Abstract
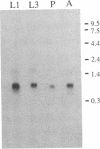
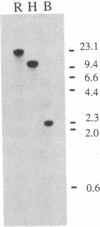
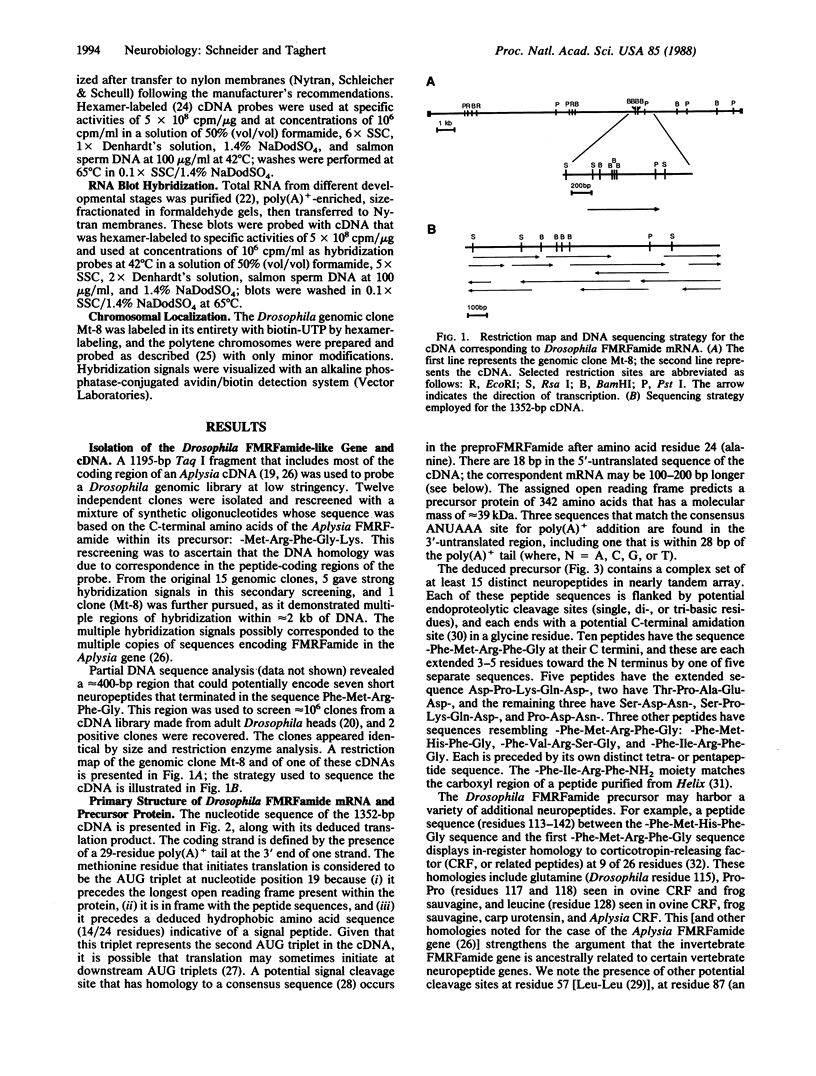
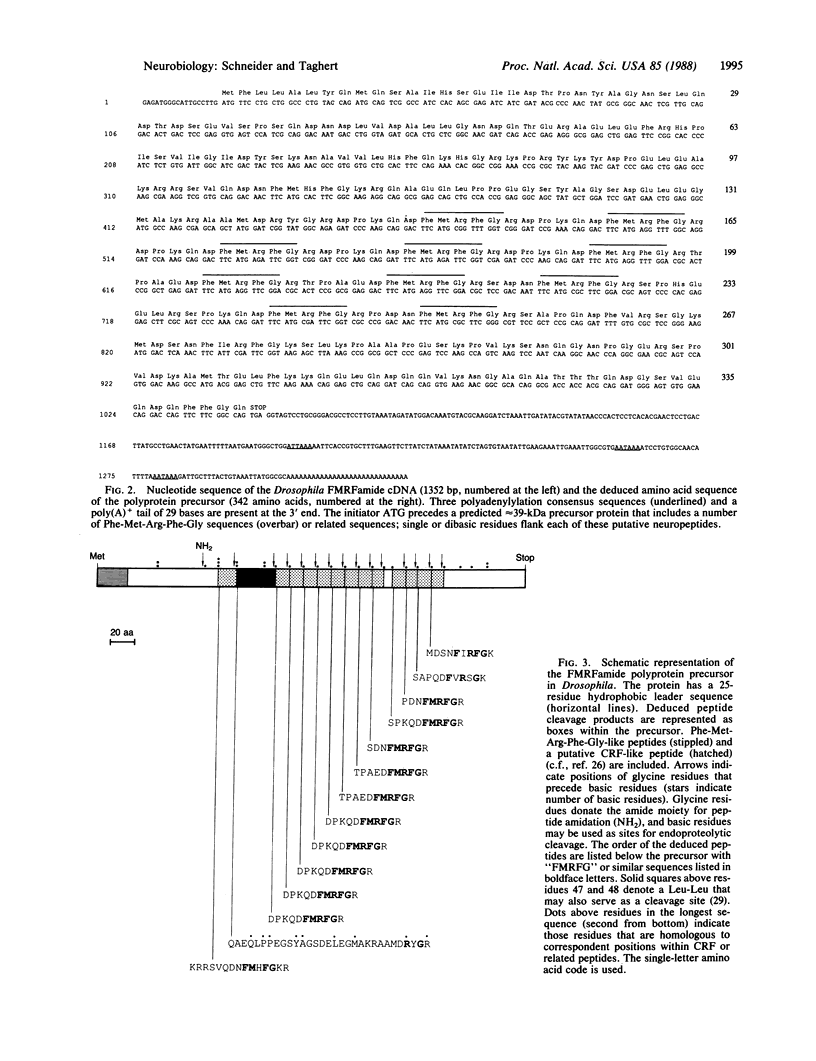
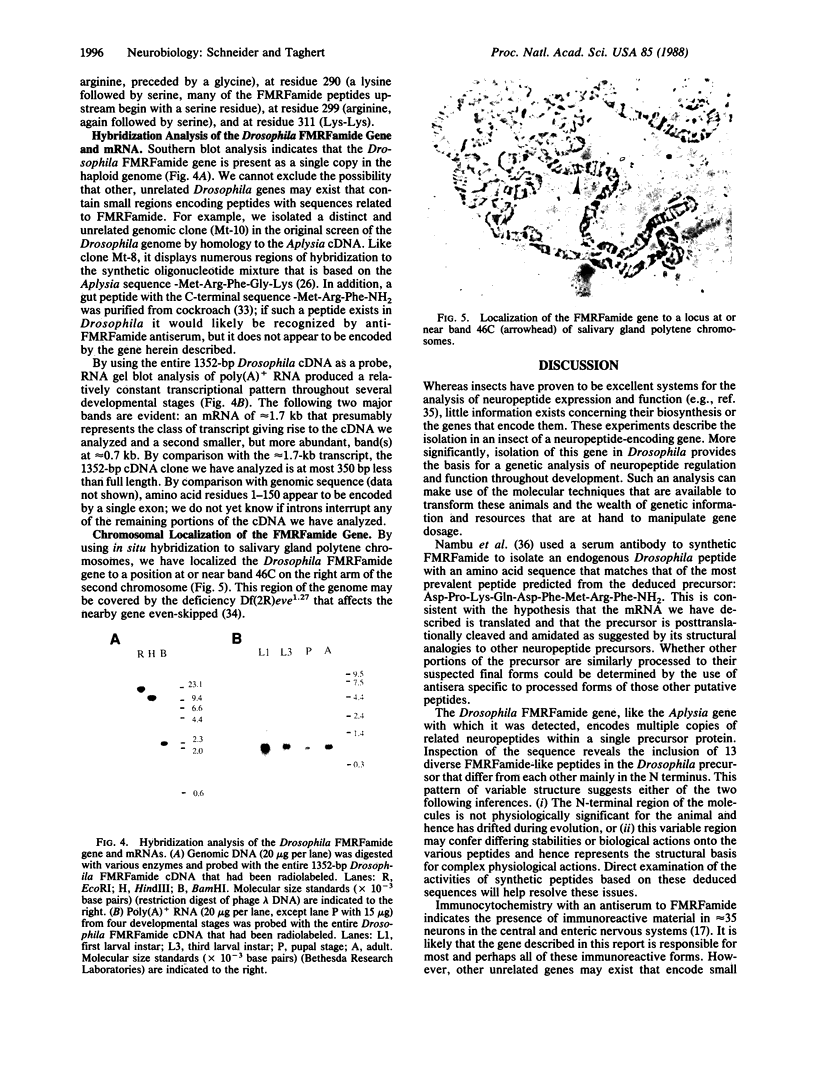
A Drosophila gene that encodes neuropeptides related to molluscan Phe-Met-Arg-Phe-NH2 (FMRFamide) was isolated by screening a genomic library with a fragment of an Aplysia Phe-Met-Arg-Phe-NH2 cDNA and with synthetic oligonucleotides. This gene was used to isolate a cDNA from a Drosophila adult head cDNA library. The cDNA was defined by sequence analysis to encode 13 peptides that have Phe-Met-Arg-Phe-NH2 or related sequences at their carboxyl termini. Other putative neuropeptides, including one that has homology to mammalian corticotropin-releasing factor, are present in the deduced approximately equal to 39-kDa precursor. Southern blot analysis suggested the presence of a single Phe-Met-Arg-Phe-NH2-like gene within the haploid genome. RNA blot analysis indicated the expression of at least two transcripts of approximately equal to 1.7 and approximately equal to 0.7 kilobases. Both transcripts are evident throughout larval, pupal, and adult developmental stages. In situ hybridization was used to localize this neuropeptide gene to band 46C on the right arm of the 2nd chromosome. These data provide the basis for utilizing the advanced genetics and molecular techniques of Drosophila to address complex aspects of neuropeptide expression and function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Carroll L. S., Carrow G. M., Calabrese R. L. Localization and release of FMRFamide-like immunoreactivity in the cerebral neuroendocrine system of Manduca sexta. J Exp Biol. 1986 Nov;126:1–14. doi: 10.1242/jeb.126.1.1. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A. FMRFamide neuropeptides simultaneously increase and decrease K+ current in an identified neurone. Nature. 1982 Mar 4;296(5852):87–89. doi: 10.1038/296087a0. [DOI] [PubMed] [Google Scholar]
