Abstract
Purpose
From February 2001 to February 2002, 946 patients with advanced GI stromal tumors (GISTs) treated with imatinib were included in a controlled EORTC/ISG/AGITG (European Organisation for Research and Treatment of Cancer/Italian Sarcoma Group/Australasian Gastro-Intestinal Trials Group) trial. This analysis investigates whether the response classification assessed by RECIST (Response Evaluation Criteria in Solid Tumors), predicts for time to progression (TTP) and overall survival (OS).
Patients and Methods
Per protocol, the first three disease assessments were done at 2, 4, and 6 months. For the purpose of the analysis (landmark method), disease response was subclassified in six categories: partial response (PR; > 30% size reduction), minor response (MR; 10% to 30% reduction), no change (NC) as either NC− (0% to 10% reduction) or NC+ (0% to 20% size increase), progressive disease (PD; > 20% increase/new lesions), and subjective PD (clinical progression).
Results
A total of 906 patients had measurable disease at entry. At all measurement time points, complete response (CR), PR, and MR resulted in similar TTP and OS; this was also true for NC− and NC+, and for PD and subjective PD. Patients were subsequently classified as responders (CR/PR/MR), NC (NC+/NC−), or PD. This three-class response categorization was found to be highly predictive of further progression or survival for the first two measurement points. After 6 months of imatinib, responders (CR/PR/MR) had the same survival prognosis as patients classified as NC.
Conclusion
RECIST perfectly enables early discrimination between patients who benefited long term from imatinib and those who did not. After 6 months of imatinib, if the patient is not experiencing PD, the pattern of radiologic response by tumor size criteria has no prognostic value for further outcome. Imatinib needs to be continued as long as there is no progression according to RECIST.
INTRODUCTION
Imatinib mesylate has become the worldwide standard first-line treatment in patients with advanced GI stromal tumors (GISTs). GIST is frequently characterized by gain of function mutations of the KIT or platelet-derived growth factor receptor α and has become the first model of a solid tumor treated efficiently by a drug targeting the initial genetic alteration of this disease.1 Imatinib has revolutionized prognosis and therapeutic strategies in patients affected by advanced GIST.2–4 This small molecule tyrosine kinase inhibitor has dramatically improved the outcome of patients presenting with incurable advanced disease, from 25% 2-year overall survival (OS) before the imatinib era to 75% since the introduction of imatinib.5,6
Imatinib treatment results in 90% early tumor control, but the patterns of drug-induced radiologic changes are heterogeneous, and the classification into formal response categories according to RECIST (Response Evaluation Criteria in Solid Tumors) is often ambiguous and complex. In fact, this targeted therapy induces changes not only in lesion size but also in lesion structure, often resulting in a cyst-like appearance with no further decrease in size.7–9 About one third of patients show such tumor tissue density changes in tumor deposits. Although many investigators have assumed that only tumor size decreases according to WHO criteria or RECIST10,11 are a sign of desired drug effect, the early trials with imatinib12 demonstrated that this was incorrect. In fact, there are many examples, including the use of cytotoxic agents in other diseases, in which stable disease or no change (NC) is related to prolonged periods of freedom from progression.13,14
The EORTC-ISG-AGITG (European Organisation for Research and Treatment of Cancer/Italian Sarcoma Group/Australasian Gastro-Intestinal Trials Group) trial included 946 patients who were randomly assigned to imatinib 400 or 800 mg daily, and the primary end point of the study was progression-free survival (PFS).5 There was no difference between the two arms in terms of objective response rate according to RECIST or PFS after a median follow-up of 3 years.15 In both arms, about one third of patients exhibited stable disease according to RECIST observed on consecutive computed tomography (CT) scans performed every 2 months during the first 6 months of treatment and every 3 months thereafter.
The objective of this retrospective analysis was to evaluate the impact of response, as assessed by RECIST and by subclassifying stable disease by RECIST into groups reflecting small tumor changes, including minor responses (MRs; 10% to 30% tumor size reduction) or slight decrease (0% to 10%) or slight increase (0% to 20%) of tumor size, on prognosis in patients with advanced GIST treated with imatinib. The impact of the time to onset of objective response was also analyzed.
