Abstract
Warfarin, an anticoagulant, is used to prevent and treat thromboembolic disease. One of the drawbacks of this agent, also known as Coumadin (Bristol-Myers Squibb), is that it is difficult to administer at the correct dose as a result of its narrow therapeutic index, its tendency to cause bleeding, and the individual variability in patient response. Achieving safe and effective doses of warfarin therapy is both an urgent and important concern for many clinicians.
Recent research has focused on single-nucleotide polymorphisms (SNPs) of genes that encode two proteins: the cytochrome P450 2C9 enzyme and VKORC1 (vitamin K epoxide reductase complex). Studies suggest that CYP 2C9 influences warfarin metabolism, whereas VKORC1 plays a role in the pharmacodynamic response in expression of the enzymatic target of warfarin. Patients who carry CYP 2C9*2 and CYP 2C9*3 alleles tend to require lower warfarin maintenance doses because of their slowed metabolism compared with patients who carry the “wild-type” allele. Patients who carry the VKORC1 A haplotype tend to require lower wafarin maintenance doses as a result of a decreased expression of messenger RNA (mRNA), which produces the proteins necessary for the formation of VKORC1.
INTRODUCTION
Pharmacogenomics has become an area of interest to clinicians because of the potential to tailor pharmacotherapy based on genetic variations in patients.1 This branch of pharmacology is concerned with the effect of a patient’s individual genetic profile on the efficacy and toxicity of drugs. Warfarin therapy is one of the many clinical situations in which a knowledge of pharmacogenomics can be applied. Traditionally, warfarin therapy is affected by the patient’s concurrent medication use, age, body weight, and liver function.2
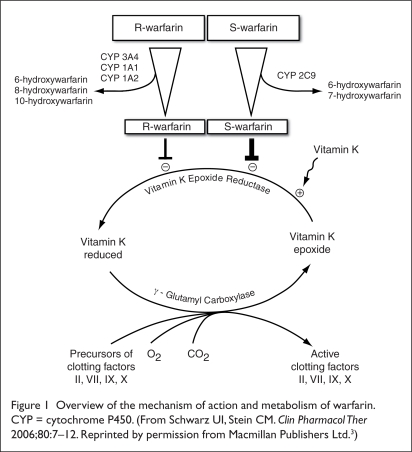
Warfarin is a racemic mixture of S and R enantiomers. The anticoagulant property of S-warfarin is much greater than that of R-warfarin. S-warfarin is metabolized mainly by CYP 2C9, which converts the drug into inactive metabolites; this is why the CYP 2C9 gene contributes to the variability in response of warfarin therapy. The metabolism of warfarin is illustrated in Figure 1.3
Figure 1.
Overview of the mechanism of action and metabolism of warfarin. CYP = cytochrome P450. (From Schwarz UI, Stein CM. Clin Pharmacol Ther 2006;80:7–12. Reprinted by permission from Macmillan Publishers Ltd.3)
SNPs are DNA variations in which a single nucleotide in a sequence differs between members of the same genome. SNPs often lead to the formation of different alleles of a gene. Of the 30 CYP 2C9 alleles discovered, CYP 2C9*2 (or 430 C>T) and CYP 2C9*3 (1075A>C) are the two alleles that are considered strong risk factors for overanticoagulation.4,5
The CYP 2C9*1 allele (wild-type) has the highest frequency, and it is the most common allele of the 30.6 The *2 allele yields CYP 2C9 proteins with 70% activity, and the *3 allele yields CYP 2C9 proteins with 20% activity.6 Patients who are carriers of these two alleles (*2 and *3) metabolize S-warfarin more slowly in vivo, which results in the need for lower warfarin maintenance doses, compared with patients who carry the wild-type allele. Moreover, it has been suggested that CYP2C9 *5, *6, *8, and *11 may also slow S-warfarin metabolism.7
Allele frequencies vary among ethnic groups. For example, the CYP 2C9*2 allele is present in approximately 10% to 12% of Caucasians but is rare in African-Americans. Alleles other than *1 are uncommon in patients of Asian ancestry. The *3 allele is less common, present in 8% of Caucasians and negligible in African-Americans.6,7
VKORC1 is the vitamin K cycle enzyme that controls the regeneration of reduced vitamin K (KH2). It is an essential cofactor in the formation of clotting factors.5 Warfarin works by noncompetitively inhibiting VKORC1, thus blocking the clotting cascade. Many polymorphisms in VKORC1 that influence warfarin dosing have been identified. The most commonly studied SNPs of VKORC1 include the 1173 C>T (CC is the wild-type) and 1639 G>A alleles (GG is the wild type). They are associated with a lower level of expression of VKORC1 because of their decreased translation of mRNA into proteins.