- Crenshaw E. B., 3rd, Russo A. F., Swanson L. W., Rosenfeld M. G. Neuron-specific alternative RNA processing in transgenic mice expressing a metallothionein-calcitonin fusion gene. Cell. 1987 May 8;49(3):389–398. doi: 10.1016/0092-8674(87)90291-1. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Reeve J. R., Jr, Shively J., Gayton R. J., Barnard C. S. A novel active pentapeptide from chicken brain identified by antibodies to FMRFamide. Nature. 1983 Sep 22;305(5932):328–330. doi: 10.1038/305328a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gayton R. J. Mammalian neuronal actions of FMRFamide and the structurally related opioid Met-enkephalin-Arg6-Phe7. Nature. 1982 Jul 15;298(5871):275–276. doi: 10.1038/298275a0. [DOI] [PubMed] [Google Scholar]
- Itoh N., Slemmon J. R., Hawke D. H., Williamson R., Morita E., Itakura K., Roberts E., Shively J. E., Crawford G. D., Salvaterra P. M. Cloning of Drosophila choline acetyltransferase cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4081–4085. doi: 10.1073/pnas.83.11.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakidani H., Furutani Y., Takahashi H., Noda M., Morimoto Y., Hirose T., Asai M., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature. 1982 Jul 15;298(5871):245–249. doi: 10.1038/298245a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Nachman R. J., Holman G. M., Haddon W. F., Ling N. Leucosulfakinin, a sulfated insect neuropeptide with homology to gastrin and cholecystokinin. Science. 1986 Oct 3;234(4772):71–73. doi: 10.1126/science.3749893. [DOI] [PubMed] [Google Scholar]
- Nelson C., Crenshaw E. B., 3rd, Franco R., Lira S. A., Albert V. R., Evans R. M., Rosenfeld M. G. Discrete cis-active genomic sequences dictate the pituitary cell type-specific expression of rat prolactin and growth hormone genes. Nature. 1986 Aug 7;322(6079):557–562. doi: 10.1038/322557a0. [DOI] [PubMed] [Google Scholar]
