Abstract
Aminolevulinic Acid (ALA)-induced Protoporphyrin IX (PpIX) fluorescence was studied as a contrast agent for noninvasive detection of murine glioma, using the fluorescence to transmission ratio measured through the cranium. Signal measured prior to administration of ALA was very similar between control animals, 9L-GFP and U251 tumor-bearing animals. However, 2 hours after ALA administration, the PpIX signal from both tumor-bearing groups was significantly higher than the control group (9L-GFP group p-value = 0.016, and U251 group p-value = 0.004, relative to control group). The variance in signal from the 9L-GFP group was much larger than either the control group or the U251 group, which was consistent with higher intrinsic PpIX fluorescence heterogeneity as seen in situ at ex vivo analysis. Decreasing the skin PpIX fluorescence via intentional photobleaching using red light (635 nm) was examined as a tool for increasing PpIX contrast between the tumor-bearing and control groups. The red light bleaching was found to increase the ability to accurately quantify PpIX fluorescence in vivo, but decreased the specificity of detection between tumor-bearing and non-tumor-bearing groups.
INTRODUCTION
Aminolevulinic acid (ALA), the precursor to protoporphyrin IX (PpIX) has been under extensive study as a photosensitizer for photodynamic therapy (PDT) and for photodiagnosis (PD) in oncology for over 20 years [1]. While much of the focus with ALA detection of tumors has been in thin squamous tissues, perhaps one of the most successful applications of ALA-PpIX for fluorescence visualization, and certainly the most important to the work presented here, has been use of ALA-induced PpIX for surgical guidance of brain tumor resection due to demonstration of high PpIX signal in the tumor tissue as compared to the normal brain [2–4]. The use of this contrast agent to detect tumors noninvasively has seen little study, yet transmission of the fluorescence through bulk tissue up to several centimeters is readily feasible. With post processing of the signal to normalize for attenuation and provide spectral filtering to remove background, it is feasible to develop noninvasive tools for detection of tumors and tracking the production of PpIX at the tissue surface for a variety of orthotopic murine tumors both implanted and of transgenic origin. This study has focused on these aspects of the problem in the context of murine glioma.
Administration of ALA overloads the heme synthesis pathway, which exists in all mammalian cells, and fluorescently detectable levels of PpIX are produced [5–7]. ALA-induced PpIX is now used extensively in dermatologic applications, with approval for treatment of actinic keratosis in the US in 1999 [8]. Methyl-Aminolevulinic acid (MAL), an ester derivative of ALA, has been approved in Europe for treatment of skin lesions [8]. One confounding factor in detection is the heterogeneity of PpIX production between tumor sub-types and even within an individual tumor. PpIX production levels between various tumor lines in vitro have been found to be quite heterogeneous [9], however most brain tumor cell lines are known to have higher PpIX production than normal brain cells [10–12], indicating that there is likely always positive contrast relative to normal brain. This data agrees with ALA-induced PpIX fluorescence in vivo in preclinical models as well as clinical data, where significant differences in tumor tissue removal can be achieved through fluorescence guided surgery over conventional white light surgery [2, 13].
Extensive study on human glioma patients has been completed by Stummer, et al culminating in a multicenter clinical trial where ALA-induced PpIX fluorescence guided resection of human gliomas was compared to conventional white light resection. The contrast-enhancing portion of the tumor, as detected by magnetic resonance imaging, was completely removed in 65% of the fluorescence guided resections as compared to 36% of the white light surgeries. This increased 6 month progression free survival to 41% in the fluorescence guided resection group as compared to 21.1% in the white light resection group [13]. The ability to visualize PpIX fluorescence in brain tumor tissue over normal brain tissue via surgical guidance was motivating for this work on noninvasive detection of brain tumors due to high tumor to normal brain tissue PpIX fluorescence signals.
