient required GnRH replacement within 24 months of irradiation.
Hearing Loss
Hearing loss was estimated for 74 of 78 patients; four were excluded because of early treatment failure. The median (±SD) hearing follow-up was 61.4 ± 27.9 months (range, 5.6 to 124.5 months). Hearing loss at any frequency occurred in nine patients, transient values greater than 25 dB were noted in 17 patients, and 48 patients never exceeded the threshold value of 25 dB. The CI (reported as mean ± SE) of hearing loss differed by ear and did not exceed 5.7% ± 3.3% (ie, 2,000 Hz) in the right ear or 4.0% ± 2.8% (ie, 1000 Hz) in the left ear when estimated at 10 years (Appendix Table A2, online only). An analysis to determine the effect of cochlear dose on hearing loss showed that irradiation to dose levels that exceeded 45 Gy in 16 patients resulted in statistically significantly higher rates of increased hearing threshold in the right ear at the following frequencies (10-year CI < 45Gy v 10-year CI > 45 Gy; reported as mean ± SE): 1 kHz (0 v 15.6% ± 10.7%; P = .0156), 4 kHz (0 v 15.6% ± 10.7%; P = .0156), 6 kHz (0 v 19.2% ± 12.9%; P = .0192), and 8 kHz (0 v 19.2% ± 12.9%; P = .0226). There was not a similar effect on the left ear, which had a similar number of cochleae that received greater than 45 Gy. Among the 23 patients treated with chemotherapy, increased hearing thresholds were estimated at 10 years (no chemotherapy v chemotherapy; reported as mean ± SD) for 1 kHz (0 v 14.1% ± 10.1%; P = .0262) and 4kHz (0 v 14.1% ± 10.0%; P = .062). This effect was limited to the right ear.
DISCUSSION
LGG is the most commonly diagnosed brain tumor in children. RT will ultimately be required for most patients, especially those with centrally located tumors that involve the diencephalon and optic pathways. Fear of adverse effects has moved caregivers and parents to consider alternatives to RT, especially for young patients or those likely to develop endocrine or cognitive deficits after RT. Surgery is clearly preferred when there is an opportunity for gross total resection with limited morbidity. Chemotherapy may be the preferred nonsurgical therapy for patients vulnerable to the effects of radiation.14–18 The indications include progressive tumor or residual disease after surgery. Chemotherapy is considered optimal when the adverse effects are minimal and a meaningful delay to irradiation can be achieved without loss of function. Recent prospective trial results show that 35% to 48% of treated patients will achieve 5 years of progression-free survival on the basis of the chosen treatment regimen.14 Choosing chemotherapy instead of RT as the primary therapy risks neurologic deterioration in patients who are difficult to monitor in the setting of stable imaging and may delay the treatment of pre-existing GH deficiency in the prepubertal patient.
This research demonstrates the importance of age when cognitive effects are estimated, and it adds to the cognitive profile of specific subsets of patients, including those with NF-1.33,34 The effect of age exceeds the effect of radiation dose. This provides guidance regarding selection of patients for treatment, and it additionally demonstrates the overarching influence of age and increasing deficits with time in younger patients.35 Extent of surgery and NF-1 status most commonly determine the pre-irradiation baseline and less often change over time. The late effects of RT may be exaggerated when the baseline function of the patient is not assessed. These results show the sensitivity of the domains of visual auditory learning, adaptive behavior, problem behavior, and academic achievement to provide guidance for the design of future studies.
With the advent of three-dimensional RT treatment planning and delivery (which includes methods of irradiation such as three-dimensional CRT, intensity-modulated RT, fractionated stereotactic RT, and other techniques used to conform the prescription dose), there is an opportunity to reassess the role of RT and to address the controversy surrounding the age at which RT might be considered safe, or the age at which the benefits of treatment outweigh the potential risks. A number of institutional studies of focal irradiation for pediatric patients with LGG have been reported.1–4 Although these studies have demonstrated excellent disease control with a reasonable number of patients, functional outcomes are lacking.