VKORC1 polymorphisms are also classified by their haplotype, a combination of alleles at multiple locations transmitted on the same chromosome.8 The AA haplotype is homozygous for variant alleles. The AB haplotype is heterozygous for variant alleles, the BB haplotype is homozygous for normal alleles (wild type), and the AA, AB, and BB haplotypes are associated with low, intermediate, and high warfarin maintenance doses, respectively.8 Variations exist in terms of ethnicity and VKORC1 polymorphisms. For example, approximately 37% of European-Americans carry either the 1173 C>T allele or the 1639 G>A allele. Approximately 85% to 90% of Asians are homozygous for low-dose VKORC1 alleles.
LITERATURE FINDINGS
Khan et al.9
In 2003, Khan and associates described two case reports that made clinicians aware of the influence of genetics on warfarin sensitivity.
Case 1. An 88-year-old woman received warfarin 10 mg daily for a pulmonary embolism. On the sixth day of therapy, her International Normalized Ratio (INR) peaked at 9.2 before returning to below 2 on day 11 following two separate 2-mg doses of intravenous (IV) vitamin K. Subsequent genotyping revealed that the patient was homozygous for the CYP 2C9*3 allele, which is implicated in overanticoagulation.9
Case 2. An 85-year-old woman who presented with an iliofemoral vein thrombosis was given warfarin 10 mg on day 0 and 5 mg on day 1. On the fourth day, her INR peaked at 13.2 before returning to below 2 on day 8 following two separate 0.5-mg doses of IV vitamin K. Subsequent genotyping revealed that the patient was heterozygous for the CYP 2C9*2 allele, which is implicated in overanticoagulation.9
At the time, these two reports suggested that a further inquiry into the role of pharmacogenomics in warfarin therapy was needed.
Schwarz et al.10
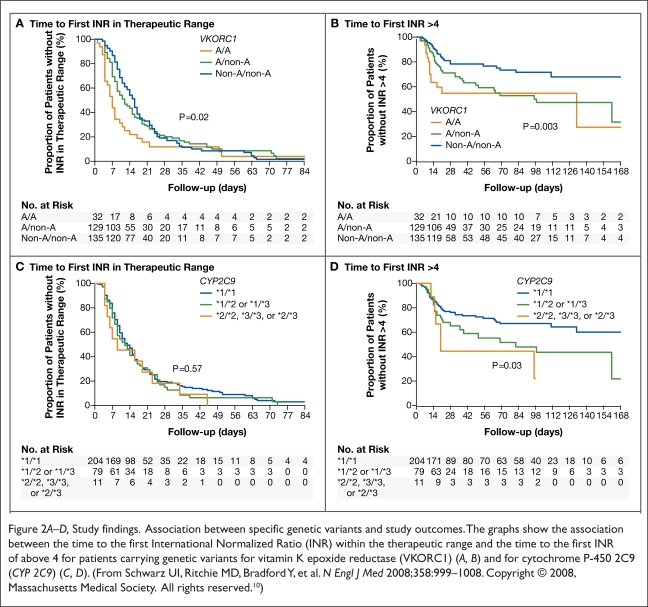
Schwarz and colleagues conducted a retrospective study at three anticoagulation clinics affiliated with Vanderbilt University Medical Center. The investigators assigned 297 patients to the VKORC1 haplotype group A, the VKORC1 haplotype group B, the CYP 2C9*2 allele group, or the CYP 2C9*3 allele group.