Following earlier studies completed in vitro [9], PpIX fluorescence was explored for its ability to delineate tumor-bearing animals from healthy animals in a noninvasive manner. Transmission fluorescence spectroscopy was used for noninvasive detection of PpIX fluorescence, while normalizing the signal to the transmitted excitation light signal [14]. Even through use of this transmission geometry, the PpIX fluorescence signal from the mouse skin was found to influence measurements, so skin photobleaching was explored as a method to increase PpIX signal contrast between tumor-bearing animals and healthy animals [15–17].
MATERIALS AND METHODS
Cell Culture
Two cell lines were used in the studies presented; the 9L rat gliosarcoma cell line which had been transfected with green fluorescent protein (9L-GFP) and the human glioma cell line U251. The 9L-GFP cell line was a generous gift from Alexi Bogdanov [18]. The cell lines were cultured in Dulbecco’s Modification of Eagle’s Medium (Cellgro, Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 1% penicillin/streptomycin from a stock solution of 10,000 IU penicillin and 10,000 µg/ml streptomycin (Mediatech, Inc., Herndon, VA), and were kept incubated at 37° Celsius in a 95% air and 5% carbon dioxide humidified environment.
Murine Glioma Model
Both cell lines (9L-GFP and U251) were used for intracranial implantation into male athymic nude mice about 6 weeks of age. Mice were anesthetized using ketamine/xylazine in a 90:10 mg/kg ratio and their body temperature was maintained during anesthesia via a heating pad. A small incision was made in the scalp exposing the top of the skull so that the landmarks on the brain were visible. A Dremel drill was used to make a 1 mm hole in the skull, located 2 mm in front of the bregma and 2 mm to the left of the midline, after which the needle was inserted 2 mm deep into the brain via guidance from a sterotactic frame. A total of 1×106 cells in 10 µl of phosphate