Our long-term objective is to correlate radiation dosimetry with functional outcomes to provide constraints for optimization of photons when using intensity-modulated RT and to provide criteria for the selection of patients for newer treatment modalities. This research includes a decade of experience of CRT use, a standardized approach to targeting, serial evaluation with a battery of standardized psychological tests, provocative endocrine testing, and the evaluation of hearing. We designed this trial to test the hypothesis that irradiation with a 10-mm clinical target volume margin would reduce the adverse effects of RT without affecting the rate of failure in patients with pediatric LGG. Indeed, the 5- and 10-year estimates of event-free survival for these patients are 87% and 74%, respectively, and the 10-year overall survival estimate is 96% (Merchant TE et al, submitted for publication). Our results show that cognitive function is largely preserved. When deficits are identified, factors other than RT are contributory. Hormone deficits are predictable for patients with centrally located tumors. Vigilance is required because of the high rate of pre-CRT endocrinopathy and because of the presence of evolving deficits during and soon after treatment. Hearing loss was uncommon on the basis of the deployed target volume margins and common central tumor location. Hearing loss will likely decrease in the future as target volume margins are reduced.
Appendix
Fig A1.
Modeled intelligence quotient (IQ) scores after conformal radiation therapy (CRT) by age and infratentorial brain dose-volume intervals for pediatric low-grade glioma. Age is measured in years, and time is measured in months after CRT. The dose-volume intervals V0-30Gy and V30-60Gy represent the percent volume of the infratentorial brain that received a dose within the specified interval. CBCL, Child Behavior Checklist.
Table A1.
Dose-Volume Data for the Total Brain and for the Supratentorial and Infratentorial Brain
| Radiation Volume by Section of Brain | Radiation Dose Data |
||||||
|---|---|---|---|---|---|---|---|
| Total No. of Patients | Mean | SD | SE | Median | Min | Max | |
| Total | |||||||
| V0-30Gy | 62 | 71.56 | 15.89 | 2.02 | 75.24 | 0 | 95.36 |
| V30-60Gy | 62 | 28.44 | 15.89 | 2.02 | 24.75 | 4.66 | 99.99 |
| Mean dose, Gy | 63 | 21.8 | 6.15 | 0.77 | 20.37 | 10.92 | 44.00 |
| Supratentorial | |||||||
| V0-30Gy | 62 | 72.8 | 17.36 | 2.2 | 75.41 | 0 | 96.38 |
| V30-60Gy | 62 | 27.19 | 17.36 | 2.2 | 24.62 | 3.6 | 100 |
| Mean dose, Gy | 63 | 20.99 | 6.877 | 0.86 | 20.74 | 0.85 | 44.01 |
| Infratentorial | |||||||
| V0-30Gy | 62 | 63.2 | 28.92 | 3.67 | 70.06 | 0 | 99.99 |
| V30-60Gy | 62 | 36.79 | 28.93 | 3.67 | 29.95 | 0 | 100 |
| Mean dose, Gy | 63 | 25.47 | 12.66 | 1.59 | 20.91 | 1.12 | 51.76 |
Abbreviations: SD, standard deviation; Min, minimum; Max, maximum; V0-30Gy, dose-volume interval of 0 to 30 Gy; V30-60Gy, dose-volume interval of 30 to 60 Gy.
Table A2.
