Abstract
We examined the role of WNT signaling in pituitary development by characterizing the pituitary phenotype of three WNT knockout mice and assessing the expression of WNT pathway components. Wnt5a mutants have expanded domains of Fgf10 and BMP expression in the ventral diencephalon and a reduced domain of LHX3 expression in Rathke's pouch. Wnt4 mutants have mildly reduced cell differentiation, reduced POU1F1 expression, and mild anterior lobe hypoplasia. Wnt4, Wnt5a double mutants exhibit an additive pituitary phenotype of dysmorphology and mild hypoplasia. Wnt6 mutants have no obvious pituitary phenotype. We surveyed WNT expression and identified transcripts for numerous Wnts, Frizzleds and downstream pathway members in the pituitary and ventral diencephalon. These findings support the emerging model that WNT signaling affects the pituitary gland via effects on ventral diencephalon signaling, and suggest additional Wnt genes that are worthy of functional studies.
Keywords: Wnt5a, Wnt4, Wnt6, Wnt11, Wnt16, mouse knockout, development
Introduction
The pituitary gland is a central organ in the endocrine system of all vertebrates and is responsible for the production and regulation of peptide hormones necessary for growth and development, regulation of thyroid function, lactation, sexual maturation and fertility, and the ability to respond to physiological stresses (Cushman and Camper, 2001). The mature gland is composed of three lobes in rodents, the posterior lobe, the intermediate lobe, and the anterior lobe which is comprised of five major pituitary hormone-producing cell types (Japon et al., 1994).
The posterior lobe is derived from neural ectoderm, whereas the anterior and intermediate lobes of the pituitary gland arise from Rathke's pouch, a primitive structure resulting from an invagination of the oral ectoderm beginning at embryonic day 9.0 (e9.0) in the mouse. The pouch pinches off from the remaining oral ectoderm at approximately e11.5 and is characterized by a domain of apoptosis at the separation point (Charles et al., 2005). In early pituitary development, the dorsal aspect of the pouch undergoes extensive cell proliferation, and cells migrate out ventrally and rostrally from Rathke's pouch and form the anterior lobe (Ikeda and Yoshimoto, 1991; Ward et al., 2005).
Pituitary development is mediated by the temporal and spatial expression of transcription factors in the pouch in response to BMP and FGF signaling in the surrounding tissues of the developing gland (Ericson et al., 1998; Dasen et al., 2001; Davis and Camper, 2007). These factors include the POU-domain transcription factor Pit1, recently renamed Pou1f1, and its predecessor, Prophet of Pit1 (Prop1), LIM homeodomain factors Islet1 (Isl1), Lhx3 and Lhx4, the pituitary homeobox genes Pitx1 and Pitx2, and the Rathke's pouch homeobox gene Rpx (Hesx1) (Watkins-Chow and Camper, 1998; Cushman and Camper, 2001). Additionally, members of the SOX family of transcription factors have also been implicated in pituitary gland size and shape formation (Camper, 2004; Rizzoti and Lovell-Badge, 2005).
In addition to the various transcription factors involved in pituitary development, WNT signaling is emerging as an important contributor. Both the canonical and non-canonical WNT pathways are highly conserved throughout evolution and are essential for proper growth, development, and organogenesis in both vertebrate and invertebrate organisms (Rijsewijk et al., 1987; Cadigan and Nusse, 1997). In the canonical pathway, a core set of proteins respond to WNT and prevent CTNNB1 (β-catenin) from being proteolyzed, thus, allowing β-catenin to activate target genes that modulate cell fate, proliferation and apoptosis. In the non-canonical pathway, WNTs function independent of β-catenin and can activate CamKII and protein kinase C (PKC), GTP-binding proteins that in turn activate phospholipase C (PLC) and phosphodiesterase (PDE), and also the planar cell polarity (PCP) pathway that activates Jun-N-terminal kinase (JNK) (reviewed in Kohn and Moon, 2005).
Several lines of investigation support roles for WNT signaling in pituitary gland organogenesis. For example, β-catenin can regulate the activity of three transcription factors with roles in pituitary development, Pitx2, Nr5a1 (Sf1) and Tcf7l2 (Tcf4) (Kioussi et al., 2002; Brinkmeier et al., 2003; Gummow et al., 2003; Brinkmeier et al., 2007). Downstream factors in the WNT pathway are also important for proper pituitary development. Tcf4-/- embryos exhibit severe pituitary overgrowth, with a 3-fold increase in anterior lobe volume (Brinkmeier et al., 2003). The mechanism underlying the overgrowth appears to involve expanded BMP and FGF expression (Brinkmeier et al., 2007). Pitx2 is expressed in many tissues where WNT signaling is active, and in the presence of LiCl, which artificially activates downstream WNT signaling, an increase in Pitx2 expression is detected in the developing Rathke's pouch at e10.5, as well as in cultured pituitary cells at this time point (Kioussi et al., 2002). Pitx2 activity is also increased in the presence of a constitutively active form of β-catenin expressed in the gonadotrope-like αT3-1 pituitary cell line. Wnt11 has been implicated as a target of Pitx2 and β-catenin in cardiac development (Zhou et al., 2007), but the WNT(s) responsible for the β-catenin-mediated activation of Pitx2 in the pituitary have yet to be identified. Moreover, the presence of activated β-catenin has not been demonstrated in the developing anterior pituitary. In addition, nuclear accumulation of β-catenin and subsequent activation of TCF/LEF transcription factors can occur after gonadotropin-releasing hormone (GnRH) stimulation in mouse pituitary gonadotrope-like cells (Gardner et al., 2007). Because GnRH receptor, like other G-protein coupled receptors (GPCRs) can activate the canonical WNT signaling pathway, β-catenin activation of Pitx2 or other critical transcription factors could be independent of a Wnt signal.
Direct evidence for WNT signaling in pituitary development stems from pituitary abnormalities arising from disruption of Wnt5a and Wnt4 (Treier et al., 1998; Cha et al., 2004). Wnt5a mRNA expression has been detected in the ventral diencephalon adjacent to the pituitary and in the pituitary primordium beginning at e9.5 (Treier et al., 1998). Wnt5a mutant embryos exhibit abnormal branching and looping of the developing pituitary, though all hormone-producing cell types are generated (Cha et al., 2004). Wnt4 is expressed from e9.5 onwards in Rathke's pouch and in the oral ectoderm. Expression becomes restricted to the dorsal aspect of the pouch by e14.5. Mice deficient in Wnt4 reportedly have a reduced population of cells producing GH, TSH, and the alpha subunit common to LH, FSH and TSH (alpha glycoprotein hormone subunit = αGSU or chorionic gonadotropin alpha = CGA) at e17.5 (Treier et al., 1998). The mechanisms underlying the defects in Wnt5a and Wnt4 mutants have not been elucidated.
Here we examine the role of WNT signaling in modulating ventral diencephalon gene expression and pituitary gland organogenesis. In the absence of Wnt5a, we show that FGF and BMP expression patterns are perturbed in the ventral diencephalon, supporting the idea that WNT and FGF signaling pathways interact in pituitary development (Wang and Shackleford, 1996; Brinkmeier et al., 2007). We confirmed that mice deficient in Wnt4 alone exhibit reduced pituitary growth, although the effect on cell type specification is less dramatic than previously suggested. Using a classical genetic double mutant analysis we tested for functional redundancy between Wnt5a and Wnt4 and found evidence that the mutant phenotypes are additive in the pituitary gland. Wnt6 is expressed near the pituitary gland during critical times in development; however, examination of embryos deficient in Wnt6 showed no obvious pituitary malformation. Because the effects of deficiencies of Wnt4, 5a, or 6 are unlikely to account for the consequences of deficiencies in the known, critical, β-catenin-regulated transcription factors in the pituitary gland, we conducted a gene expression survey and identified several WNTs, FZDs, and WNT pathway molecules expressed in the pituitary and/or neighboring tissues in early development (e12.5-e14.5). We catalogued their spatial and temporal expression in the developing and adult pituitary gland, producing several candidate genes for future studies. In conclusion, our data suggest that the Wnt signaling pathway regulates pituitary development, in part, through functional intersection with other signaling pathways. The identification of additional Wnt pathway components in the pituitary and ventral diencephalon provides additional targets for investigation in order to more fully understand pituitary development.