The two primary endpoints were the time from initiation of warfarin to the first therapeutic INR (defined as T1) and the time from initiation of warfarin to first INR measured above 4 (defined as T2). Patients with one or two VKORC1 haplotype A alleles had a shorter T1 and T2 than patients with two haplotype B alleles. By contrast, the CYP 2C9 genotype did not significantly affect T1. However, patients who were carriers of CYP 2C9*2 and CYP 2C9*3 alleles had a shorter T2 than carriers of the wild-type allele CYP2C9*1. The results are summarized in Figure 2 and Table 1. Patients with two VKORC1 haplotype A alleles were more prone to supratherapeutic INRs than patients with the wild-type allele.
Figure 2.
A–D, Study findings. Association between specific genetic variants and study outcomes. The graphs show the association between the time to the first International Normalized Ratio (INR) within the therapeutic range and the time to the first INR of above 4 for patients carrying genetic variants for vitamin K epoxide reductase (VKORC1) (A, B) and for cytochrome P-450 2C9 (CYP 2C9) (C, D). (From Schwarz UI, Ritchie MD, Bradford Y, et al. N Engl J Med 2008;358:999–1008. Copyright © 2008, Massachusetts Medical Society. All rights reserved.10)
Table 1.
Study Findings: Median Times to the First International Normalized Ratio (INR) Within the Therapeutic Range and to the First INR of More Than 4
| Haplotype or Genotype | Median time to First INR within Therapeutic Range (Days) | Median Time to First INR > 4 (Days) |
|---|---|---|
| VKORC1 | ||
| Non-A/Non-A | 15 | 23 |
| Non-A/A | 11 | 20 |
| A/A | 7 | 17 |
| CYP 2C9 | ||
| *1/*1 | 12 | 22 |
| *1/*2 or *1/*3 | 11 | 19 |
| *2/*2, *3/*3, or *2/*3 | 9 | 18 |
From Schwarz UI, Ritchie MD, Bradford Y, et al. N Engl J Med 2008;358:999–1008. Copyright © 2008, Massachusetts Medical Society. All rights reserved.10
Li et al.11
Li and coworkers conducted a prospective cohort study based in Chapel Hill, North Carolina, to evaluate the association between SNPs in VKORC1 and CYP 2C9 and the average weekly warfarin dose required to maintain a target INR. In this study, 93 European-American patients were selected from an anticoagulation clinic database. This database included the indications, duration, the average dose, dose adjustments, and INR for warfarin therapy. Data for mean weekly warfarin doses were collected over a period of nearly 21 months. The target INR in most patients was reported as 2 to 3.
The authors found that the VKORC1 *1173, *1542, and *2255 alleles (three of the six alleles tested) were significantly associated with lower warfarin maintenance doses needed to achieve the target INR. There was no evidence to suggest that a CYP 2C9 polymorphism (*2 and *3 alleles) was associated with an average weekly dose of warfarin.11
Higashi et al.12
Higashi et al. conducted a retrospective cohort study at two anticoagulation clinics in Seattle to determine whether the CYP 2C9 variants *2 and *3 were associated with overanti-coagulation and bleeding events in warfarin-treated patients. Two hundred patients were enrolled in the study and received long-term warfarin therapy from April 30, 1990, to May 31, 2001. The main outcome measures were the patient’s anticoagulation status, measured by the time to the therapeutic INR; the rate of above-range INRs; and the time to stable warfarin dosing. It was concluded that patients with at least one variant allele had an increased risk of above-range INRs and a possible risk of overanticoagulation. Moreover, these patients, compared with patients carrying wild-type alleles, needed more time to achieve stable dosing, with an average difference of 95 days.
Finally, patients with CYP 2C9 variant alleles experienced a first bleeding event sooner than patients with wild-type alleles. The variant genotype increased the risk of bleeding during both the initiation and the maintenance phases of warfarin therapy.