Cumulative Incidence of Hearing Loss by Frequency and Ear
| Frequency, Hz | Cumulative Incidence of Hearing Loss |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total No. of Patients | Right Ear |
Left Ear |
|||||||
| Year 5 |
Year 10 |
Year 5 |
Year 10 |
||||||
| Estimate (%) | SE | Estimate (%) | SE | Estimate (%) | SE | Estimate (%) | SE | ||
| 250 | 72 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 500 | 73 | 0 | 0 | 0 | 0 | 4.0 | 2.8 | 4.0 | 2.8 |
| 1,000 | 73 | 4.1 | 2.9 | 4.1 | 2.9 | 4.0 | 2.8 | 4.0 | 2.8 |
| 2,000 | 72 | 5.7 | 3.3 | 5.7 | 3.3 | 1.6 | 1.6 | 1.6 | 1.6 |
| 3,000 | 71 | 2.4 | 2.4 | 2.4 | 2.4 | 0 | 0 | 0 | 0 |
| 4,000 | 73 | 4.1 | 2.9 | 4.1 | 2.9 | 1.6 | 1.6 | 1.6 | 1.6 |
| 6,000 | 73 | 2.4 | 2.4 | 5.3 | 3.7 | 1.6 | 1.6 | 1.6 | 1.6 |
| 8,000 | 73 | 2.5 | 2.5 | 5.5 | 3.8 | 0 | 0 | 0 | 0 |
Footnotes
Supported in part by the National Cancer Institute, Cancer Center Support Grant No. 5 P30 CA21765-28, by the American Cancer Society, and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas E. Merchant, Robert H. Lustig, Xiaoping Xiong
Provision of study materials or patients: Thomas E. Merchant
Collection and assembly of data: Thomas E. Merchant, Heather M. Conklin
Data analysis and interpretation: Thomas E. Merchant, Heather M. Conklin, Shengjie Wu, Robert H. Lustig, Xiaoping Xiong
Manuscript writing: Thomas E. Merchant, Heather M. Conklin, Shengjie Wu, Robert H. Lustig, Xiaoping Xiong
Final approval of manuscript: Thomas E. Merchant, Heather M. Conklin, Shengjie Wu, Robert H. Lustig, Xiaoping Xiong
REFERENCES
- 1a.Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–3604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Marcus KJ, Goumnerova L, Billett AL, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: Final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61:374–379. doi: 10.1016/j.ijrobp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Saran FH, Baumert BG, Khoo VS, et al. Stereotactically guided conformal radiotherapy for progressive low-grade gliomas of childhood. Int J Radiat Oncol Biol Phys. 2002;53:43–51. doi: 10.1016/s0360-3016(02)02734-7. [DOI] [PubMed] [Google Scholar]
- 3.Grabenbauer GG, Schuchardt U, Buchfelder M, et al. Radiation therapy of optico-hypothalamic gliomas (OHG)-radiographic response, vision and late toxicity. Radiother Oncol. 2000;54:239–245. doi: 10.1016/s0167-8140(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 4.Erkal HS, Serin M, Cakmak A. Management of optic pathway and chiasmatic-hypothalamic gliomas in children with radiation therapy. Radiother Oncol. 1997;45:11–15. doi: 10.1016/s0167-8140(97)00102-3. [DOI] [PubMed] [Google Scholar]
- 5.Chadderton RD, West CG, Schuller S, et al. Radiotherapy in the treatment of low-grade astrocytomas: II—The physical and cognitive sequelae. Childs Nerv Syst. 1995;11:443–448. doi: 10.1007/BF00334961. [DOI] [PubMed] [Google Scholar]
- 6.Brauner R, Malandry F, Rappaport R, et al. Growth and endocrine disorders in optic glioma. Eur J Pediatr. 1990;149:825–828. doi: 10.1007/BF02072067. [DOI] [PubMed] [Google Scholar]
- 7.Lustig RH, Post SR, Srivannaboon K, et al. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88:611–616. doi: 10.1210/jc.2002-021180. [DOI] [PubMed] [Google Scholar]
- 8.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45:393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Epstein MA, Packer RJ, Rorke LB, et al. Vascular malformation with radiation vasculopathy after treatment of chiasmatic/hypothalamic glioma. Cancer. 1992;70:887–893. doi: 10.1002/1097-0142(19920815)70:4<887::aid-cncr2820700427>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Ferroir JP, Marro B, Belkacemi Y, et al. Cerebral infarction related to intracranial radiation arteritis twenty-four years after encephalic radiation therapy [in French] Rev Neurol. 2007;163:96–98. doi: 10.1016/s0035-3787(07)90361-6. [DOI] [PubMed] [Google Scholar]
- 11.Parsa CF, Givrad S. Juvenile pilocytic astrocytomas do not undergo spontaneous malignant transformation: Grounds for designation as hamartomas. Br J Ophthalmol. 2008;92:40–46. doi: 10.1136/bjo.2007.125567. [DOI] [PubMed] [Google Scholar]
