Abstract
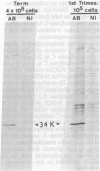
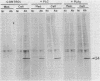
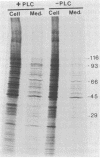
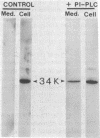
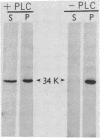
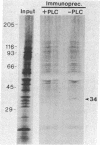
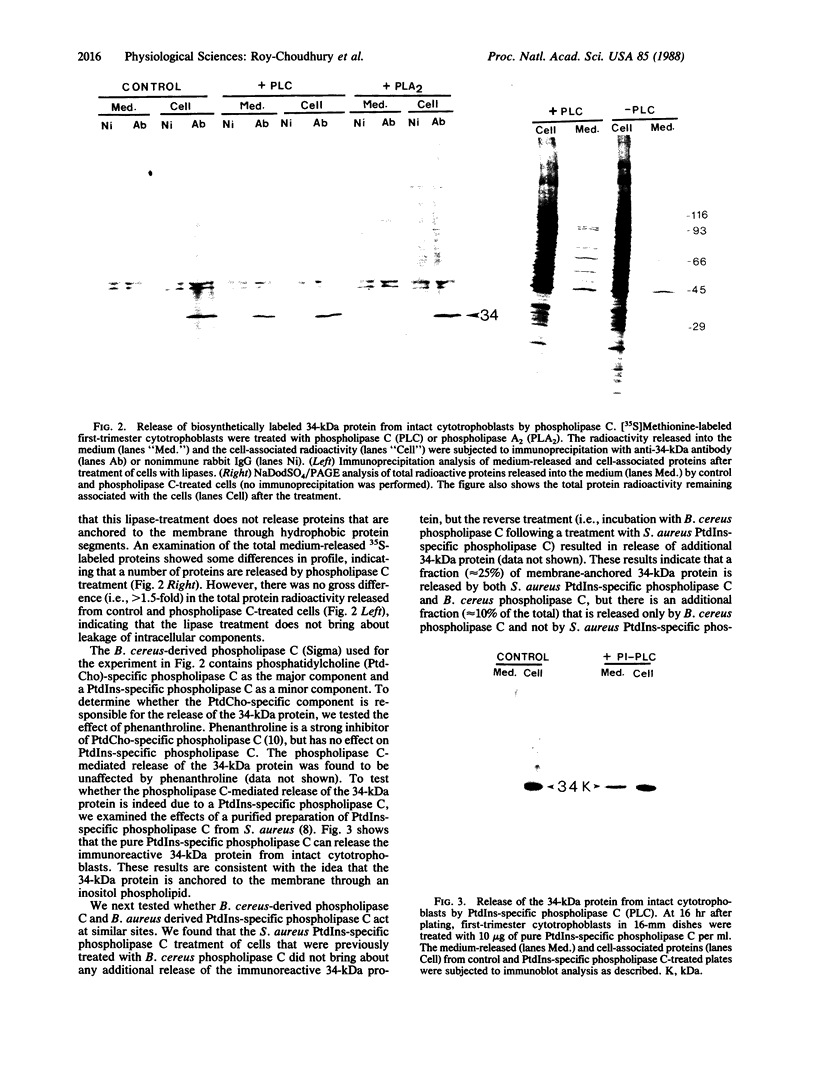
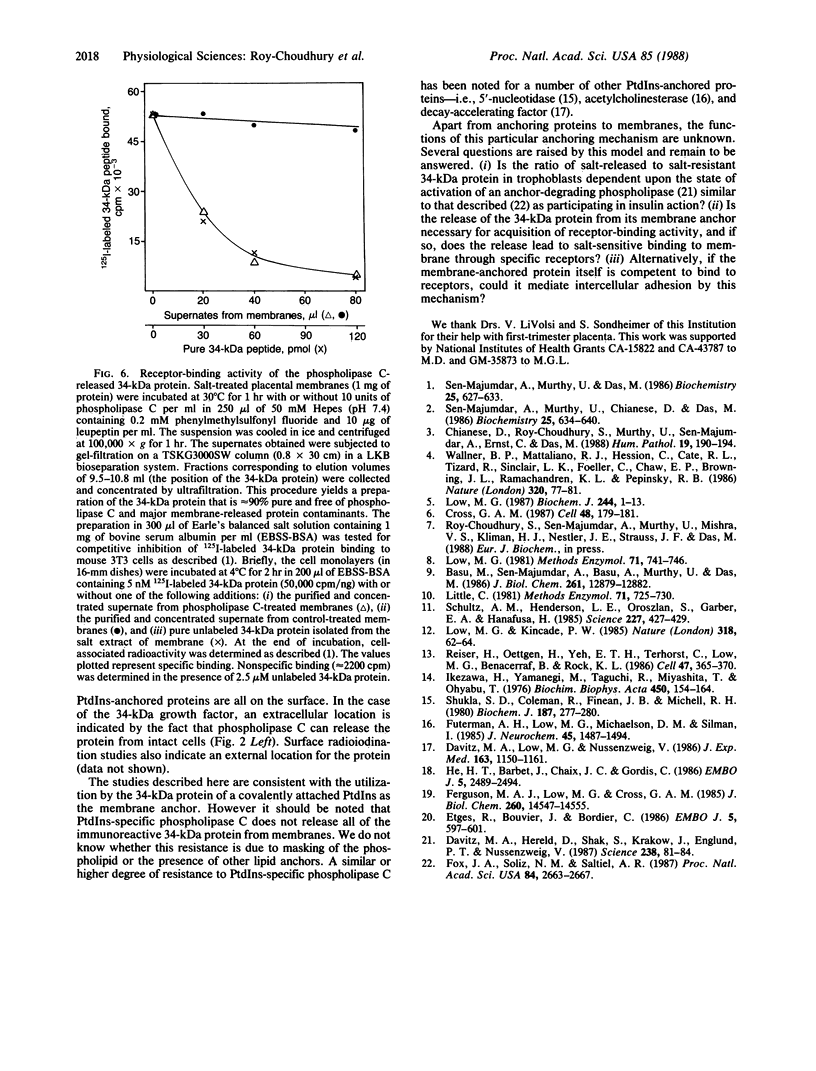
Recently we isolated a protein growth factor of 34 kDa from trophoblastic membranes of human placenta. A fraction (approximately equal to 50%) of the membrane-associated 34-kDa protein is peripherally associated--i.e., it can be released by high salt treatment. The remainder shows the characteristics of an integral membrane protein--i.e., its release requires detergent treatment. Here we report studies on the structural basis for membrane anchorage of the protein. Phospholipase C was found to release an immunoreactive 34-kDa polypeptide from intact isolated cytotrophoblasts. Studies with isolated trophoblastic membranes showed that phospholipase C specifically released the salt-resistant fraction of the 34-kDa polypeptide. The polypeptide released by phospholipase C showed the same electrophoretic mobility in NaDodSO4/PAGE as the polypeptide prior to phospholipase C treatment. The identity of the released protein with the 34-kDa growth factor has been established by both immunologic and receptor-binding assays. Other studies show that there is biosynthetic incorporation of [3H]myristate into the 34-kDa protein. The myristate label is labile to phospholipase C treatment. These results suggest that some of the 34-kDa protein is anchored to the plasma membrane via a posttranslationally added phospholipid. This mode of anchorage has been observed for some other membrane proteins and raises interesting questions regarding the role of this novel linkage in the mitogenic function of the 34-kDa polypeptide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu M., Sen-Majumdar A., Basu A., Murthy U., Das M. Regulation of kinase and intermolecular bonding in intact and truncated epidermal growth factor receptor. J Biol Chem. 1986 Sep 25;261(27):12879–12882. [PubMed] [Google Scholar]
- Chianese D., Roy-Choudhury S., Murthy U., Sen-Majumdar A., Ernst C., Das M. Cell- and tissue-specific expression of a 34,000-molecular-weight peptide growth factor in humans. Hum Pathol. 1988 Feb;19(2):190–194. doi: 10.1016/s0046-8177(88)80348-4. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Hereld D., Shak S., Krakow J., Englund P. T., Nussenzweig V. A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science. 1987 Oct 2;238(4823):81–84. doi: 10.1126/science.2443973. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Fox J. A., Soliz N. M., Saltiel A. R. Purification of a phosphatidylinositol-glycan-specific phospholipase C from liver plasma membranes: a possible target of insulin action. Proc Natl Acad Sci U S A. 1987 May;84(9):2663–2667. doi: 10.1073/pnas.84.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Michaelson D. M., Silman I. Solubilization of membrane-bound acetylcholinesterase by a phosphatidylinositol-specific phospholipase C. J Neurochem. 1985 Nov;45(5):1487–1494. doi: 10.1111/j.1471-4159.1985.tb07217.x. [DOI] [PubMed] [Google Scholar]
- He H. T., Barbet J., Chaix J. C., Goridis C. Phosphatidylinositol is involved in the membrane attachment of NCAM-120, the smallest component of the neural cell adhesion molecule. EMBO J. 1986 Oct;5(10):2489–2494. doi: 10.1002/j.1460-2075.1986.tb04526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa H., Yamanegi M., Taguchi R., Miyashita T., Ohyabu T. Studies on phosphatidylinositol phosphodiesterase (phospholipase C type) of Bacillus cereus. I. purification, properties and phosphatase-releasing activity. Biochim Biophys Acta. 1976 Nov 19;450(2):154–164. [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Low M. G. Phosphatidylinositol-specific phospholipase C from Staphylococcus aureus. Methods Enzymol. 1981;71(Pt 100):741–746. doi: 10.1016/0076-6879(81)71087-5. [DOI] [PubMed] [Google Scholar]
- Reiser H., Oettgen H., Yeh E. T., Terhorst C., Low M. G., Benacerraf B., Rock K. L. Structural characterization of the TAP molecule: a phosphatidylinositol-linked glycoprotein distinct from the T cell receptor/T3 complex and Thy-1. Cell. 1986 Nov 7;47(3):365–370. doi: 10.1016/0092-8674(86)90593-3. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Sen-Majumdar A., Murthy U., Chianese D., Das M. A specific antibody to a new peptide growth factor from human placenta: immunocytochemical studies on its location and biosynthesis. Biochemistry. 1986 Feb 11;25(3):634–640. doi: 10.1021/bi00351a018. [DOI] [PubMed] [Google Scholar]
- Sen-Majumdar A., Murthy U., Das M. A new trophoblast-derived growth factor from human placenta: purification and receptor identification. Biochemistry. 1986 Feb 11;25(3):627–634. doi: 10.1021/bi00351a017. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Coleman R., Finean J. B., Michell R. H. Selective release of plasma-membrane enzymes from rat hepatocytes by a phosphatidylinositol-specific phospholipase C. Biochem J. 1980 Apr 1;187(1):277–280. doi: 10.1042/bj1870277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]