Results
Wnt5a mutant embryos exhibit altered expression of BMP and FGF in the ventral diencephalon
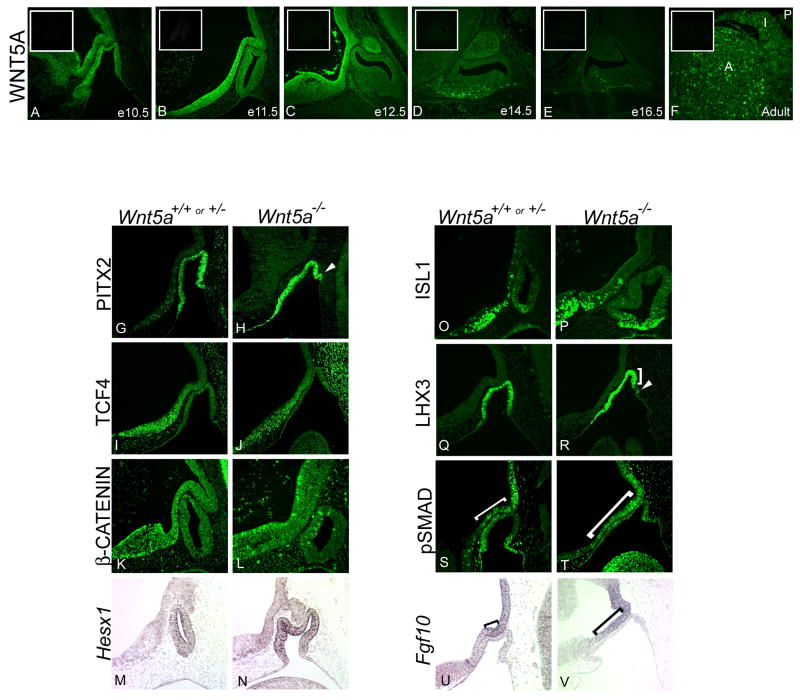
Wnt5a is required for normal pituitary morphology, but the expression pattern of the protein has not been reported and the mechanism of action has been elusive (Cha et al., 2004). WNT5A protein is detectable as early as e10.5 in Rathke's pouch in the cells lining the presumptive lumen (Figure 1A). WNT5A is also present in the ventral diencephalon, particularly in the basal cells lining the lumen, with little or no expression in the cells on the apical aspect. At e11.5, WNT5A is expressed in both the rostral domain and caudal domain of the ventral diencephalon, and in Rathke's pouch (Figure 1B). The rostral domain is demarcated by the normal expression of Bmp4 and Fgf10, and the caudal domain includes diencephalon tissue that strongly expresses TCF4 (Brinkmeier et al., 2007; Davis and Camper, 2007). This pattern of protein immunoreactivity is consistent with the reported expression pattern of Wnt5a mRNA (Treier et al., 1998). Protein expression persists in the infundibulum and ventral diencephalon through e14.5, and it is detectable in the ventral region of the anterior lobe at e14.5 (Figure 1D). The major expression domain of WNT5A in the diencephalon corresponds with the major expression domain of TCF4 (Brinkmeier et al., 2007). Additionally, some protein expression is detected in the anterior and intermediate lobes of the adult pituitary, but no expression is detected in the posterior lobe of the adult (Figure 1F).
Figure 1. Loss of Wnt5a may alter pituitary gland patterning in early development.
Immunohistochemical staining for WNT5A protein was performed on wild-type paraffin sections from e10.5 to e16.5 and adult pituitary glands (A-F). Expression is detected throughout the caudal and rostral domains of the ventral diencephalon from e10.5-e12.5. WNT5A immunostaining is also present in Rathke's pouch at e10.5 and e11.5, but the signal is no longer evident in the pouch by e12.5. At e14.5, WNT5A is expressed in the developing posterior lobe, as well as in the ventral anterior lobe, extending into the rostral tip. WNT5A expression is waning by e16.5, but is evident again in the adult pituitary in the anterior and intermediate lobes (A,I), but not in the mature posterior lobe (P). Insets are slides at each time point without primary antibody. Expression of signaling molecules and transcription factors was performed at e10.5 on Wnt5a wild type and mutant embryos (G-V). PITX2 antibody staining is detected throughout Rathke's pouch at e10.5, and in extra oral ectoderm invaginations (G-H, arrows). TCF4 and β-CATENIN are expressed normally in the ventral diencephalon at this time (I-L). Hesx1 mRNA expression in the oral ectoderm is unaffected (M-N), as is ISL1 protein (O-P). LHX3 expression is truncated on the caudal side compared to wild type (Q-R, bracket). Extra invaginations of oral ectoderm do not express LHX3 (R, arrow). Phosphorylated SMAD1 (pSMAD1) is detected by immunohistochemistry (S-T). Brackets are used to mark the boundary of pSMAD1 expression from the infundibulum to where expression extends into the ventral diencephalon. Fgf10 is detected in the developing ventral diencephalon in e10.5 embryos by in situ hybridization, with brackets used to demarcate its domain of expression (U-V).
We examined expression of several transcription factors in Wnt5a mutants in an effort to elucidate the mechanism underlying the dysmorphology. PITX2 protein expression is present in Rathke's pouch at e10.5, indicating that the cells comprising the characteristic dysmorphic oral ectoderm have committed to pituitary cell fate (Figure 1H). Wnt5a mutants show unaltered expression of TCF4 in the lower domain of the ventral diencephalon (Figure 1I-J). Unlike previous reports of TCF4 expression (Cha et al., 2004), immunoreactivity detected with this antibody correctly mimics Tcf4 mRNA expression from e10.5-e18.5, and no protein is detected in the Tcf4 knockout (Brinkmeier et al., 2007; Davis and Camper, 2007). Expression of an activated form of β-catenin, which should be detected in cells responding to canonical WNT signaling, is unaltered in the Wnt5a mutant (Figure1K-L).
Sox3 mutants and heterozygotes share a similar dysmorphic pituitary phenotype with the Wnt5a mutants at e10.5 and e11.5. The dysmorphology in Sox3 mutants has been attributed to the expanded domains of BMP and FGF signaling and Hesx1 expression (Rizzoti et al., 2004). Hesx1 expression is unaltered in Wnt5a mutants at e11.5 (Figure 1M-N) or at e12.5 (Cha et al., 2004), suggesting that Wnt5a is not upstream of Hesx1. Wnt5a mutants exhibit truncated expression of another homeobox gene, LHX3, at the caudal aspect of the pouch, where multiple invaginations of oral ectoderm tissue occur. Expression of another LIM homeodomain factor, ISL1 is unaltered in the Wnt5a mutants (Figure 1O-R).
Signals emanating from the ventral diencephalon form distinct boundaries of expression around Rathke's pouch. Early in development, the transcription factor SIX3 and signaling molecules such as BMP4, FGF8 and FGF10 are expressed in the portion of the ventral diencephalon that evaginates to become the infundibulum, and later the posterior lobe of the pituitary gland (Treier et al., 1998; Cha et al., 2004; Davis and Camper, 2007). This domain of expression in the ventral diencephalon forms a distinct rostral-caudal boundary of expression with Sonic hedgehog (Shh) (Davis and Camper, 2007). Immunohistochemistry with an antibody that detects the phosphorylated form of SMAD1 (pSMAD1) was used as an indicator of active BMP signaling. In normal mice expression of pSMAD1 is detected in the caudal domain of the ventral diencephalon in the same area as Fgf10 transcripts. In the absence of Wnt5a, pSMAD1 expression extends rostrally, beyond the normal border of the caudal domain (Figure 1S-T). Fgf10 expression is mutually exclusive with TCF4 expression and demarcates the boundaries of the caudal and rostral domains of the ventral diencephalon in wild-type e10.5 embryos. In Wnt5a mutant embryos, however, Fgf10 expression is no longer limited to the caudal domain. The expression domain is expanded along the ventral diencephalon into the area that would normally constitute the rostral domain (Figure 1U-V). Thus, Wnt5a deficiency causes expansion of both BMP and FGF signaling domains in the ventral diencephalon.
Wnt4 has a mild effect on pituitary cell specification
Previous studies report a dramatic decrease in levels of GH, TSHβ, and αGSU immunoreactivity at e17.5 in Wnt4 deficient animals (Treier et al., 1998). To explore the mechanism that underlies the hormone reduction in these animals, we examined the pituitary phenotype of the Wnt4 mutants. To ensure our analysis would be comparable to the previous study, we utilized the same mutant mice on the same genetic background (Treier et al., 1998). We confirmed that there is no reduction in pro-opiomelanocortin (POMC) immunoreactivity in the anterior or intermediate lobes of mutant embryos, where it marks differentiated corticotropes and melanotropes, respectively (Figure 2A-B). Equal SF1 expression at e16.5 in wild type and mutants suggests that the pre-gonadotrope population is unaffected by loss of Wnt4 (Figure 2C-D). In contrast to previous reports, we observed no appreciable reduction in chorionic gonadotropin alpha (CGA or αGSU) immunoreactivity at 16.5 or e18.5 (Treier et al., 1998). Furthermore, we observed no reduction in levels of FOXL2 (Figure 2I-J), a protein that is co-expressed with αGSU in pre-gonadotropes and pre-thyrotropes, and activates αGSU transcription in cell culture and transgenic mice (Ellsworth et al., 2006). At e16.5 and e18.5 we observed a slight reduction in GH and TSHβ immunoreactivity in Wnt4 mutant pituitaries (Figure 2K-N). PIT1-positive cells were reduced in the mutant embryos relative to wild type at e16.5 and e18.5 (Figure 2O-R), which could underlie the reduced number of somatotropes and thyrotropes. The consistent reduction in PIT1 expression suggests that the differentiated cell populations are reduced and not simply developmentally delayed.
Figure 2. Wnt4 has a mild effect on pituitary cell specification.
Immunostaining for the pituitary hormones reveals that each of the major cell types have begun to differentiate properly by e16.5 (n=2) and e18.5 (n≥3). Sagittal sections of embryos were stained with antibodies that recognize the pituitary hormones. Pro-opiomelanocortin (POMC) and its cleavage product adrenocorticotropic hormone (ACTH) are unchanged in the mutant at e16.5 (A-B). Steroidogenic factor 1 (SF1), marking pre-gonadotropes, is also unchanged at e16.5 (C-D). The alpha subunit shared by thyrotropes and gonadotropes, αGSU, appears unchanged both at e16.5 (E-F) and e18.5 (G-H), as does FOXL2 at e18.5 (I-J). Growth hormone (GH) is present at e16.5 in the wild type and mutant (K-L). Arrowheads indicate positive growth hormone cells. Thyroid-stimulating hormone β subunit (TSHβ) is also present by e16.5 (M-N). Insets show enlarged picture of anterior lobe region. PIT1 staining identifies pre-somatotropes, pre-lactotropes, and pre-thyrotropes in the wild type and mutant at e16.5 (O-P) and e18.5 (Q-R). A slight dysmorphology is seen in anterior lobe tissue surrounding the lumen in mutants at e16.5 (B,D,L,P) and e18.5 (H,R). Immunostaining was developed with FITC or diaminobenzidine (DAB), and sections are counterstained with methyl green or hematoxylin.