buffered saline (PBS) were injected over 5 minutes using a Hamilton syringe. After the injection, the needle was slowly retracted, the skull was cleaned to ensure cells were not deposited outside the brain and bone wax was used to cover the hole drilled in the skull. Finally, the incision in the scalp was closed with a small amount of Vetbond Tissue Adhesive (J.A. Webster, Inc, Sterling, MA). Mice were examined daily following surgery to ensure proper healing of the scalp. Control mice were implanted with 10 µl PBS without cells to allow similar surgical procedures to be performed on all mice.
Single Channel Fluorescence Spectroscopy System
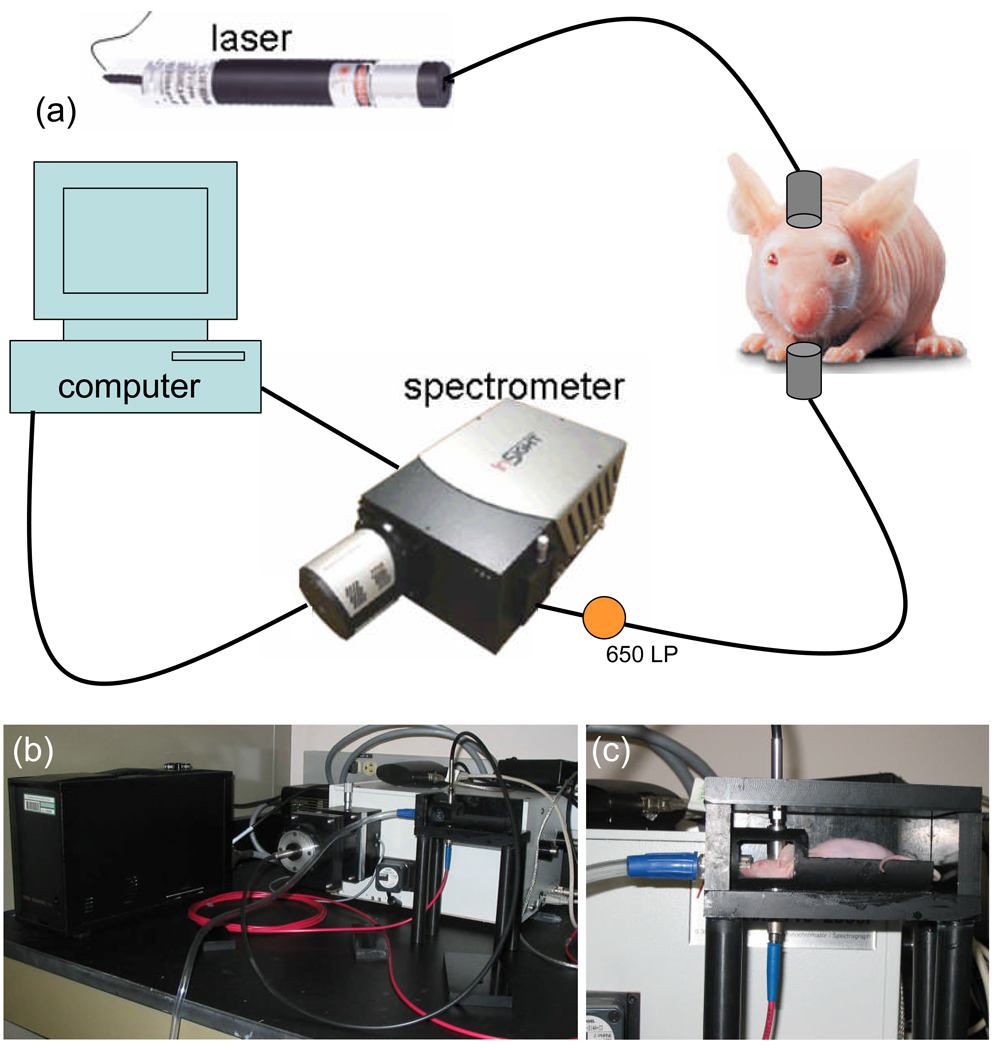
A schematic of the single channel spectroscopy system can be seen in figure 1(a). The system consisted of a 250 mW, 635 nm diode laser (Power Technology, Inc.) for excitation collimated onto the chin of the mouse. The light transmitted through the mouse head was collected through a second collimator, and passed through a 650 nm LP filter prior to spectrally resolved detection through a spectrometer (1200 l/mm grating, SpectraPro 300, Acton Research, Acton MA) and onto a cooled CCD camera (Spec-10:400BR/XTE, Princeton Instruments, Acton MA). Data from the camera was captured and transferred using commercial software (Winspec, Acton Research). The spectrometer was centered at 705 nm to collect the PpIX fluorescence emission peak and at 615 nm to collect the transmitted intensity from the 635 nm laser. A mouse holder consisted of a bed in a light tight box with holes 180° apart from one another to hold the collimators, for collection of spectroscopy data (Figure 1(b) & 1(c)).
Figure 1.
(a) A schematic of the single channel spectroscopy system is shown containing a 635 nm laser, a spectrometer, a 650 nm LP filter and a computer. In (b) a photograph of the system with the light tight box open is shown. In (c) a sample mouse is shown in the holder in position for measurement.