Sconce et al.13
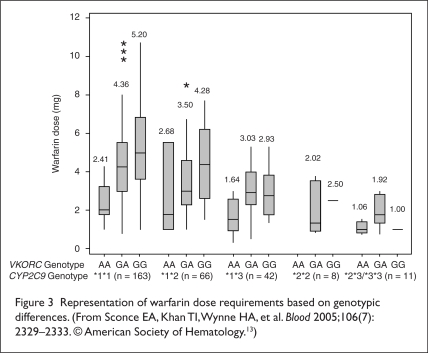
Although most warfarin dosing algorithms today don’t account for genetic variability or clinical characteristics such as age, height, and weight, the Sconce group conducted a retrospective cohort study that tried to incorporate both the genetic variability and the clinical characteristics into a potentially useful dosing algorithm. Patients were recruited from the Newcastle upon Tyne Hospitals National Health Service Trust. The study enrolled 297 patients.
The authors noted that the dose requirement for warfarin decreased as age increased, whereas this requirement increased as body weight, height, and body surface area increased. The authors concluded that the mean warfarin dose requirement was highest in patients with CYP 2C9 homozygous wild-type alleles compared with those with variant *2 and *3 alleles. The mean warfarin dose requirement was significantly higher in patients with the VKORC1 (−1639) GG (wild-type) genotype than in patients with the VKORC1 (−1639) AA genotype. The results are summarized in Figure 3.
Figure 3.
Representation of warfarin dose requirements based on genotypic differences. (From Sconce EA, Khan TI, Wynne HA, et al. Blood 2005;106(7): 2329–2333. © American Society of Hematology.13)
The patient’s warfarin dose, age, body surface area, and CYP 2C9 genotype contributed significantly to S-warfarin clearance. Only age, body size, and warfarin dose contributed to R-warfarin clearance. The patient’s sex, as well as the VKORC1 genotype, did not contribute significantly to overall warfarin clearance. The results are summarized in Table 2.
Table 2.
Representation of P Values of Characteristics Affecting Warfarin Clearance From Regression Analysis
| Variable | R-Warfarin Clearance | S-Warfarin Clearance | Total Warfarin Clearance |
|---|---|---|---|
| Warfarin dose | 0.001 | 0.001 | 0.001 |
| Age | 0.001 | 0.04 | 0.001 |
| Body surface area | 0.001 | 0.002 | 0.001 |
| CYP 2C9 genotype | 0.696 | 0.001 | 0.005 |
| VKORC1 genotype | 0.426 | 0.777 | 0.426 |
| Sex | 0.297 | 0.665 | 0.450 |
| Height | 0.241 | 0.999 | 0.472 |
| Weight | 0.205 | 0.727 | 0.961 |
CYP = cytochrome P450.
From Sconce EA, Khan TI, Wynne HA, et al. Blood 2005;106(7):2329–2333. © American Society of Hematology.13
Zhu et al.4
Zhu and associates performed a retrospective data review in 2007 to evaluate the role of CYP 2C9 and VKORC1 polymorphisms in maintenance doses of warfarin. Sixty-five Caucasian patients were enrolled, and all were receiving warfarin for atrial fibrillation. The target INR was between 2 and 3. The authors determined that there was a statistically significant difference in average daily warfarin doses between the various VKORC1 (−1639) G>A genotypes as follows: 6.3 mg for the GG genotype (wild type), 4.3 mg for the GA genotype, and 2.7 mg for the AA genotype.
This finding supports the suggestion that patients with the AA genotype have a lower expression of VKORC1 compared with the wild-type genotype, thus necessitating lower warfarin doses. Of the 25 patients who required warfarin doses of less than 4 mg, 23 were found to be carriers of either CYP 2C9 *2 or *3 alleles, suggesting that patients with a reduced metabolism require lower warfarin doses.4
Lindh et al.14
A prospective case–control study involving patients in the Warfarin Genetics Study (WARG) was conducted to analyze the genetic risk factors for bleeding events. Lindh’s team recruited warfarin-naive patients at anticoagulation clinics. Patients who were younger than 18 years old, those with contraindications to warfarin therapy, and those previously treated with warfarin were excluded from the study.