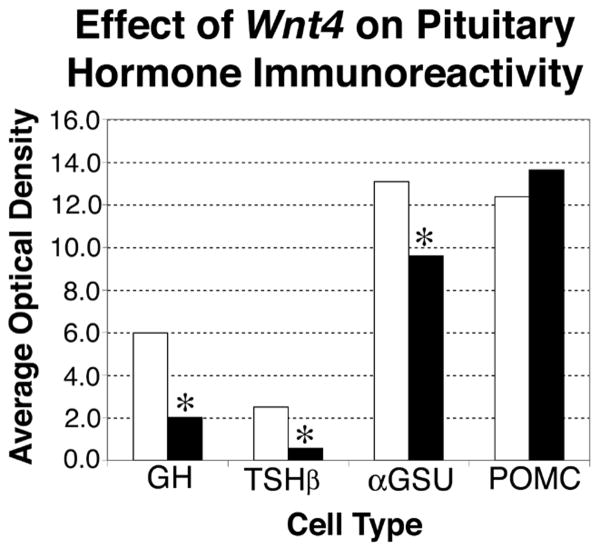
To confirm these qualitative results we quantified the immunoreactivity of each hormone at e18.5 (Figure 3). GH immunoreactivity is significantly reduced in the mutants with a 66% reduction in activity relative to wild type (P=0.03). TSH immunoreactivity in the anterior lobe of the Wnt4 mutants is reduced 78%, which is significant (P=0.04). The difference in αGSU immunoreactivity at e18.5 is not obvious, but quantification of αGSU levels in mutants revealed a mild 27% reduction of immunoreactivity compared with wild type, with a borderline level of significance (P=0.0495), suggesting that there could be a subtle reduction. As expected, POMC immunoreactivity in the anterior lobe, which did not appear to be grossly altered in Wnt4 mutants, exhibited no statistically significant change when quantified; the 9% difference between wild type and mutant yields a P value of 0.86.
Figure 3. Effect of Wnt4 on pituitary hormone immunoreactivity.
Sagittal sections taken at e18.5 from three wild type and three mutant e18.5 Wnt4 embryos were stained for GH, TSHβ, αGSU and POMC. White bars represent wild types and black bars represent mutants. Average optical density (OD) for each genotype was obtained from three slides and repeated three times using ImagePro Plus software. Optical density parameters were set independently for each hormone, and therefore OD levels are not comparable between the different hormones. OD units are arbitrary and represented in the graph as 1×10E6. Repeated measures ANOVA analyses of the average optical density for each hormone were performed to determine statistical significance. For GH, P=0.0317; for TSHβ, P=0.0402; for αGSU, P=0.0495; for POMC, P=0.8635. * Indicates significant P-values at 0.05.
Independent roles of Wnt4 and Wnt5a in development
The canonical and non-canonical Wnt signaling pathways may interact and influence one another in development (Topol et al., 2003; Zhou et al., 2007). Wnt4 is frequently associated with the non-canonical class of WNT molecules, although some WNTs can activate different signaling pathways depending on context (Mikels and Nusse, 2006). To determine if loss of Wnt4-mediated signaling affects the canonical pathway in the pituitary and ventral diencephalon, we analyzed expression of the activated form of β-catenin in the rostral domain of the ventral diencephalon and found it was undisturbed. We also found no changes in TCF4 immunoreactivity in the ventral diencephalon at e12.5 or Lef1 mRNA levels in Rathke's pouch at e16.5 in Wnt4 mutants (data not shown).
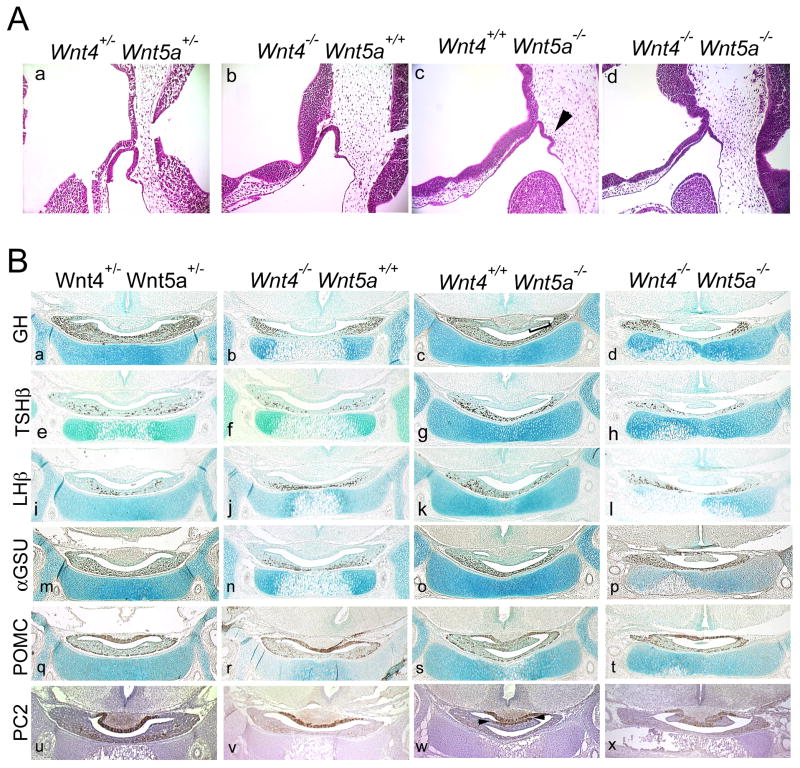
Wnt5a mutant pituitaries begin to show signs of abnormal development at e10.5. In these mutants, Rathke's pouch tissue expands rostrally, with extra invaginations of pouch tissue on the caudal side (Figure 4A, c). This dysmorphology persists through e18.5 (Figure 4B, c, bracket), though hormone immunoreactivity remains largely unaffected. The dysmorphic region of Wnt5a mutants contains some somatotropes (Figure 4B, c) (Cha et al., 2004), and a portion of differentiated corticotropes and melanotropes as indicated by the POMC and prohormone convertase 2 (PC2) staining in the region (Figure 4B, s, w). This expression of PC2 was used to assess the cell specificity of POMC-positive cells in the dysmorphic intermediate lobe of the Wnt5a mutants. Patches of cells in the Wnt5a mutant are positive for PC2 immunoreactivity, suggesting that only part of the dysmorphic tissue is truly intermediate lobe. POMC staining appears normal in the intermediate lobes of all genotypes examined. These hormone-positive cell types cannot account for all of the cells in the dysmorphic region, however, suggesting some of the cells fail to complete a hormone-producing differentiation program.
Figure 4. A mild additive pituitary phenotype in a Wnt4, Wnt5a double mutant.
A. Hematoxylin and eosin stained paraffin sections of a Wnt4, Wnt5a double mutant at e10.5. Sections are oriented sagittally, with rostral to the left and caudal to the right. Arrowhead indicates dysmorphology of Wnt5a mutant. B. Coronal sections of embryos at day e18.5 were stained with antibodies to anterior pituitary hormones to examine cell specification. The dysmorphology of the Wnt5a mutant (c) is indicated by the bracket. Prohormone convertase 2 (PC2) immunostaining shows differentiation of parts of the dysmorphology as of Wnt5a mutants into corticotropes (w, arrows). A Wnt4,+/-Wnt5a+/- embryo is shown for comparison. Sections are developed with DAB and counterstained with methyl green or hematoxylin.
Surviving Wnt4, Wnt5a double mutants reveal no overlapping function in pituitary development
To assess the potential genetic interaction of Wnt4 and Wnt5a in the pituitary, we produced F1 double heterozygotes and intercrossed them to generate Wnt4, Wnt5a double mutants. The progeny had a genetically heterogeneous background, which arose from the different mixed backgrounds that constituted the stocks for the Wnt4 and Wnt5a mutants. Only two homozygous double mutant embryos were obtained at e10.5 and neither embryo appeared to be viable. Wnt4, Wnt5a double mutants appear to die by an unknown interaction of the two genes early in development. At e10.5 double mutant embryos appear dead or dying, but their pituitaries resemble the Wnt5a single mutant phenotypically at this age (Figure 4A, c, d). At e12.5 most Wnt4,+/-Wnt5a-/- embryos are necrotic, with development arrested at or before e10.5 (n=6/8). One double mutant was obtained from 93 embryos at e18.5. Results of a χ2 test show that this distribution of genotypes is not attributable to chance at either developmental stage; at e12.5 P<0.05 and at e18.5 P<0.005 (Table 1).