PATIENTS AND METHODS
Patient Population
Patients eligible for this analysis were included in a large randomized phase III trial comparing two daily doses of imatinib (400 v 800 mg) in patients with advanced and/or metastatic GIST characterized by c-KIT expression. A total of 946 patients (473 in each arm) were randomly assigned between February 2001 and February 2002.
Follow-Up Investigations
According to the protocol, the efficacy of imatinib was evaluated by CT scans performed after 2, 4, and 6 months, and every 3 months thereafter, until progressive disease (PD) in both therapeutic arms. Standard RECIST was used for evaluating response.11
Results of the Clinical Trial
Results of this trial were published elsewhere.5 Briefly, 5% of patients achieved a complete response (CR), 47% achieved a partial response (PR), and 32% had stable disease, with no difference between the two arms. Median time to first documentation of objective CR or PR was 4 months, and the cumulative incidence of response showed that 80% of those patients achieved their maximal tumor size regression in the first 9 months of imatinib treatment. At a median follow-up of 3 years, the difference between the arms in PFS is not significant,15 despite a statistically significant advantage in favor of the higher dose after a median follow-up of 2 years.5
Evaluation of Response
This analysis investigates which anatomic changes in tumor size enable prediction of further duration of PFS and OS in patients with advanced GIST treated with imatinib. The first three disease measurements were scheduled at 2, 4, and 6 months, and results of these three measurements were analyzed. Patients evaluable for each time point were those who were still being observed at that time point and who had not previously experienced PD. Results of the measurements were classified into six categories: CR and PR according to RECIST,11 MR (10% to 30% reduction), NC regression (NC−; 0% to 10% reduction), NC size increase (NC+; 0% to 20% increase), objective progression (PD; > 20% increase/new lesions), and subjective PD (clinical progression/no measurements).
Statistical Design
According to the landmark method, PFS and OS were computed from the date of disease measurement, and all patients experiencing PD or lost to follow-up before this date were subsequently excluded from the analysis.
PFS was measured from the date of measurement to the date of documented PD or death (whatever the cause). Patients alive and progression free at the last follow-up were censored. OS was measured from the date of measurement to the date of death. Patients alive at the time of analysis were censored at the date of last follow-up. Both end points were estimated by the Kaplan-Meier method. The log-rank test was used for comparison tests.
RESULTS
Pattern of Responses
A total of 946 patients were included in the analysis, and the median follow-up was 3.5 years. A total of 906 patients had measurable disease at entry, of whom 852 were evaluable for tumor size changes at 2 months, 681 at 4 months, and 642 at 6 months. The results of the radiologic response evaluation at the first three measurement points are summarized in Table 1.
Table 1.
Radiologic Response Evaluation at the First Three Measurement Points for Patients Still Observed and Without Prior Progression
| Response Category | 2 Months(n = 835) | 4 Months(n = 659) | 6 Months(n = 557) |
|---|---|---|---|
| CR/PR (> 30%) | 250 | 320 | 318 |
| MR (10%-30%) | 299 | 201 | 149 |
| NC− (0%-10%) | 76 | 48 | 31 |
| NC+ (0%-20% increase) | 123 | 60 | 46 |
| PD (> 20% increase/new lesions) | 83 | 30 | 12 |
| PD (clinical assessment) | 4 | 0 | 1 |
Abbreviations: CR, complete response; PR, partial response; MR, minor response; NC, no change; PD, progressive disease.
TTP and OS
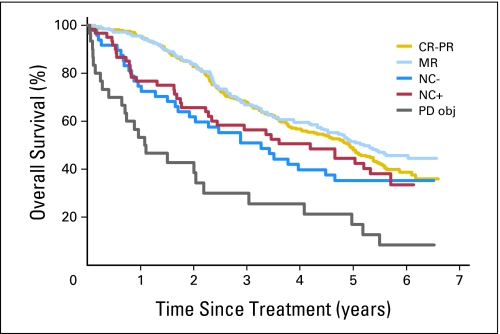
At all measurement time points, CR, PR, and MR resulted in similar time to progression (TTP) and OS; this was also true for NC− and NC+, and for objective as well as subjective progression. The results observed for all measurement time points are summarized in Table 2. As an example, for the evaluation at 4 months, the median PFSs in the six groups of patients were 2.16, 2.21, 1.19, 1.33, 0, and 0 years, respectively, whereas the 3-year OS estimates were 68.0%, 66.8%, 50.9%, 56.4%, 29.9%, and 0%, respectively. The OS according to response at 4 months of imatinib treatment is shown in Figure 1.