The primary endpoint was overanticoagulation during the first three weeks of warfarin treatment. Overanticoagulation was defined as the occurrence of at least one supratherapeutic INR in any given week. The *2 and *3 allele groups were compared with the *1 allele group for over-anticoagulation.
In week 1, patients with the *3 allele were six times more likely to have an INR above 3 and patients with the *2 allele were almost three times more likely to have an INR above 3. During week 3, patients with the *2 and *3 alleles had nearly the same incidence of INRs above 3 as did the patients with the wild-type allele.
Overall, the incidence of having an INR above 3 was 2.2 times higher for *3 group and 1.8 times higher for *2 group. The results are summarized in Table 3. This study suggests that pharmacogenetic monitoring of warfarin is more useful during the initial stages of therapy.
Table 3.
Study Results
| CYP 2C9*1 | CYP 2C9*2 | CYP 2C9*3 | |
|---|---|---|---|
| Week 1 | 1.0 | 2.8 (1.2–6.7) | 6.1 (2.7–13.6) |
| Week 2 | 1.0 | 2.1 (1.2–3.7) | 3.5 (2.1–5.8) |
| Week 3 | 1.0 | 1.0 (0.5–1.8) | 1.1 (0.6–2.0) |
| Weeks 1 to 3 | 1.0 | 1.8 (1.2–2.5) | 2.2 (1.6–3.0) |
CYP = cytochrome P450.
Reproduced from Lindh JD, Lundgren S, Holm L, et al. Clin Pharmacol Ther 2005;78:540–550. Reprinted by permission from Macmillan Publishers Ltd.14
Anderson et al.15
A prospective, randomized clinical trial of 200 patients was undertaken to evaluate the differences between genotype-guided warfarin therapy and the warfarin nomogram standard 10-mg regimen of Kovacs et al. In the Kovacs nomogram, 10 mg of warfarin is given daily on days 1 and 2, followed by a subsequent daily maintenance dose of 5 mg. Based on an INR measurement at day 5, an adjusted dose is given on days 5 to 7. The INR is checked on day 8, and the dose may be adjusted again. INRs after day 8 are measured on days 21, 60, and 90. Patients were enrolled in the Anderson study if they were older than 18 years of age, had an indication for anticoagulation with a target INR of 2 to 3, and had given written informed consent. Patients who were pregnant or lactating, who had participated in other trials within 30 days, who were taking rifampin within the previous three weeks, and who had conditions not conducive to a target INR of 2 to 3 were excluded from the study.
The Anderson trial was designed to give both the control arm (the Kovacs 10-mg warfarin nomogram group) and the pharmacogenetic-guided arm twice the maintenance dose on days 1 and 2, followed by the respective daily maintenance dose based on INR levels. INRs were measured on days 0, 3, 5, 8, 21, 60, and 90. Dosing for the pharmacogenetic-guided group was based on regression analysis incorporating the patient’s CYP 2C9 genotype, VKORC1 allele, age, weight, and sex. This trial was designed to last up to three months.
The primary endpoint was the reduced percentage of out-of-range INRs (between 1.8 and 3.2). Secondary endpoints included the time to the first supratherapeutic INR or the use of vitamin K; the percentage of time within the therapeutic INR range; the percentage of patients reaching therapeutic INR on days 5 and 8; the total number of INR measurements; the number of dose adjustments made; and the percentage of patients with serious adverse events in each group (e.g., INR of 4 or above, the use of vitamin K, and major bleeding events). This study failed to show that the pharmacogenetic-guided dosing regimen could reduce out-of-range INRs (P = 0.47). Results are summarized in Table 4.
Table 4.