- Price D. A., Greenberg M. J. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977 Aug 12;197(4304):670–671. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Picciotto M. R., Kreiner T., Kaldany R. R., Taussig R., Scheller R. H. Aplysia neurons express a gene encoding multiple FMRFamide neuropeptides. Cell. 1985 Jun;41(2):457–467. doi: 10.1016/s0092-8674(85)80019-2. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Jackson J. F., McAllister L. B., Rothman B. S., Mayeri E., Axel R. A single gene encodes multiple neuropeptides mediating a stereotyped behavior. Cell. 1983 Jan;32(1):7–22. doi: 10.1016/0092-8674(83)90492-0. [DOI] [PubMed] [Google Scholar]
- Scholnick S. B., Bray S. J., Morgan B. A., McCormick C. A., Hirsh J. CNS and hypoderm regulatory elements of the Drosophila melanogaster dopa decarboxylase gene. Science. 1986 Nov 21;234(4779):998–1002. doi: 10.1126/science.3095924. [DOI] [PubMed] [Google Scholar]
- Shyamala M., Fisher J. M., Scheller R. H. A neuropeptide precursor expressed in Aplysia neuron L5. DNA. 1986 Jun;5(3):203–208. doi: 10.1089/dna.1986.5.203. [DOI] [PubMed] [Google Scholar]
- Smyth D. G., Massey D. E. A new glycopeptide in pig, ox and sheep pituitary. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1006–1010. doi: 10.1016/s0006-291x(79)80007-8. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Taussig R., Scheller R. H. The Aplysia FMRFamide gene encodes sequences related to mammalian brain peptides. DNA. 1986 Dec;5(6):453–461. doi: 10.1089/dna.1.1986.5.453. [DOI] [PubMed] [Google Scholar]
- Walther C., Schiebe M. FMRF-NH2-like factor from neurohaemal organ modulates neuromuscular transmission in the locust. Neurosci Lett. 1987 Jun 15;77(2):209–214. doi: 10.1016/0304-3940(87)90588-x. [DOI] [PubMed] [Google Scholar]
- White K., Hurteau T., Punsal P. Neuropeptide-FMRFamide-like immunoreactivity in Drosophila: development and distribution. J Comp Neurol. 1986 May 22;247(4):430–438. doi: 10.1002/cne.902470403. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]