Spectroscopy Data Collect & Processing
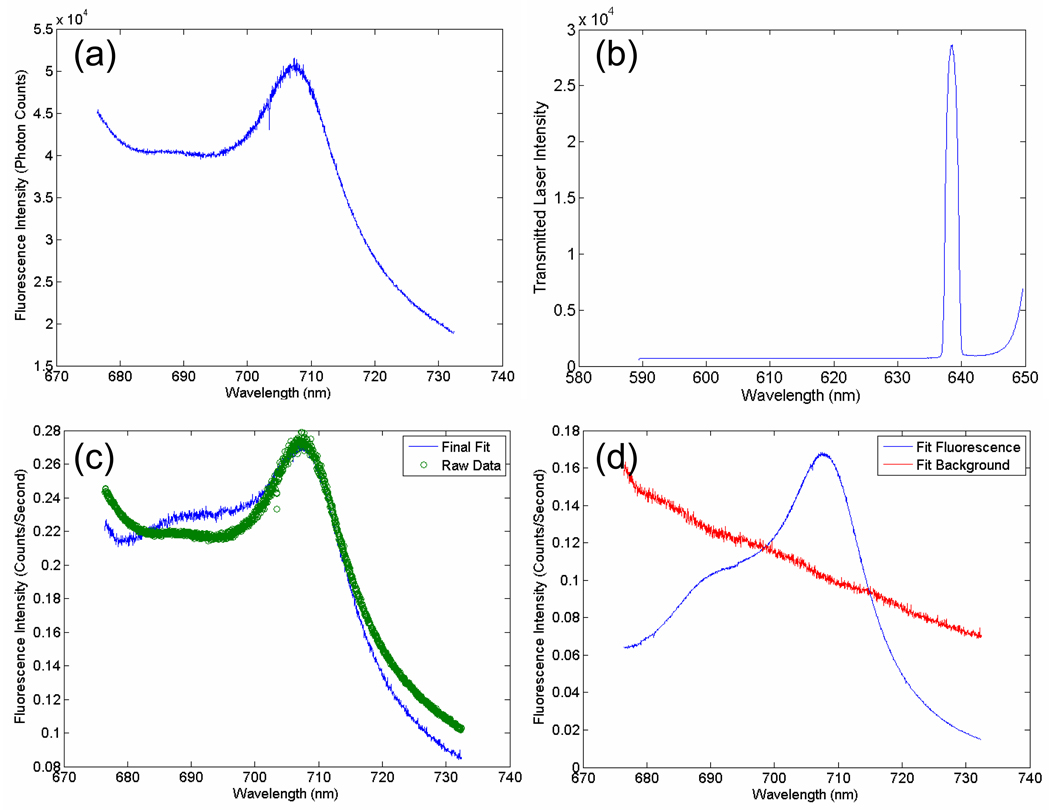
At each time point the mouse was placed in the mouse holder with the collimators in contact with the head. Fluorescence emission data was collected with the spectrometer centered at 705 nm and the exposure time adjusted to obtain signals in the linear range of the spectrometers with maximal signal to noise level. The light was passed through the 650 nm LP filter to collect the PpIX fluorescence emission prior to detection by the spectrometer (figure 2(a)). Prior to any movement of the mouse, transmittance data was also collected, where the spectrometer was centered at 615 nm to allow detection of the 635 nm laser intensity through the 650 nm LP filter and the exposure time was again adjusted to ensure detection in the linear range of the spectrometers (figure 2(b)).
Figure 2.
In (a) the PpIX fluorescence emission data is plotted for a sample mouse. In (b) the transmittance spectrum from the 635 nm laser through the head of a sample mouse is shown. In (c) the PpIX fluorescence data is spectrally fitted to a reference PpIX spectrum from tissue-simulating phantom data. In (d) the deconvolved fluorescence and non-specific background signals are shown.
The raw spectral excitation and emission data were post-processed by a two-step process involving spectral fitting and then normalization. PpIX fluorescence data was collected using a liquid tissue simulating phantom composed of 1% Intralipid to simulate scattering, 1 mg/L India ink to simulate absorption, 1 µg/mL PpIX in dimethyl sulfoxide to simulate in vivo PpIX concentrations and 5% Tween 20 to decrease aggregation of PpIX, a hydrophobic molecule, in solution. The spectral shapes of PpIX obtained from these tissue simulating phantoms were normalized to one and used for spectral fitting of in vivo PpIX data. The fluorescence data was spectrally fitted using a MatLab program to perform a linear least squares fit to the PpIX phantom data, so that the non-specific background signal could be deconvolved from the PpIX fluorescence signal (figure 2(c) & 2(d)), as it had a distinctly different spectral shape. The area under the deconvolved PpIX fluorescence curve was than calculated and reported as a single number. Both the fluorescence emission data and the transmittance data were normalized to counts/second to account for differences in exposure time. Then the integrated fluorescence intensity was normalized to the integrated transmitted laser intensity to account for positional differences between measurements of a single mouse as well as variations in optical properties [14], resulting in the fluorescence to transmittance ratio.