Table 1. Wnt4, Wnt5a double mutants are underrepresented1.
| E10.5 | E12.5 | E18.5 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ratios | Wnt5a | Wnt4 | Observed | Expected | Observed | Expected | Observed | Expected |
| 1/16 | +/+ | +/+ | 2 | 5 | 1 | 3 | 11 | 6 |
| 2/16 | +/- | +/+ | 6 | 10 | 8 | 6 | 13 | 12 |
| 1/16 | -/- | +/+ | 5 | 5 | 1 | 3 | 1 | 6 |
| 2/16 | +/+ | +/- | 15 | 10 | 8 | 6 | 15 | 12 |
| 4/16 | +/- | +/- | 24 | 20 | 18 | 12 | 36 | 23 |
| 2/16 | -/- | +/- | 17 | 10 | 2* | 6 | 7 | 12 |
| 1/16 | +/+ | -/- | 4 | 5 | 5 | 3 | 5 | 6 |
| 2/16 | +/- | -/- | 5 | 10 | 5 | 6 | 4 | 12 |
| 1/16 | -/- | -/- | 2 | 5 | 0 | 3 | 1 | 6 |
| Total | 80 | P < 0.9 | 48 | P < 0.05 | 93 | P < 0.005 | ||
Results of a χ2 test show that the distribution of these genotypes is likely not due to chance at e12.5 (P<0.05) and at e18.5 (P<0.005).
Six severely necrotic embryos were also found of this genotype.
The Wnt4,-/-Wnt5a-/- pituitary exhibits an additive phenotype. Somatotropes and thyrotropes appear to be slightly reduced in the double mutant relative to wild type, mimicking the findings for Wnt4 (Figure 4B, d, h), while αGSU immunoreactivity does not appear drastically reduced. LHβ and POMC immunoreactivity appears normal at all genotypes examined. The dysmorphology evident around the lumen of the Wnt5a mutants is also apparent in the double mutant (Figure 4B, d, h, l, p, t, x). While hormone immunoreactivity remains largely unaffected, both Wnt5a single mutants and the Wnt4, Wnt5a double mutant contain a dysmorphic cleft crossing the lumen between the intermediate and anterior lobes. The intermediate lobe of the double mutant expresses PC2 in the same subset of cells as the single Wnt5a mutant (Figure 4B, w, x), suggesting the cell specification process in the double mutant is not altered beyond that of the single mutant. The combined phenotypes among the rare viable animals observed at either age do not appear more severe than either single mutant, which suggests separate roles for Wnt4 and Wnt5a in pituitary development, despite the interaction in other developing organs that causes reduced viability.
Wnt6 does not affect pituitary gland development
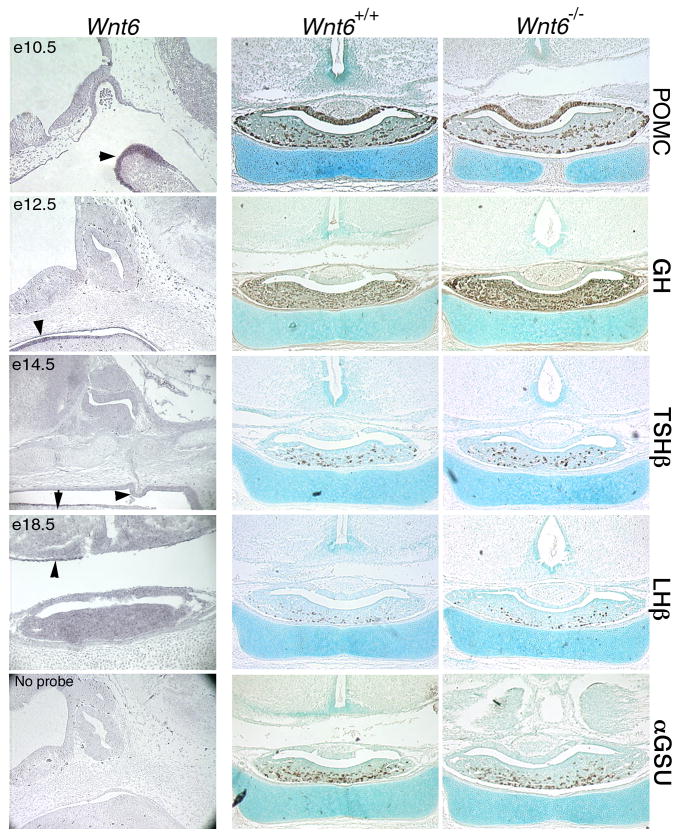
A cDNA library generated from dissected Prop1df/df Rathke's pouch tissue at e14.5 contained Wnt6 cDNA (Carninci et al., 2003). Wnt6 transcripts are detected in tissues surrounding the developing pituitary beginning at e10.5. At this stage Wnt6 is present in the pharyngeal arch. Expression in the oral ectoderm underlying the pituitary is detectable at e12.5 and to a lesser extent at e14.5 (Figure 5, arrowheads). No expression is detectable in Rathke's pouch or its derivatives. Expression is extinguished in the oral ectoderm by e18.5. Expression of POMC, GH, TSHβ, LHβ and αGSU is unchanged in Wnt6-/- embryos at e18.5. This suggests that Wnt6 is dispensable for normal differentiation of pituitary cell lineages and morphogenesis.
Figure 5. Wnt6 is expressed near the pituitary during formation of Rathke's pouch, but is not required for pituitary development.
Expression of Wnt6 at critical time points of pituitary organogenesis is detected by in situ hybridization using NBT and BCIP for development of the purple precipitate. Sagittal sections of e10.5-e14.5 embryos are oriented with dorsal at the top and rostral at the left. Overnight hybridization without a riboprobe served as a negative control. Coronal sections of normal and Wnt6 mutant embryos at e18.5 were immunostained and developed with DAB to assess cell specification with antibodies to POMC, GH, TSHβ, luteinizing hormone β subunit (LHβ), and αGSU. Sections are counterstained with methyl green.
Wnt pathway expression in the pituitary gland
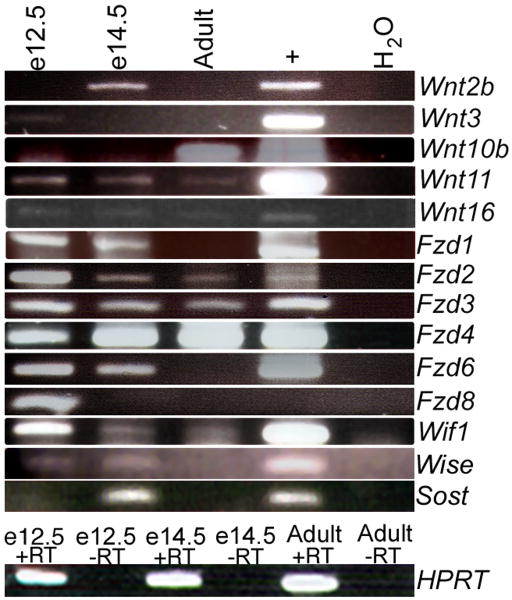
Since Wnt4, Wnt5a and Wnt6 were not critical for pituitary differentiation, we performed a PCR screen for the presence of other Wnts in the developing pituitary gland. Intron spanning primers were used to amplify Wnt and Fzd transcripts from Rathke's pouch RNA collected at e12.5 and e14.5, and from adult pituitaries. The cDNAs generated from the pituitary RNA, as well as from control tissues, were used as templates. Tissues for positive controls were selected based on previous reports of Wnt or Fzd expression in that tissue (www.informatics.jax.org). Adult testis, kidney, and lung, and e12.5 head, e12.5 body and e14.5 body RNA were used as positive controls for this assay. From this RT-PCR screening, transcripts from several Wnt and Fzd genes were identified in the pituitary with varying temporal expression patterns (Figure 6). Identities of the RT-PCR products were confirmed by DNA sequencing.
Figure 6. Many Wnt pathway genes are expressed in the developing pituitary.
RT-PCR was used to detect expression of Wnt signaling pathway members and regulators during various times of pituitary gland development. RNA from the specified ages was analyzed using intron-spanning primers specific to each Wnt and Frizzled gene. For positive controls, cDNAs generated from adult and embryonic tissues were chosen that had previously been reported to express each Wnt or Fzd gene. Water was used as a negative control. HPRT PCR products showing +RT and −RT reactions confirm no genomic contamination in the e12.5, e14.5 or adult pituitary cDNA. PCR products were sequenced to confirm the identity of each gene.
Wnt11 and Wnt16 were expressed at all time points examined. Other Wnts such as Wnt2b, Wnt3 and Wnt10b were expressed only at one time point (e14.5, e12.5, adult, respectively). Wnt5b, Wnt7a, and Wnt7b expression were not detected in the pituitary gland at any of the times examined (data not shown).
Several receptors of the WNT signaling pathway were also detected in the survey. Fzd1 expression is detected in the embryonic cDNA, and Fzd2, Fzd3, and Fzd4 expression is observed at all three times in pituitary development. Fzd6 and Fzd8 are detected in developing e12.5 and e14.5 pituitary cDNA, but not in the adult tissue. Fzd9 is not detected in the pituitary at any of the times examined (data not shown).
Regulators of the WNT pathway are also present in the pituitary. Wnt inhibitory factor-1 (Wif1) is detected throughout pituitary gland development and in the adult. Wise, the Wnt inhibitor in the surface ectoderm, and its highly related counterpart Sclerostin, Sost, were also detected during embryonic stages.