Table 2.
Median PFS and 3-Year OS Estimates According to Response Status (landmark method)
| Response Category | 2 Months |
4 Months |
6 Months |
|||
|---|---|---|---|---|---|---|
| Median PFS (years) | 3-Year OS (%) | Median PFS (years) | 3-Year OS (%) | Median PFS (years) | 3-Year OS (%) | |
| CR/PR | 2.27 | 66.8 | 2.16 | 68.0 | 2.09 | 71.6 |
| MR | 2.23 | 67.4 | 2.21 | 66.8 | 2.14 | 71.4 |
| NC− | 1.63 | 56.7 | 1.19 | 50.9 | 1.58 | 73.3 |
| NC+ | 1.07 | 50.1 | 1.33 | 56.4 | 1.16 | 63.8 |
| PD (objective) | — | 17.7 | 0 | 29.9 | 0 | 33.3 |
| PD (subjective) | — | 0 | 0 | 0 | 0 | 0 |
Abbreviations: PFS, progression-free survival; OS, overall survival; CR/PR, complete response/partial response; MR, minor response; NC−, no change (0% to 10% reduction); NC+, no change (0% to 20% size increase); PD, progressive disease.
Fig 1.
Overall survival according to response at 4 months of treatment with imatinib. CR, complete response; PR, partial response; MR, minor response; NC−, no change (0% to 10% reduction); NC+, no change (0% to 20% size increase); PD obj, objective progressive disease.
Novel Category of Response
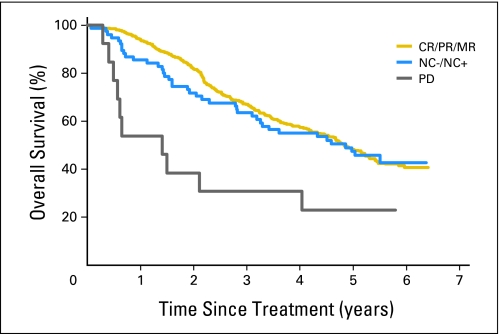
Patients were subsequently regrouped according to observed changes in tumor size or clinical progression. After 2 and 4 months of treatment, patients could be classified as responders (> 10% reduction of the tumor load), NC (< 10% reduction and < 20% increase), and nonresponders (> 20% increase, new lesions, or clinical progression). This new regrouping resulted in a high predictive value of further PFS and OS for the first two measurement time points. As an example, the 3-year OS estimates in these three categories of response after 4 months of imatinib treatment were 67.5%, 53.9%, and 29.9%, respectively (Table 3; Fig 2). Moreover, the outcome of patients according to these categories of response was similar in both therapeutic arms (data not shown) and at each measurement time point.
Table 3.
Median PFS and 3-Year OS Estimates in Grouped Categories According to Response Status (landmark method)
| Response Category | 2 Months |
4 Months |
6 Months |
|||
|---|---|---|---|---|---|---|
| Median PFS (years) | 3-Year OS (%) | Median PFS (years) | 3-Year OS (%) | Median PFS (years) | 3-Year OS (%) | |
| CR/PR/MR | 2.24 | 67.1 | 2.18 | 67.5 | 2.11 | 67.0 |
| NC | 1.32 | 52.7 | 1.33 | 53.9 | 1.43 | 63.5 |
| PD | — | 16.8 | — | 29.9 | — | 30.8 |
Abbreviations: PFS, progression-free survival; OS, overall survival; CR, complete response; PR, partial response; MR, minor response; NC, no change; PD, progressive disease.
Fig 2.
Overall survival according to grouped categories of response at 4 months of imatinib. CR, complete response; PR, partial response; MR, minor response; NC−, no change (0% to 10% reduction); NC+, no change (0% to 20% size increase); PD, progressive disease.
For the 6-month response assessments in the six groups of patients, the median TTP estimates were 2.09, 2.14, 1.58, 1.16, 0, and 0 years, respectively, while the 3-year OS estimates were 71.6%, 71.4%, 73.3%, 63.8%, 33.3%, and 0%, respectively (Table 2). Responders to imatinib had the same survival prognosis as patients with NC for both median TTP and 3-year OS. The median OS was not yet reached and was similar for each category of response in patients achieving a CR and those exhibiting a less than 20% progression of their disease. According to our new regrouping, responders (CR/PR/MR) to imatinib at 6 months have the same 3-year OS as those patients exhibiting stabilization (NC−/NC+)—67% v 63.5%, respectively (Fig 3). All patients exhibiting at least a nonprogression of their disease at 6 months according to RECIST have the same ultimate treatment outcome.