PpIX Detection of Brain Tumors
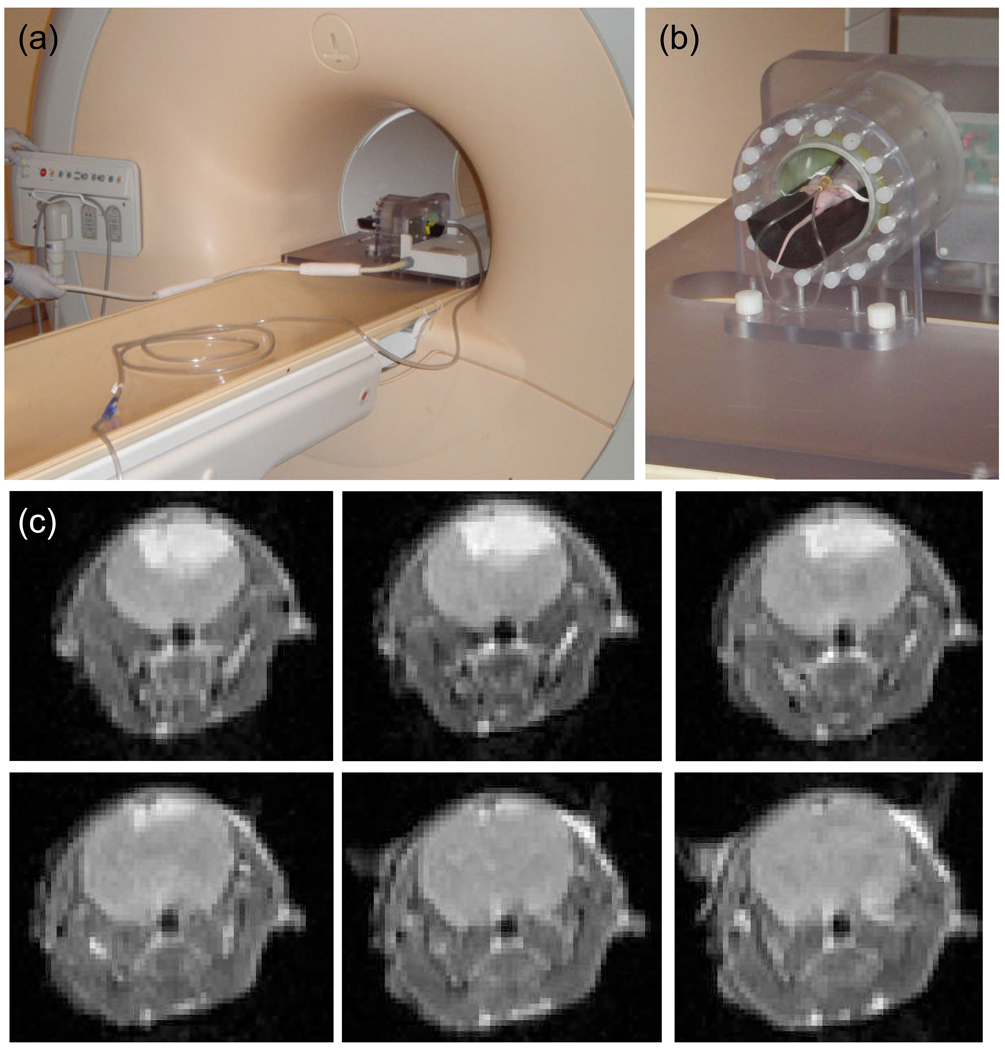
At 10 – 14 days after tumor implantation, the mice were imaged via a large bore Philips 3T Magnetic Resonance Imaging (MRI) Achieva system, using a modified rodent body coil insert [19]. A plastic insert was used in the rodent coil to raise the mouse into the isocenter of the magnetic field (Figure 3(a) & 3(b)). Intracranial tumors were identified using T1 turbo spin echo (TSE) images with and without Gadolinium enhancement (1 µl/mg body weight) (figure 3(c)) as well as T2 TSE imaging sequences. T1 TSE and T2 TSE images were also collected prior to sacrifice for in vivo and ex vivo tumor comparison.