In situ hybridization analysis for expression of Wnt pathway members
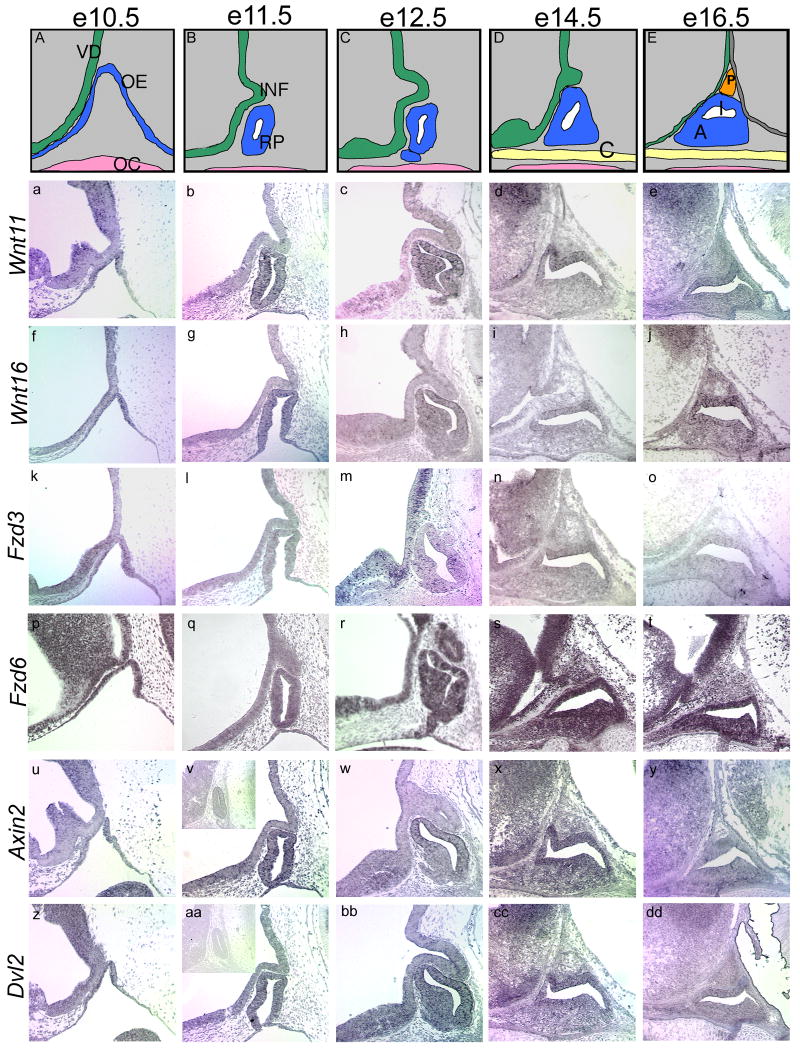
In situ hybridization analysis of Wnts and Frizzleds identified in the initial RT-PCR survey reveal temporally and spatially restricted expression of these genes in the pituitary gland and ventral diencephalon (Figure 7). Wnt11 and Wnt16 are expressed in Rathke's pouch beginning at e10.5 and continuing through e16.5. Expression can also be detected in the rostral, lower domain of the ventral diencephalon early in development, from e10.5-e12.5. Wnt11 and Wnt16 transcripts, however, appear to be excluded from the forming infundibulum at all ages examined. Their expression within Rathke's pouch is concentrated dorsally, with less hybridization signal in the area of differentiating cells in the anterior lobe. Expression of Fzd3 is detected in the lower domain of the ventral diencephalon with no detectable expression in the infundibulum. This is similar to Wnt11 and Wnt16 expression patterns. Fzd6 expression is detectable throughout the ventral diencephalon and Rathke's pouch through e16.5. Dvl2 and the negative regulator of WNT signaling, Axin2, are expressed in the ventral diencephalon with Wnt11, Wnt16, and Fzd3. Dvl2 and Axin2 expression are also concentrated in the dorsal aspect of Rathke's pouch.
Figure 7. Spatial expression patterns of Wnt pathway members in the developing pituitary and ventral diencephalon.
Formation of Rathke's pouch is illustrated from e10.5 to e16.5 (A-E). At e10.5, the oral ectoderm begins to invaginate and pinches off around e11.5 to form Rathke's pouch. Meanwhile, the ventral diencephalon evaginates to form the infundibulum. From e12.5 to e14.5, the anterior lobe begins to form as cells surrounding the lumen rapidly divide and migrate out of the pouch. By e16.5, the three distinct lobes of the developing pituitary are evident, with the posterior lobe arising from the infundibulum. In situ hybridizations were performed on wild-type sagittal sections from e10.5 to e16.5 to determine temporal and spatial patterns of expression. Slides are oriented with dorsal to the top and rostral to the left. Inset panels (v, aa) show sense slides for negative controls. VD, ventral diencephalon; OE, oral ectoderm; OC, oral cavity; INF, infundibulum; RP, Rathke's pouch; C, cartilage plate of hard palate; P, developing posterior lobe; I, developing intermediate lobe; A, developing anterior lobe.
Discussion
Wnt5a affects patterning of the ventral diencephalon
Wnt5a mutant animals exhibit pituitary dysmorphology (Cha et al., 2004). Our data implicate expanded FGF and BMP signaling as the underlying mechanism for the dysmorphology. Bmp4 is required for the invagination of Rathke's pouch (Takuma et al., 1998), but noggin expression is required to attenuate this dorsal BMP activity (Davis and Camper, 2007). The excess BMP activity that we observed in Wnt5a-/- embryos may induce additional oral ectoderm to invaginate, contributing to the dysmorphology. Sox3 and Tcf4 deficient mice exhibit expanded BMP and FGF signaling and abnormalities in Rathke's pouch (Rizzoti et al., 2004; Brinkmeier et al., 2007). Taken together, these observations support the idea of cross talk between the signaling pathways.
Elevated levels or temporal expansion of Hesx1 transcription can cause inappropriate induction of Rathke's pouch tissue from the oral ectoderm (Dattani et al., 1998). We examined Hesx1 expression in the oral ectoderm of Wnt5a mutants at e11.5, but we noted no change, suggesting that Hesx1 does not contribute to the dysmorphology in Wnt5a mutants. Sox3 is expressed early in development in the ventral diencephalon and presumptive hypothalamus in a similar pattern to that of Wnt5a (Solomon et al., 2004), and mutations in both of these genes result in similar expansion of BMP and FGF signals. Wnt5a and Sox3 may function in a similar pathway or parallel pathways because Sox3 expression is unaltered in Wnt5a mutants (data not shown).
FGF signaling induces proliferation and expansion of pituitary cell types in pituitary explants, and overexpression of FGF in transgenic mice causes excess proliferation and pouch dysmorphology (Ericson et al., 1998; Treier et al., 1998). Based on these observations we suggest that the expansion of the Fgf10 expression domain in Wnt5a mutants may induce additional oral ectoderm tissue to differentiate into Rathke's pouch. The excess tissue could result in extra folds along the lumen, causing the characteristic dysmorphology.
FGF overexpression interferes with cell specification and causes striking dysmorphology in transgenic mice, but Wnt5a mutants have normal cell specification and mild dysmorphology (Treier et al., 1998; Cha et al., 2004). This discrepancy could be due to the fact that expansion of FGF expression is transient and within the physiological range in Wnt5a mutants, and the overall level of excess FGF produced in transgenic mice is much higher and potentially non-physiological. Wnt5a can antagonize the canonical WNT signaling pathway (Topol et al., 2003), and it can stabilize β-catenin under certain circumstances (Mikels and Nusse, 2006). Though the spatial and temporal expression patterns of WNT5A and activated β-catenin overlap in tissues adjacent to the pituitary gland, we observe no difference in levels of activated β-catenin immunoreactivity in the Wnt5a mutant (Figure 1K-L). Thus, Wnt5a is not likely to be the activating signal for β-catenin in this context.
Lhx3 has been shown to be important for pituitary cell survival (Zhao et al., 2006; Ellsworth et al., 2008). For proper LHX3 expression, other pituitary factors such as Lhx4, or the combination of Pitx1 and Pitx2 transcripts are required (Raetzman et al., 2002; Charles et al., 2005). Additionally, inhibition of BMP signaling (Noggin) and Notch activation of transcriptional repressors (Hes1) are required for normal expression of LHX3 (Davis and Camper, 2007; Raetzman et al., 2007). We observe the same exclusion of LHX3 expression from the caudal side of Rathke's pouch in Wnt5a mutants as was observed in Hes1 mutants. This implicates the interplay of several different signaling pathways in the activation of critical pituitary transcription factors.
The dysmorphology characteristic of Wnt5a mutants at e18.5 has a striking similarity to that of the groucho-related Aes mutant mice, which exhibit an abnormal connection of pituitary tissue to the intermediate lobe (Brinkmeier et al., 2003), and some phenotypic similarity to the Prop1 mutant (Ward et al., 2005). The dysmorphic tissue of Wnt5a mutants is comprised of some GH-positive cells, and cells destined to become intermediate lobe, though the dysmorphic region contains some incompletely differentiated cells. These undifferentiated cells may result from an abnormal connection of neural tissue to the intermediate lobe, as seen in the Uncx4.1 mutant mice at birth (Asbreuk et al., 2006).
Interaction between WNT5A, FGF, and BMP signaling pathways has been observed in other organs. Fgf10 is upregulated in the Wnt5a mutant lung, and overexpression of Wnt5a in developing lung causes increased Fgf10 expression, altered spatial pattern of Bmp4 expression, and reduced epithelial branching (Li et al., 2002; Li et al., 2005). In addition, expression of Fgf8 can reduce Wnt5a expression in the developing mouse cerebral cortex (Shimogori et al., 2004). Together, these findings support our hypothesis that Wnt5a regulates Fgf10 in the ventral diencephalon, which has an indirect effect on Rathke's pouch. Thus, the balance between each signaling pathway is critical for normal pituitary gland organogenesis.