Fig 3.
Overall survival according to grouped categories of response at 6 months of imatinib. CR, complete response; PR, partial response; MR, minor response; NC−, no change (0% to 10% reduction); NC+, no change (0% to 20% size increase); PD, progressive disease.
Time to Onset of Response
Finally, we investigated whether the kinetics of tumor shrinkage influenced final OS of the patients. CRs and PRs documented for the first time at 2, 4, 6, 9, and 12 months resulted in similar TTP and OS values in both study arms. The kinetics of response had no prognostic value in patients with advanced GIST; the majority of CRs and PRs according to RECIST were documented during the first year of treatment.
DISCUSSION
RECIST has been specifically designed to document changes in tumor size in response to new drugs in early screening studies. With a few exceptions, RECIST has been found most useful in these studies.13 However, there has also been the misconception that only CR and PR according to RECIST are relevant signals of drug activity in a screening study. Screening phase II studies are used to identify agents that warrant further pivotal phase III studies. Even for cytotoxic drugs, it has become evident that lasting absence of progression can be a meaningful sign of drug activity.14 As a consequence, absence of progression is even more important than classifying the exact level decrease in tumor size in these screening studies.16,17 Response criteria such as RECIST were never intended to provide a surrogate end point for palliative effect in further stages of drug development (late phase II and III trials), although they have frequently been misused in this sense.
Given that clinical practice may be different from study practice, RECIST specifically stated11 that any one of the criteria may not be optimal for use in daily clinical practice as a single decision criterion to determine whether a patient is benefiting from a therapy. Yet, they have been widely adopted for this last purpose, in the absence of any other objective measure to identify patients who are benefiting from therapy. This study investigates whether and how radiologic assessments (CT scans) can be used to identify patients with GIST who do (and those who do not) benefit from treatment with imatinib.
Measuring tumor size only, as difficult as it may be, does not take into account the duration of any changes observed. The Soft Tissue and Bone Sarcoma Group of EORTC has highlighted the prognostic impact of the absence of tumor progression, which seems to be a highly relevant measure of benefit in patients with advanced soft tissue sarcomas (STS) treated either with new agents18 or with classic cytotoxic drugs.19,20 Actually, patients showing a prolonged stable disease have an outcome similar to those who experience a PR in STS, and the notion of tumor control rate measured by the progression-free rate or progression arrest rate has been implemented in recent prospective trials in STS.16,21
Although worldwide health authorities require objective assessment of the absence of tumor progression as a reproducible and quantifiable surrogate end point for survival in the evaluation of the efficacy of new compounds in the field of oncology, measurement of tumor size shrinkage has, for reasons that are unclear, remained an element of phase III studies, including studies exploring targeted therapies such as monoclonal antibodies and small molecule inhibitors, such as imatinib in GIST.
Similar to some cytotoxic agents,18 most molecularly targeted treatments have been reported to induce changes in the tumor structure, such as decreased tumor vascularity, hemorrhage, necrosis, or cystic or myxoid degeneration, that are consistent with therapeutic activity with or without a change in tumor volume.22 Combinations of morphologic (CT) and functional imaging techniques, such as [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET), have highlighted the discrepancy between the biologic (cellular level) and clinical (radiologic level) activities of imatinib in GIST.3,23,24 The median time to achieve a RECIST PR in our trial was 4 months,5 whereas a complete metabolic response, as measured by FDG PET, can be observed as early as 24 hours after the first intake of imatinib and in the vast majority of responding patients within the first 8 weeks of treatment, whereas the tumor volume only moderately decreases or even remains unaltered during this time.25–27
When these two different radiologic tumor assessments were applied to the same patients, imatinib was found to induce tumor response in only 23% of patients according to changes in tumor size (rate of RECIST objective response in our trial with CT) but in approximately 90% of patients according to metabolic response using FDG PET after 2 months of treatment with imatinib in patients with advanced GIST.7 The same findings are observed in the imatinib resistance period where a biologic resistance (increase of standardized uptake value on FDG PET; nodule in a mass) may be apparent between 6 and 12 months before a radiologic progression is confirmed according to RECIST.9
Our current exploratory analysis of the largest imatinib GIST trial published to date clearly confirms that absence of PD as assessed by RECIST indicates survival benefit and therefore can be used for treatment continuation decisions. However, within categories other than progression, RECIST less adequately distinguishes subgroups of patients with a different expectation of survival and therefore distinct imatinib activity/efficacy in GIST.