Primary Endpoint Results
| Endpoint |
Pharmacogenetic Group (n = 101) Mean (SD) (%) |
Standard Group (n = 99) Mean (SD) (%) |
PValue |
|---|---|---|---|
| Out-of-range INRs* (all patients) | 30.7 (22.9) | 33.1 (22.9) | 0.47 |
| Out-of-range INRs (multiple-variant patients) | 31.0 (21.4) | 40.4 (25.4) | 0.14 |
| Out-of-range INRs (wild-type patients) | 28.1 (24.8) | 36.9 (25.3) | 0.21 |
| Out-of-range INRs (wild-type and multiple-variant patients) | 29.3 (23.4) | 39.1 (25.2) | 0.03 |
| Out-of-range INRs (single-variant patients) | 33.6 (22.1) | 27.0 (17.8) | 0.14 |
INR = International Normalized Ratio; SD = standard deviation.
Primary endpoint, intent-to-treat population.
From Anderson JL, Horne BD, Stevens SM, et al. Circulation 2007;116(22):2563–2570.15
For secondary endpoints, the pharmacogenetic-guided group had a statistically significant reduction in the number of required dose adjustments (P = 0.035). Differences in time to the first supratherapeutic INR, time in therapeutic range, and achieving a therapeutic range on days 5 and 8 favored the pharmacogenetic-guided group, but they were not statistically significant. The study did show that patients with multiple variant alleles were significantly more likely to have an INR above 4 and, therefore, adverse events (P = 0.029). Despite the shortcoming of not validating the primary endpoint, this study is an important stepping stone for further studies in warfarin pharmacogenomics.
DISCUSSION
Warfarin is a widely prescribed anticoagulant with a very narrow therapeutic index. It is important to recognize that dosing can be improved by identifying individual genetic properties. As our understanding of the various clinical characteristics of warfarin and of CYP 2C9 and VKORC1 genotypes has increased, as much as 60% of the variability in warfarin doses has been identified.5 In particular, VKORC1 haplotypes account for a three-fold greater effect on an individual’s warfarin dose requirements than CYP 2C9 polymorphisms.15 Studies have demonstrated that these genetic polymorphisms are associated with an increased risk of excess anticoagulation and unanticipated bleeding events.
Both VKORC1 and CYP 2C9 play an active role in the potential for predicting therapeutic warfarin doses. The role of genetic testing in warfarin patients is an important yet controversial topic. To date, no significant benefit of genetic testing in warfarin therapy has been determined by a prospective randomized clinical trial.
Genetic testing can be costly as well. It is estimated that about two million patients in the U.S. start taking warfarin every year. The annual cost of performing the genetic testing to identify CYP 2C9 and VKORC1 polymorphisms in these two million patients is approximately $1 billion.17
A pharmacoeconomic study, published by Eckman et al. in 2009,18 concluded that for the average 69-year-old man with nonvalvular atrial fibrillation at average risk for stroke and without specific bleeding risk factors, genotype-guided dosing of warfarin resulted in a gain of 0.0026 quality-adjusted life-years (QALY), or an additional day during the patient’s life span when compared with standard dosing. The Eckman article considered genetic testing to cost about $400 per patient and concluded that this modest QALY gain came at a high price with a marginal cost-effectiveness ratio of $172,000 per QALY gained. This far exceeds the accepted societal threshold of willingness to pay for medical therapy ($50,000 per QALY gained). It is notable that evidence supporting the efficacy of genetic-guided dosing is still lacking in the clinical literature and at present appears too costly to be recommended as a routine procedure at the initiation of warfarin therapy.