Wnt4 causes reduction in the PIT1 lineage
Wnt4 is expressed early in development, from e9.5 in Rathke's pouch, with limited expression in the dorsal aspect of the pouch through e14.5 (Treier et al., 1998; Olson et al., 2006). Wnt4 is also expressed in the oral ectoderm throughout development. Wnt4 was implicated as a necessary element in anterior pituitary precursor cell expansion and αGSU expression (Treier et al., 1998).
Our analysis of Wnt4 mutants at e16.5 and e18.5 reveals a mild reduction of anterior lobe size, although, in contrast to the previous report, we see no drastic reduction in αGSU cell number at either e16.5 or e18.5, and quantification of αGSU immunostaining results in a barely significant change in expression (P=0.0495).
The pituitary transcription factor PIT1 is responsible for differentiation and expansion of somatotropes, thyrotropes and lactotropes (Camper et al., 1990; Li et al., 1990). We have found Wnt4 mice have reduced PIT1, which is likely the cause for the reduction in numbers of somatotropes and thyrotropes. Pit1 deficiency does not alter the size of the pituitary gland until several days after birth, so the delay in Pit1 expression does not account for the pituitary hypoplasia in Wnt4 mutants during gestation (Ward et al., 2006).
Wnt4 and Wnt5a function independently in the pituitary gland
Currently, 19 WNT related genes have been identified in the mouse (http://www.stanford.edu/∼rnusse/wntwindow.html). Due to the large number of mammalian WNT family members, WNTs are likely to compensate for one another in development. Wnt4 and Wnt5a may both function in the non-canonical WNT/Ca2+ pathway (reviewed in Kuhl et al., 2000). Since Wnt4 and Wnt5a are expressed in complementary regions in and surrounding the pituitary gland, and have overlapping temporal expression patterns, we conducted a classic double mutant analysis to test for interaction between the two genes.
Conditional mutants for Wnt4 and Wnt5a would be ideal for studying genetic interaction in the pituitary gland because double mutants were underrepresented at e10.5 and e18.5, suggesting that Wnt4 and Wnt5a are interacting early in development, resulting in lethality of some embryos before e10.5. Because pituitary hormones are not necessary for fetal growth or survival to term, the lethality must arise from a requirement for either Wnt4 or Wnt5a in other organs. We do not observe a genetic interaction between Wnt4 and Wnt5a in the pituitary glands of the surviving double mutants. Wnt4 mutants do not have an observable morphological phenotype at e10.5, and a double mutant at this age exhibits the characteristic Wnt5a phenotype. At e18.5, an additive phenotype is observed in the double mutant. This suggests that the roles of Wnt4 and Wnt5a in the pituitary gland are functionally distinct and not synergistic or overlapping.
Wnt6 is not required for pituitary gland development
Using in situ hybridization, expression of Wnt6 was localized to the oral ectoderm, but Wnt6 was not detected in Rathke's pouch tissue at any time examined throughout development. Expression of Wnt6 was detected by RT-PCR in e12.5 laser-captured Rathke's pouch cDNA (Olson et al., 2006). This may indicate that there are low levels of Wnt6 expression in Rathke's pouch that precluded detection by in situ hybridization, or the laser capture may have included oral ectoderm outside the pituitary anlage. Despite this discrepancy in expression patterns, it is clear that Wnt6 mutants exhibit no obvious pituitary morphological abnormalities and undergo cell differentiation appropriately. Thus, the expression of Wnt6 is not required for pituitary gland development.
Multiple opportunities for Wnt signaling to regulate pituitary growth and development
TCF4 was detected in Rathke's pouch, and expression was localized by immunohistochemistry to the rostral domain of the ventral diencephalon (Douglas et al., 2001; Brinkmeier et al., 2007). In the absence of Tcf4, the pituitary gland exhibits a profound increase in anterior lobe size, demonstrating an important role for TCF4 in repressing pituitary gland growth (Brinkmeier et al., 2003).
It is not known which WNT, if any, regulates Tcf4 expression. An RT-PCR survey performed on laser-captured e12.5 Rathke's pouch cDNA also reports expression of Wnts 3, 11 and 16 at e12.5. Additionally, it was reported that Wnts 5b, 7a and 7b were present at e12.5 (Olson et al., 2006), though these transcripts were not detected in our survey. Our analysis suggests that Wnt11 and Wnt16 are expressed in a pattern that might permit them to activate Tcf4 expression (Figure 8). Such a role has been suggested for Wnt16 in synovial joint formation (Guo et al., 2004) where activated β-catenin is detected in the rostral domain concurrent with Wnt11 and Wnt16 expression. Furthermore, LEF/TCF reporter expression is activated in the caudal domain of the ventral diencephalon in an overlapping pattern with Fgf10 and Bmp4, suggesting that canonical WNT signaling may modulate FGF and BMP signaling in that region (Maretto et al., 2003).
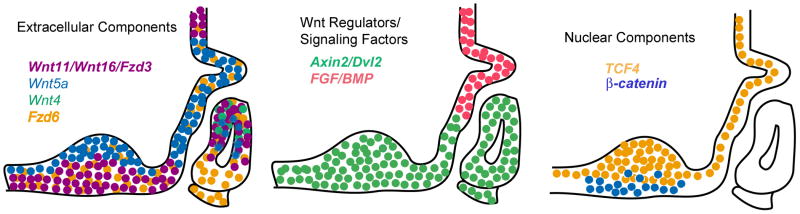
Figure 8. Gene expression summary of BMP, FGF and WNT signaling molecules.
Signals from the ventral diencephalon and from Rathke's pouch early in development are important for proper patterning of the pituitary gland. These signals are expressed in a spatially restricted manner that is important for regulating pituitary shape and growth. Wnt4 has a limited window of expression in Rathke's pouch (Treier et al., 1998). WNT5A is expressed throughout the ventral diencephalon and in Rathke's pouch early in development. Wnt11, Wnt16, and Fzd3 are expressed in the rostral domain of the ventral diencephalon, and excluded from the caudal domain and infundibulum. Their expression is also seen in Rathke's pouch, where it becomes dorsally concentrated by e14.5. These factors, along with Axin2 and Dvl2, are expressed in a mutually exclusive pattern relative to FGF and BMP, suggestive of an antagonistic regulation. Fzd6 has no such restriction and is expressed throughout the pouch and ventral diencephalon. Downstream effecter TCF4 is also expressed throughout the ventral diencephalon, particularly on the apical side. Activated β-CATENIN expression is seen in the rostral domain of the ventral diencephalon. Taken together, these expression studies suggest extensive Wnt activity in the developing pituitary gland, with potential for overlapping functions among different members of the family.
The spatial and temporal pattern of Wnt11 expression mimics the expression of Wnt16 (Figure 8). While Wnt16 is thought to activate the canonical WNT/β-catenin pathway, Wnt11 is often classified with Wnt5a in the non-canonical WNT pathway. It is possible that both canonical and non-canonical signaling pathways are actively participating in patterning of the ventral diencephalon and Rathke's pouch. Wnt11 is proposed to have overlapping functions with FGF ligands and receptors and members of the BMP family in regulation of ureteric branching (Majumdar et al., 2003). Wnt11 and Wnt5a regulate convergent extension movements in the zebrafish, and their activities are negatively regulated by a gradient of BMP signaling (Myers et al., 2002). This suggests that Wnt11 and Wnt5a may similarly pattern the pituitary gland, in part by influencing BMP signaling, but may not be involved in specification of the hormone-producing cell types.
The expression patterns of Axin2 and Dvl2 are also similar to the expression patterns of Wnt11 and Wnt16 (Figure 8). AXIN2, known to be an inhibitor of canonical WNT signaling, was reportedly expressed in the ventral aspect of Rathke's pouch from e11.5 to e14.5 (Olson et al., 2006). Our Axin2 probe was generated from a full-length cDNA that was completely sequenced. This probe revealed an expression pattern beginning at e10.5 in Rathke's pouch, and continuing through e16.5. The specific pattern of expression, including concentrated mRNA signal in the dorsal aspect of the pouch, as well as in the rostral domain of the ventral diencephalon, supports our hypothesis that Wnt signaling is active in the pituitary and adjacent ventral diencephalon. While Olsen et al. (2006) report no change in expression of Axin2 in e14.5 Prop1 null embryos, Axin2 expression has been shown to be down-regulated in Prop1df/df P1 cDNA compared to wild type via gene expression microarray analysis, suggesting more than just a circumstantial connection between Prop1 and WNT signaling (Mortensen and Camper, unpublished).
Wnt10b is unique among the WNTs we surveyed in that it is expressed only in the adult pituitary gland. Wnt10b expression is detected in a high fraction (11/14) of human pituitary adenomas (Howng et al., 2002). WNT expression in adult pituitary might affect BMP signaling in the same way that we observed in pituitary development. This could be significant because BMP4 promotes cell proliferation in the prolactinomas, the most common type of pituitary adenoma (Paez-Pereda et al., 2003).
In conclusion, we have clearly defined the roles of Wnt5a, Wnt4, and Wnt6 in development of the pituitary gland, and implicate additional WNT family members that may play functional roles. The ability of WNTs to influence BMP and FGF signaling pathways emerges as a common theme in the pituitary gland (Camper, 2004; Rizzoti et al., 2004; Brinkmeier et al., 2007; Davis and Camper, 2007). Disruption of this balance in two WNT mutants results in patterning defects, dysmorphology, and a developmental delay in cell specification. The discovery of numerous other WNT family members expressed in the pituitary gland and ventral diencephalon suggests that there may be multiple roles for WNT genes in pituitary development and a great deal of overlapping function among WNT family members.