After 2 and 4 months of treatment, a reduction of at least 10% of the tumor load indicates major treatment benefit, and this confirms previously reported data.8 There is evidence of benefit even in patients showing a reduction of less than 10% or an increase of less than 20% of the tumor size, with a few possible exceptions, such as the appearance of a nodule in the mass9 that did not seem to affect the results in the larger group (intermediate sensitivity to imatinib according to our classification at 2 and 4 months). An FDG PET scan could be useful in such patients to discriminate reliably between presence or absence of benefit at an early stage.
After 6 months of treatment, patients with CR or PR according to RECIST had the same survival prognosis as patients classified in the stable disease category by RECIST (from < 30% reduction to < 20% increase). A similar trend existed at 2 and 4 months, respectively. Consequently, imatinib should be continued at the same dose even in patients who exhibit less than 20% tumor size increase after the second, fourth, or sixth month of treatment. Interestingly, the time to onset of CR or PR according to RECIST has no influence on PFS and OS.
In contrast to other advanced solid tumor models, achievement of a CR does not yield improved survival. Although CR may be related to cure in a few patients in some other diseases,28 it may not be the case in GIST. Interruption of imatinib in patients with a CR after 1 or 3 years of treatment results in a high risk of rapid (median 6 months) progression in patients with advanced GIST.29,30 The results of the BFR14 trial thus confirmed that absence of residual radiologic lesions in patients with GIST who respond to treatment with imatinib is not really relevant.
Patients experiencing a RECIST PD, whatever the time of assessment, have a significantly worse prognosis and are candidates for the high-dose imatinib regimen6,31 or a second-line targeted therapy,32 with the exception of the so-called false radiologic progression due to the appearance of new cystic lesions indicating imatinib-induced tumor necrosis in small nodules previously undetected with conventional imaging.
The use of the word response will have to be reconsidered. In essence, even a PD is a response to treatment, albeit an undesired one. What we are looking for is treatment-related benefit. Thus, we may have to redefine the description of this benefit.
As previously alluded to, assessing absence or presence of progression by RECIST has been helpful in identifying clinical benefit early with both cytotoxic drugs and molecularly targeted therapy, where a positive impact of these compounds has been observed on PFS despite a low tumor regression rate (< 10%).18,33,34 Given that the histologic response is not necessarily correlated to size changes in locally advanced STS treated with induction chemotherapy but significantly influences outcome of patients,35 the tissue response or the biologic response had to be evaluated and incorporated into radiologic assessments in the future, even in metastatic situations. The recently reported Choi criteria aimed to combine tumor volume response and biologic response.36 A decrease by at least 15% of tumor density on contrast-enhanced CT, as measured using Hounsfield units, and a diminution by at least 10% of the tumor size are sufficient to identify the patients with GIST benefiting from imatinib.36,37 Validation of these criteria on a large independent data set is urgently needed. Conversely, although using these more complex criteria identified the 88% of patients who benefitted from imatinib, our analysis shows that the same results can be achieved by the much simpler RECIST if we aim to distinguish only between absence or presence of PD.
To demonstrate that an agent has antitumor activity before any morphologic change in size can be observed, the assessment of activity may have to combine functional and morphologic imaging. In addition to the well-known role of FDG PET in the early evaluation of small molecule inhibitors in GIST, dynamic contrast-enhanced ultrasonography may be a less expensive and more reproducible tool that assesses both tumor size and structure with a functional study of macro- and microvascularization and perfusion using contrast agents and perfusion software.38 Indeed, a decrease of contrast uptake, assessed by this technique 7 and 14 days after the beginning of treatment, correlated with a good response and prolonged PFS. Similarly to PET, dynamic contrast-enhanced ultrasonography is capable of early detection of biologic resistance a few months before radiologic resistance during treatment and after imatinib interruption, and therefore could be implemented prospectively in imatinib trials, such as early dose-optimization trials with prospective mutation analysis.39 A limitation may be the dependence on the experience of the radiologist using the equipment. Major interobserver differences in standard ultrasound techniques have until now been the reason for excluding ultrasonography techniques as acceptable tools for response assessment. However, it is not inconceivable that with adequate training this problem may be overcome.