In August 2007, the FDA approved a label change to the warfarin package insert stating that “lower initiation doses should be considered for patients with certain genetic variations in CYP 2C9 and VKORC1 enzymes.”10 Warfarin has been added to a list of drugs, including 6-mercaptopurine (Purinethol, Gate), azathioprine (Imuran, GlaxoSmithKline), atomoxetine (Strattera, Lilly), and irinotecan (Camptosar, Pfizer), for which the FDA encourages health care professionals to consider the use of pharmacogenetic testing at the initiation of therapy.7
CONCLUSION
The role of VKORC1 and CYP 2C9 polymorphisms in warfarin therapy has been studied. The new and growing field of pharmacogenetics may one day enable clinicians to tailor a patient’s warfarin regimen. However, because pharmacogenetics is still in its infancy, more clinical trials, especially prospective randomized studies, are needed to gain a full understanding of the true ramifications in terms of the efficacy and cost of such gene-guided drug therapy.
Footnotes
Disclosure: The authors indicate that they have no financial or commercial relationships to report in regard to this article.
REFERENCESSS
- 1.Shurin SB, Nabel EG. Pharmacogenomics: Ready for prime time? N Engl J Med. 2008;358(10):1061–1063. doi: 10.1056/NEJMe0800801. [DOI] [PubMed] [Google Scholar]
- 2.Kamali F, King BP, Frearson R, et al. Contribution of age, body size, and CYP2C9 genotype to anticoagulant response to warfarin. Clin Pharmacol Ther. 2004;75:204–212. doi: 10.1016/j.clpt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz UI, Stein CM. Genetic determinants of dose and clinical outcomes in patients receiving oral anticoagulants. Clin Pharmacol Ther. 2006;80:7–12. doi: 10.1016/j.clpt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Shennan M, Reynolds KK, et al. Estimation of warfarin maintenance dose based on VKORC1 (−1639 G>A) and CYP 2C9 genotypes. Clin Chem. 2007;53(7):1199–1205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 5.Rettie AE, Tai GY. The pharmacogenomics of warfarin: Closing in on personalized medicine. Mol Interv. 2006;6(4):223–226. doi: 10.1124/mi.6.4.8. [DOI] [PubMed] [Google Scholar]
- 6.Hill CE, Duncan A. Overview of pharmacogenetics in anticoagulation therapy. Clin Lab Med. 2008;28(4):513–524. doi: 10.1016/j.cll.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Gage BF, Lesko LJ. Pharmacogenetics of warfarin: Regulatory, scientific, and clinical issues. J Thromb Thrombol. 2008;25:45–51. doi: 10.1007/s11239-007-0104-y. [DOI] [PubMed] [Google Scholar]
- 8.Limdi NA, Veenstra DL. Warfarin pharmacogenetics. Pharmacotherapy. 2008;28(9):1084–1097. doi: 10.1592/phco.28.9.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan T, Kamali F, Daly A, et al. Warfarin sensitivity: Be aware of genetic influence. Age Aging. 2003;32:226–227. doi: 10.1093/ageing/32.2.226. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz UI, Ritchie MD, Bradford Y, et al. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Lange LA, Li X, et al. Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. J Med Genet. 2006;43:740–744. doi: 10.1136/jmg.2005.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287(13):1690–1698. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 13.Sconce EA, Khan TI, Wynne HA, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: Proposal for a new dosing regimen. Blood. 2005;106(7):2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 14.Lindh JD, Lundgren S, Holm L, et al. Pharmacogenetics and genomics: Several-fold increase in risk of overanticoagulation by CYP2C9 mutations. Clin Pharmacol Ther. 2005;78:540–550. doi: 10.1016/j.clpt.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Horne BD, Stevens SM, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116(22):2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 16.Singh AJ, Emery JO. Pharmacogenomics: The potential of genetically guided prescribing. Aust Fam Physician. 2007;36(10):820–824. [PubMed] [Google Scholar]
- 17.Bussey HI, Wittkowsky AK, Hylek EM, et al. Genetic testing for warfarin dosing? Not yet ready for prime time. Pharmacotherapy. 2008;28(2):141–143. doi: 10.1592/phco.28.2.141. [DOI] [PubMed] [Google Scholar]
- 18.Eckman MH, Rosand J, Greenberg SM, et al. Cost effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med. 2009;150:73–83. doi: 10.7326/0003-4819-150-2-200901200-00005. [DOI] [PubMed] [Google Scholar]