Experimental Procedures
Mouse care and embryo preparation
Wnt5a mutant mice on a mixed 129SvBrd and C57BL/6 background were obtained from Stephen Jones (Yamaguchi et al., 1999) and maintained at the University of Michigan by heterozygote matings. 129-Wnt4tm1Amc/J mutant mice were obtained from The Jackson Laboratory and maintained at the University of Michigan by heterozygote matings and matings with wild-type C57BL/6J females, also from The Jackson Laboratory. We will refer to these mice as Wnt4-/-. Wnt4, Wnt5a double mutants were obtained by intercrossing double heterozygous animals obtained from a Wnt4+/- × Wnt5a+/- parental cross. A Wnt6 null allele was generated by Andreas Kispert at the Institute for Molecular Biology at Hannover Medical School, Germany, where e18.5 embryos were generated by heterozygous matings. Exon 3 and exon 4 were deleted from the Wnt6 locus to assure generation of a null allele. Integrity of the null allele was ascertained by RFLP analysis. The generation of the Wnt6 allele will be fully reported elsewhere. Mice were housed under the supervision of the Unit for Laboratory Animal Medicine and the University Committee for Usage and Care of Animals, and all procedures were in compliance with the principles outlined in the NIH Guidelines for the Care and Use of Experimental Animals. Genotyping was performed as previously described for Wnt4 (Stark et al., 1994) and Wnt5a (Cha et al., 2004). Noon of the day of the vaginal plug is designated as embryonic day 0.5. Embryos were dissected and fixed 30 min-overnight in 3.7% formaldehyde in PBS at 4°C. Embryos were dehydrated to 100% ethanol, embedded in a Citadel 1000 (Thermo Electric, Chesire, England) paraffin embedding machine and sectioned sagittally or coronally at 6μm thickness for immunohistochemistry and in situ hybridization.
Immunohistochemistry, in situ hybridizations and histology
Immunohistochemistry for the pituitary hormones was performed on paraffin sections as previously described (Kendall et al., 1994), and visualized with diaminobenzadine (DAB) chromogen. Antibodies used in fluorescent immunohistochemistry were incubated at 4°C overnight. Rabbit anti-phosphorylated SMAD1 (pSMAD1) (Cell Signaling Technology, Inc., Danvers, MA) was used at 1:200 dilution overnight, and goat anti-WNT5A (R&D Systems Inc., Minneapolis, MN) at a 1:100 dilution overnight. Mouse anti-Lim3 (LHX3) monoclonal antibody (Developmental Studies Hybridoma Bank, U of Iowa) was used at a 1:200 dilution and mouse anti-TCF4 (Upstate Cell Signaling, Charlottesville, VA) was applied at 1:100. Mouse anti-activated β-CATENIN monoclonal antibody (Millipore, Billerica, MA) was used at a 1:100 dilution. The purified antibody for PITX2 was a gift from Dr. Phil Gage (University of Michigan), and was generated by Dr. Tord Hjalt, (Lund University, Sweden). Antibody staining was performed after treating slides with 3% H2O2:methanol 1:1 for 20 minutes followed by boiling 10 minutes in 0.1M citric acid pH 6.0. Primary antibodies were added after a 30 minute block in TSA blocking reagent. For pSMAD1 and PITX2, biotin-conjugated anti-rabbit secondary antibody at 1:200 (Jackson Immunoresearch, West Grove, PA) was used and for WNT5A, biotin-conjugated anti-goat secondary antibody at 1:200 was used (Vector Labs, Burlingame, CA). For LHX3, TCF4, and β-CATENIN, biotin-conjugated anti-mouse secondary antibody from the M.O.M Kit (Vector Labs, Burlingame, CA) was used. Amplification and detection were carried out with the TSA Fluorescein System (Perkin-Elmer, Wellesley, MA) according to the manufacturer's directions.
Quantification of immunostained pituitary sections was performed using the ImagePro Plus 6.2 software program (Media Cybernetics, In., Bethesda, MD) (Meynen et al., 2007; Mitchell et al., 2007). Three sagittal slides were taken from each of three Wnt4 mutant and three wild type or heterozygous embryos at e18.5. The anterior lobes of these sections were selected as the area of interest (AOI) and immunoreactive sites exclusively in the anterior lobes were identified using the program's histogram based color selection, which was set to recognize the DAB stained cells. For GH, TSHβ, αGSU and ACTH, the ImagePro Plus integrated optimal density (IOD) was chosen to quantify the amount of DAB staining for each slide, and the sums of optical density for each section were recorded, resulting in a calculation in arbitrary units for the amount of each hormone present. Each section was analyzed three separate times to ensure the software program was reading the samples consistently, and therefore the optical density for each slide was averaged. The averaged IOD values for the three slides for each of the six embryos examined were analyzed for significance using a repeated measures ANOVA test in StatView 5.0.1 from SAS Institute, Inc. (Cary, NC), and P-values were generated using P<0.05 as significant.
In situ hybridizations were performed on paraffin sections using digoxygenin-labeled antisense riboprobes (Roche, Indianapolis, IN) as previously described (Douglas et al., 2001). Probes for Wnt11, Wnt16 and Fzd3 were generated from RT-PCR products as described below, cloned into pGEM-T Easy cloning vector (Promega, Madison, WI), and verified by DNA sequencing. Wnt11 was subcloned into pBluescript (Stratagene, La Jolla, CA) and the antisense probe was linearized with KpnI and labeled with T3 polymerase. Wnt16 was linearized with NcoI and labeled with SP6 polymerase, and Fzd3 was linearized with SphI and labeled with SP6 polymerase. Probes were diluted 1:50 and hybridized at 50°C overnight. Probes for Wnt6, Fzd6, Axin2 and Dvl2 were obtained from embryonic pituitary cDNA libraries (Carninci et al., 2003). Wnt6 was linearized with NotI and labeled with T3 polymerase and hybridized at 55°C. Fzd6, Axin2, and Dvl2 were linearized with SalI, labeled with T3, and hybridized at 53°C. A plasmid containing Fgf10 was a gift from Brigid Hogan (Duke University Medical Center) and a probe was generated using BamHI to linearize and T3 polymerase to label. A plasmid containing Hesx1 was a gift from Paul Q. Thomas (University of Adelaide, Australia). An anti-sense probe was generated by BamHI linearization and labeling with T3 polymerase, and sense probe generated with EcoRI and T7. For negative controls, sense probes were generated or no probe was added for hybridization. Hematoxylin and eosin staining were performed as previously described (Cha et al., 2004).
RT-PCR
RNA was isolated from Rathke's pouches dissected from wild-type embryos at e12.5 and e14.5, and from wild-type adult pituitaries using the Trizol method per manufacturer directions (Invitrogen, Carlsbad, CA). 5μg total RNA was treated for 30 minutes with DNaseI (Promega) and purified with the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was generated using a SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) as suggested by the manufacturer. 1μl of resulting cDNA was used as template in a 25μl PCR reaction. Intron-spanning primers were used to amplify PCR products on a Mastercycler gradient PCR machine (Eppendorf, New York, NY). The PCR was performed under the following conditions: 92°C for 3 minutes, followed by 40 cycles of 92°C for 30 seconds, annealing temperature for 45 seconds, and 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. Primer sets are listed 5′-3′ as follows: Wnt2b GAGGAGGCGATATGATGG, AGTCAGAGGCTTGAAGTG; Wnt3 GCTGCCAAGAGTGTATTCG, CCTGTTCTGTTGCGGTAG; Wnt10b CTGTTCTTGGCTTTGTTCAGTCG, CAGAGTTGCGGTTGTGGGTATC; Wnt11 AAGGACTCAGAACTTGTGTATC, CCTGGTGTGGTGTCTTCC; Wnt16 GACCGAATGTTCCTGTGAC, CGTAGCAGCACCAGATAAAC; Fzd1 CCGGCCGGCTGAGCTTGGAACT, CAGGCGCGTACATGGAGCACAGGA; Fzd2 TCGCCTGCTACTTCTATGAG, ACCTGGGAGAGGGGAAAG; Fzd3 GGATGACCAAAGAAGCAAAGC, GGATGACCAAAGAAGCAAAGC; Fzd4 TACATCTGGGTGAAGAGGAGCCTG, CTGCCAAAAACCAAGTGAGTGTC; Fzd6 CGGAATGGCAGGGAAAGC, TGTACCACTGGGCTACTCTC; Fzd8 TGCCCTGCCACAACCCCTTCTTTA, CAGCGCGGGGCCAGTGGTCTCATA. Wif1 primers were as described (Heller et al., 2002). Wise and Sost primers were as described (Yanagita et al., 2006). PCR products were purified using a QiaExII Gel Extraction Kit (Qiagen) and sequenced to confirm their identity. Positive control cDNA was generated from e12.5 head, e12.5 body, e14.5 head, e14.5 body, liver, kidney, lung and testis and used as positive controls for each primer set. HPRT primers were used to determine quality of the cDNA and as a negative control on non-transcribed RNA.