In conclusion, absence of progression according to RECIST turned out to be an excellent predictive marker of benefit in terms of PFS and OS. However, within the benefiting populations, the CR, PR, and MR classifications are not different from the NC classification, which strengthens recent suggestions that only discriminating between progression and nonprogression is relevant in this disease. After 6 months of therapy with imatinib, if the patient is not experiencing PD, the pattern of radiologic response has no prognostic value for further outcome. Imatinib needs to be continued as long as there is no PD according to RECIST. RECIST assessment can thus be used for screening studies as well as for practical decision making, as long as one only assesses PD according to the indicated criteria. The 6-month PFS rate is a more relevant activity screening end point than objective response in GIST.
Footnotes
Supported by an unrestricted grant from Novartis Oncology for European Organisation for Research and Treatment of Cancer/Italian Sarcoma Group/Australasian Gastro-Intestinal Trials Group Clinical Trial 62005; by Grant No. 5U10 CA11488-37 (through 5U10 CA011488-38) from the National Cancer Institute, Bethesda, MD; and by funding from Fonds Cancer (FOCA) in Belgium.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Peter Reichardt, Novartis (C), Pfizer (C); Paolo G. Casali, Novartis (C); Ian Judson, Novartis (C); Jean-Yves Blay, Novartis (C) Stock Ownership: None Honoraria: Axel Le Cesne, Novartis; Jaap Verweij, Novartis; Peter Reichardt, Novartis, Pfizer; Paolo G. Casali, Novartis; Ian Judson, Novartis; Serge Leyvraz, Novartis; Jean-Yves Blay, Novartis Research Funding: Ian Judson, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Axel Le Cesne, Jaap Verweij
Provision of study materials or patients: Axel Le Cesne, Jaap Verweij, Paolo G. Casali, Michael Findlay, Peter Reichardt, Rolf Issels, Ian Judson, Patrick Schoffski, Serge Leyvraz, Binh Bui, Jean-Yves Blay
Collection and assembly of data: Axel Le Cesne, Martine Van Glabbeke, Jaap Verweij, Paolo G. Casali, Peter Reichardt, Rolf Issels, Ian Judson, Patrick Schoffski, Raf Sciot, Jean-Yves Blay
Data analysis and interpretation: Axel Le Cesne, Martine Van Glabbeke, Jaap Verweij, Ian Judson, Pancras C.W. Hogendoorn, Jean-Yves Blay
Manuscript writing: Axel Le Cesne, Martine Van Glabbeke, Jaap Verweij, Ian Judson, Jean-Yves Blay
Final approval of manuscript: Axel Le Cesne, Martine Van Glabbeke, Jaap Verweij, Paolo G. Casali, Michael Findlay, Peter Reichardt, Rolf Issels, Ian Judson, Patrick Schoffski, Serge Leyvraz, Binh Bui, Pancras C.W. Hogendoorn, Raf Sciot, Jean-Yves Blay
REFERENCES
- 1.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 2.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 3.Demetri G, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 4.van Oosterom A, Judson I, Verweij J, et al. Safety and efficacy of imatimib (STI571) in metastatic gastrointestinal stromal tumors: A phase I study. Lancet. 2001;358:1421–1423. doi: 10.1016/s0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- 5.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 6.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 7.Choi H, Charnsangavej C, de Castro Faria S, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: A quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619–1628. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]
- 8.Holdsworth CH, Manola J, Badawi RD, et al. Use of computerized tomography (CT) as an early prognostic indicator of response to imatinib mesylate in patients with gastrointestinal stromal tumors. Proc Am Soc Clin Oncol. 2004;23:197s. abstr 3011. [Google Scholar]
- 9.Vanel D, Albiter M, Shapeero L, et al. Role of computed tomography in the follow-up of hepatic and peritoneal metastases of GIST under imatinib mesylate treatment: A prospective study of 54 patients. Eur J Radiol. 2005;54:118–123. doi: 10.1016/j.ejrad.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: WHO Offset Publication No. 48; 1979. [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors–expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Le Cesne A, Van Glabbeke M, et al. RECIST vs. WHO: Prospective comparison of response criteria in an EORTC phase II clinical trial investigating ET-743 in advanced soft tissue sarcoma. Eur J Cancer. 2005;41:1426–1430. doi: 10.1016/j.ejca.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Casali PG, Verweij J, Kotasek D, et al. Imatinib mesylate in advanced gastrointestinal stromal tumors (GIST): Survival analysis of the intergroup EORTC/ISG/AGITG randomized trial in 946 patients. Eur J Cancer. 2005;3(suppl):201. abstr 711. [Google Scholar]