- 12.Mittelbronn M, Schittenhelm J, Lemke D, et al. Low grade ganglioglioma rapidly progressing to a WHO grade IV tumor showing malignant transformation in both astroglial and neuronal cell components. Neuropathology. 2007;27:463–467. doi: 10.1111/j.1440-1789.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 13.Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 14.Ater J, Holmes E, Zhou T, et al. Results of COG protocol A9952: A randomized phase 3 study of two chemotherapy regimens for incompletely resected low-grade glioma in young children. Neuro Oncol. 2008;10:451. abstr LGG18. [Google Scholar]
- 15.Gnekow AK, Kortmann RD, Pietsch T, et al. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy: Report from the multicenter treatment study for children and adolescents with a low-grade glioma, HIT-LGG 1996, of the Society of Pediatric Oncology and Hematology (GPOH) Klin Padiatr. 2004;216:331–342. doi: 10.1055/s-2004-832355. [DOI] [PubMed] [Google Scholar]
- 16.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 17.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 18.Prados MD, Edwards MS, Rabbitt J, et al. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 19.Merchant TE, Zhu Y, Thompson SJ, et al. Preliminary results from a phase II trial of conformal radiation therapy for pediatric patients with localised low-grade astrocytoma and ependymoma. Int J Radiat Oncol Biol Phys. 2002;52:325–332. doi: 10.1016/s0360-3016(01)01807-7. [DOI] [PubMed] [Google Scholar]
- 20.The Psychological Corporation. The Bayley Scales of Infant Development. ed 2. New York, NY: Harcourt, Brace, Jovanovich; 1993. [Google Scholar]
- 21.The Psychological Corporation. New York, NY: Harcourt, Brace, Jovanovich; 1989. The Wechsler Preschool and Primary Scales of Intelligence: Revised. [Google Scholar]
- 22.The Psychological Corporation. The Wechsler Intelligence Test for Children. ed 3. New York, NY: Harcourt, Brace, Jovanovich; 1992. [Google Scholar]
- 23.The Psychological Corporation. New York, NY: Harcourt, Brace, Jovanovich; 1989. The Wechsler Adult Intelligence Scale: Revised. [Google Scholar]
- 24.Delis DC, Kramer JH, Kaplan E, et al. New York, NY: Harcourt, Brace, Jovanovich; 1994. California Verbal Learning Test: Children's Version. [Google Scholar]
- 25.Delis DC, Kramer JH, Kaplan E, et al. New York, NY: Harcourt, Brace, Jovanovich; 1987. California Verbal Learning Test. [Google Scholar]
- 26.The Psychological Corporation. New York, NY: Harcourt, Brace, Jovanovich; 1992. The Wechsler Individual Achievement Test. [Google Scholar]
- 27.Sparrow SS, Balla DA, Cicchetti DV. Circle Pines, MN: American Guidance Services; 1984. Vineland Adaptive Behavior Scales. [Google Scholar]
- 28.Woodcock RW, Johnson MB. New York, NY: Riverside Publishing; 1989. Woodcock-Johnson Tests of Cognitive Ability: Revised. [Google Scholar]
- 28a.Achenbach TM. Burlington, VT: Department of Psychology; 1991. Integrative Guide to the 1991 CBCL/4-18, YSSR, and TRF Profiles. [Google Scholar]
- 29.Merchant TE, Williams T, Smith JM, et al. Preirradiation endocrinopathies in pediatric brain tumor patients determined by dynamic tests of endocrine function. Int J Radiat Oncol Biol Phys. 2002;54:45–50. doi: 10.1016/s0360-3016(02)02888-2. [DOI] [PubMed] [Google Scholar]
- 30.Hua C, Bass JK, Khan R, et al. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int J Radiat Oncol Biol Phys. 2008;72:892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 31.Little RC, Milliken GA, Stroup WS, et al. Cary, NC: SAS Institute; 1996. SAS System for Mixed Models. [Google Scholar]
- 32.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1140–1154. [Google Scholar]
- 33.North KN, Riccardi V, Samango-Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis: I—Consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121–1127. doi: 10.1212/wnl.48.4.1121. [DOI] [PubMed] [Google Scholar]
- 34.Levine TM, Materek A, Abel J, et al. Cognitive profile of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:8–20. doi: 10.1016/j.spen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Taylor HG, Alden J. Age-related differences in outcomes following childhood brain insults: An introduction and overview. J Int Neuropsychol Soc. 1997;3:555–567. [PubMed] [Google Scholar]