Acknowledgments
We would like to thank Dr. Brian Gummow in the lab of Dr. Gary Hammer and Dr. Blair Madison in the lab of Dr. Deborah Gumucio for providing RT-PCR primer sequences. We are grateful to Dr. Nicola Solomon, Dr. Shannon Davis and Dr. Gary Hammer for their helpful comments, Dr. Kristin Douglas for RNA samples, Dr. Donald Swidersky for statistical analysis, and the National Hormone Pituitary Program and Dr. Parlow for pituitary hormone-specific antibodies. This work was supported by NIH grants R37-HD30428 and R01-HD34283 (S.A.C.), the Cellular and Molecular Approaches to Systems and Integrative Biology Training Grant 2-T32-BM0832213 (K.B.C.) and the Medical Scientist Training Program Grant GM07863 (K.B.C.).
Grant Sponsor: NIH; Grant number R37-HD30428 to S.A.C.
Grant Sponsor: NIH; Grant number R01-HD34283 to S.A.C.
Grant Sponsor: Cellular and Molecular Approaches to Systems and Integrative Biology; Grant number 2-T32-BM0832213 to K.B.C.
Grant Sponsor: Medical Scientist Training Program; Grant number GM07863 to K.B.C.
References
- Asbreuk CH, van Doorninck JH, Mansouri A, Smidt MP, Burbach JP. Neurohypophysial dysmorphogenesis in mice lacking the homeobox gene Uncx4.1. J Mol Endocrinol. 2006;36:65–71. doi: 10.1677/jme.1.01831. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Cha KB, Gridley T, Stifani S, Meeldijk J, Clevers H, Camper SA. TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17:2152–2161. doi: 10.1210/me.2003-0225. [DOI] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Davis SW, Camper SA. TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Camper SA. Sox3 and sexual dysfunction: it's in the head. Nat Genet. 2004;36:217–219. doi: 10.1038/ng0304-217. [DOI] [PubMed] [Google Scholar]
- Camper SA, Saunders TL, Katz RW, Reeves RH. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics. 1990;8:586–590. doi: 10.1016/0888-7543(90)90050-5. [DOI] [PubMed] [Google Scholar]
- Carninci P, Waki K, Shiraki T, Konno H, Shibata K, Itoh M, Aizawa K, Arakawa T, Ishii Y, Sasaki D, Bono H, Kondo S, Sugahara Y, Saito R, Osato N, Fukuda S, Sato K, Watahiki A, Hirozane-Kishikawa T, Nakamura M, Shibata Y, Yasunishi A, Kikuchi N, Yoshiki A, Kusakabe M, Gustincich S, Beisel K, Pavan W, Aidinis V, Nakagawara A, Held WA, Iwata H, Kono T, Nakauchi H, Lyons P, Wells C, Hume DA, Fagiolini M, Hensch TK, Brinkmeier M, Camper S, Hirota J, Mombaerts P, Muramatsu M, Okazaki Y, Kawai J, Hayashizaki Y. Targeting a complex transcriptome: the construction of the mouse full-length cDNA encyclopedia. Genome Res. 2003;13:1273–1289. doi: 10.1101/gr.1119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mech Dev. 2004;121:183–194. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19:1893–1903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Camper SA. Molecular basis of pituitary dysfunction in mouse and human. Mamm Genome. 2001;12:485–494. doi: 10.1007/s003350040002. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Barbera JP, Herman TS, Connell SO, Olson L, Ju B, Tollkuhn J, Baek SH, Rose DW, Rosenfeld MG. Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes Dev. 2001;15:3193–3207. doi: 10.1101/gad.932601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattani MT, Martinez-Barbera JP, Thomas PQ, Brickman JM, Gupta R, Martensson IL, Toresson H, Fox M, Wales JK, Hindmarsh PC, Krauss S, Beddington RS, Robinson IC. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet. 1998;19:125–133. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305:145–160. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas KR, Brinkmeier ML, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH, Jr, MacDougald OA, Camper SA. Identification of members of the Wnt signaling pathway in the embryonic pituitary gland. Mamm Genome. 2001;12:843–851. doi: 10.1007/s00335-001-2076-0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol. 2006;20:2796–2805. doi: 10.1210/me.2005-0303. [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Butts DL, Camper SA. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol. 2008;313:118–129. doi: 10.1016/j.ydbio.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Mol Endocrinol. 1996;10:1570–1581. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Gardner S, Maudsley S, Millar RP, Pawson AJ. Nuclear Stabilization of {beta}-catenin and Inactivation of Glycogen Synthase Kinase-3{beta} by Gonadotropin-Releasing Hormone: Targeting Wnt Signaling in the Pituitary Gonadotrope. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0268. [DOI] [PubMed] [Google Scholar]
- Gummow BM, Winnay JN, Hammer GD. Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin alpha gene. J Biol Chem. 2003;278:26572–26579. doi: 10.1074/jbc.M212677200. [DOI] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller RS, Dichmann DS, Jensen J, Miller C, Wong G, Madsen OD, Serup P. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev Dyn. 2002;225:260–270. doi: 10.1002/dvdy.10157. [DOI] [PubMed] [Google Scholar]
- Howng SL, Wu CH, Cheng TS, Sy WD, Lin PC, Wang C, Hong YR. Differential expression of Wnt genes, beta-catenin and E-cadherin in human brain tumors. Cancer Lett. 2002;183:95–101. doi: 10.1016/s0304-3835(02)00085-x. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Yoshimoto T. Developmental changes in proliferative activity of cells of the murine Rathke's pouch. Cell Tissue Res. 1991;263:41–47. doi: 10.1007/BF00318398. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993;7:852–860. doi: 10.1210/mend.7.7.8413309. [DOI] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Kendall SK, Gordon DF, Birkmeier TS, Petrey D, Sarapura VD, O'Shea KS, Wood WM, Lloyd RV, Ridgway EC, Camper SA. Enhancer-mediated high level expression of mouse pituitary glycoprotein hormone alpha-subunit transgene in thyrotropes, gonadotropes, and developing pituitary gland. Mol Endocrinol. 1994;8:1420–1433. doi: 10.1210/mend.8.10.7531821. [DOI] [PubMed] [Google Scholar]
- Kennell JA, O'Leary EE, Gummow BM, Hammer GD, MacDougald OA. T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with beta-catenin to coactivate C/EBPalpha and steroidogenic factor 1 transcription factors. Mol Cell Biol. 2003;23:5366–5375. doi: 10.1128/MCB.23.15.5366-5375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JM, Gordon DF, Woodmansee WW, Sarapura VD, Ridgway EC, Wood WM. Growth arrest of thyrotropic tumors by thyroid hormone is correlated with novel changes in Wnt-10A. Mol Cell Endocrinol. 2005;238:57–67. doi: 10.1016/j.mce.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Liu W, Selever J, Lu MF, Martin JF. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development. 2003;130:6375–6385. doi: 10.1242/dev.00849. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynen G, Unmehopa UA, Hofman MA, Swaab DF, Hoogendijk WJ. Relation between corticotropin-releasing hormone neuron number in the hypothalamic paraventricular nucleus and depressive state in Alzheimer's disease. Neuroendocrinology. 2007;85:37–44. doi: 10.1159/000100582. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PJ, Hanson JC, Quets-Nguyen AT, Bergeron M, Smith RC. A quantitative method for analysis of in vitro neurite outgrowth. J Neurosci Methods. 2007;164:350–362. doi: 10.1016/j.jneumeth.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Paez-Pereda M, Giacomini D, Refojo D, Nagashima AC, Hopfner U, Grubler Y, Chervin A, Goldberg V, Goya R, Hentges ST, Low MJ, Holsboer F, Stalla GK, Arzt E. Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proc Natl Acad Sci U S A. 2003;100:1034–1039. doi: 10.1073/pnas.0237312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–4239. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36:247–255. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Lovell-Badge R. Early development of the pituitary gland: induction and shaping of Rathke's pouch. Rev Endocr Metab Disord. 2005;6:161–172. doi: 10.1007/s11154-005-3047-7. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H. Multistep control of pituitary organogenesis. Science. 1997;278:1809–1812. doi: 10.1126/science.278.5344.1809. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Solomon NM, Ross SA, Morgan T, Belsky JL, Hol FA, Karnes PS, Hopwood NJ, Myers SE, Tan AS, Warne GL, Forrest SM, Thomas PQ. Array comparative genomic hybridisation analysis of boys with X linked hypopituitarism identifies a 3.9 Mb duplicated critical region at Xq27 containing SOX3. J Med Genet. 2004;41:669–678. doi: 10.1136/jmg.2003.016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–337. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke's pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shackleford GM. Murine Wnt10a and Wnt10b: cloning and expression in developing limbs, face and skin of embryos and in adults. Oncogene. 1996;13:1537–1544. [PubMed] [Google Scholar]
- Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- Ward RD, Stone BM, Raetzman LT, Camper SA. Cell Proliferation and Vascularization in Mouse Models of Pituitary Hormone Deficiency. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- Watkins-Chow DE, Camper SA. How many homeobox genes does it take to make a pituitary gland? Trends Genet. 1998;14:284–290. doi: 10.1016/s0168-9525(98)01476-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yanagita M, Okuda T, Endo S, Tanaka M, Takahashi K, Sugiyama F, Kunita S, Takahashi S, Fukatsu A, Yanagisawa M, Kita T, Sakurai T. Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J Clin Invest. 2006;116:70–79. doi: 10.1172/JCI25445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Morales DC, Hermesz E, Lee WK, Pfaff SL, Westphal H. Reduced expression of the LIM-homeobox gene Lhx3 impairs growth and differentiation of Rathke's pouch and increases cell apoptosis during mouse pituitary development. Mech Dev. 2006;123:605–613. doi: 10.1016/j.mod.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]