- 16.Van Glabbeke M, Verweij J, Judson I, et al. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcoma. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 17.Booth CM, Calvert AH, Giaccone G, et al. Design and conduct of phase II studies of targeted anticancer therapy: Recommendations from the task force on methodology for the development of innovative cancer therapies (MDICT) Eur J Cancer. 2008;44:25–29. doi: 10.1016/j.ejca.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: A European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23:576–584. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 19.Le Cesne A, Judson I, Crowther D, et al. Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: A trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18:2676–2684. doi: 10.1200/JCO.2000.18.14.2676. [DOI] [PubMed] [Google Scholar]
- 20.Van Glabbeke M, van Oosterom A, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 21.Van Glabbeke M, Le Cesne A, Radford J, et al. Late responders to first line chemotherapy for advanced or metastatic soft tissue sarcomas (STS) have a longer survival than early responders: A retrospective analysis of the EORTC soft tissue and bone sarcoma group (STBSG) Proc Am Soc Clin Oncol. 2000;19:552a. abstr 2176. [Google Scholar]
- 22.Chen MY, Bechtold RE, Savage PD. Cystic changes in hepatic metastases from gastrointestinal stromal tumors (GISTs) treated with Gleevec (imatinib mesylate) AJR Am J Roentgenol. 2002;179:1059–1062. doi: 10.2214/ajr.179.4.1791059. [DOI] [PubMed] [Google Scholar]
- 23.Van den Abbeele AD, Badawi RD. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs) Eur J Cancer. 2002;38(suppl 5):S60–S65. doi: 10.1016/s0959-8049(02)80604-9. [DOI] [PubMed] [Google Scholar]
- 24.Stroobants S, Goeminne J, Seegers M, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–2020. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 25.Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal tumours: Best monitored with FDG PET. Nucl Med Commun. 2004;25:433–438. doi: 10.1097/00006231-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Antoch G, Kanja J, Bauer S, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–365. [PubMed] [Google Scholar]
- 27.Gayed I, Vu T, Iyer R, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17–21. [PubMed] [Google Scholar]
- 28.Blay JY, Van Glabbeke M, Verweij J, et al. Advanced soft-tissue sarcoma: A disease that is potentially curable for a subset of patients treated with chemotherapy. Eur J Cancer. 2003;39:64–69. doi: 10.1016/s0959-8049(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 29.Blay JY, Le Cesne A, Ray-Coquard I. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumours comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107–1113. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- 30.Le Cesne A, Ray-Coquard I, Bui BN, et al. Continuous vs interruption of imatinib in responding patients with advanced GIST alter three years of treatment: A prospective randomized phase III trial of the French Sarcoma Group. J Clin Oncol. 2007;25:546s. doi: 10.1200/JCO.2006.09.0183. abstr 10005. [DOI] [PubMed] [Google Scholar]
- 31.Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751–1757. doi: 10.1016/j.ejca.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 33.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: A retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 34.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Soto R, Auger N, Castaing M, et al. Can ERCC1 and topoisomerase II-alpha predict histological response and outcome after induction chemotherapy in locally advanced soft tissue sarcomas? J Clin Oncol. 2007;25:563s. abstr 10074. [Google Scholar]
- 36.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 37.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 38.Lassau N, Lamuraglia M, Chami L, et al. Gastrointestinal stromal tumors treated with imatinib: Monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol. 2006;187:1267–1273. doi: 10.2214/AJR.05.1192. [DOI] [PubMed] [Google Scholar]
- 39.Lassau N, Chami L, Benatsou B, et al. Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: A new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol. 2007;17(suppl 6):F89–F98. doi: 10.1007/s10406-007-0233-6. [DOI] [PubMed] [Google Scholar]