Figure 3.
In (a) the rodent coil inside the 3T MRI is shown, and in (b) an example mouse in the rodent coil with IP catheter for Gd-DTPA injection is shown. In (c) a sample set of T1 coronal turbo spin echo contrast enhanced (TSE-CE) images are shown, for a 9L-GFP tumor.
Prior to ALA administration the mice were placed in the single channel spectroscopy mouse holder and their background PpIX fluorescence was measured while they were under inhaled isofluorane anesthesia. The mice were then administered 100 mg/kg ALA dissolved in PBS by intraperitonal (IP) injection. Two hours after ALA administration, the PpIX fluorescence was again measured using the single channel spectroscopy system. The mice were then sacrificed, their brains extracted and placed back into the spectroscopy system for ex vivo measurement of the tumor in situ. Following collection of all spectroscopy measurements, the brain was sectioned in a coronal plane and imaged on a fluorescence plate scanner (Typhoon 9410, GE Healthcare Life Sciences) with the cut faces of the brain facing the imaging plane of the scanner. The tissue slices were imaged for PpIX fluorescence using a 633 nm excitation laser and a 650 nm LP emission filter, and when appropriate then scanned for GFP fluorescence using a 488 nm laser with a 526 nm SP emission filter. Following fluorescence imaging, the brain slices were sent to pathology for routine H+E staining.
Red Light Time Course Photobleaching
Four control mice, nine 9L-GFP tumor-bearing mice and seven U251 tumor-bearing mice were compared in this study. In each group the tumors were implanted, identified via MRI and the PpIX fluorescence of each mouse was then measured using the single channel spectroscopy system as described previously. Following the measurement 2 hours after the administration of ALA, the 635 nm laser was left running and subsequent measurements were collected after 1, 2, 4, 8, 16, 24 and 32 minutes to allow for photobleaching of the skin PpIX fluorescence signal.
Statistical Analysis
Differences between all groups were established through box and whisker plots and unpaired 2-tailed students-t tests to establish p-values for comparison of all groups of interest. All box and whisker plots shown illustrate the median as the center line and the interquartile range as the shaded box showing 75% of the data. The upper whisker represents Q3 + 1.5(Q3 − Q1) and the lower whisker represents Q1 − 1.5(Q3 −Q1), where Q is the quartile. Each open circle represents the fluorescence to transmittance ratio of one mouse.
The ability to quantify PpIX fluorescence spectroscopy as a method to detect tumors was assessed by receiver operator characteristic (ROC) analysis. The values for sensitivity and specificity were estimated based upon the number of true positives, false positives, true negatives and false negatives, where tumor status was confirmed through MRI and histology. Sensitivity was calculated as the number of true positive (TP) cases divided by the number of true positive and false negative (FN) cases (Sensitivity = TP/(TP + FN)). Specificity was calculated as the number of true negative cases (TN) divided by the number of true negative and false positive (FP) cases (Specificity = TN/(TN + FP)) [20].
ROC curves were constructed using different intensity threshold levels on the PpIX fluorescence to transmittance ratio collected from the control, 9L-GFP and U251 tumor-bearing mice. As the intensity threshold was changed the true positive fraction (TPF) and false positive fraction (FPF) were calculated, where TPF is synonymous with sensitivity and FPF is representative of 1 minus the specificity of the tumor detection modality. The area under the curve (AUC) was calculated from the ROC curves and used as a direct measure of sensitivity and specificity of the spectroscopy system for tumor detection. An AUC of 1 would indicate that the tumor detection modality had 100% sensitivity and specificity of detection while an AUC of 0.5 would indicate there was only 50% sensitivity and specificity, equivalent to random guessing of tumor status.
RESULTS
PpIX Fluorescence Detection of Brain Tumors
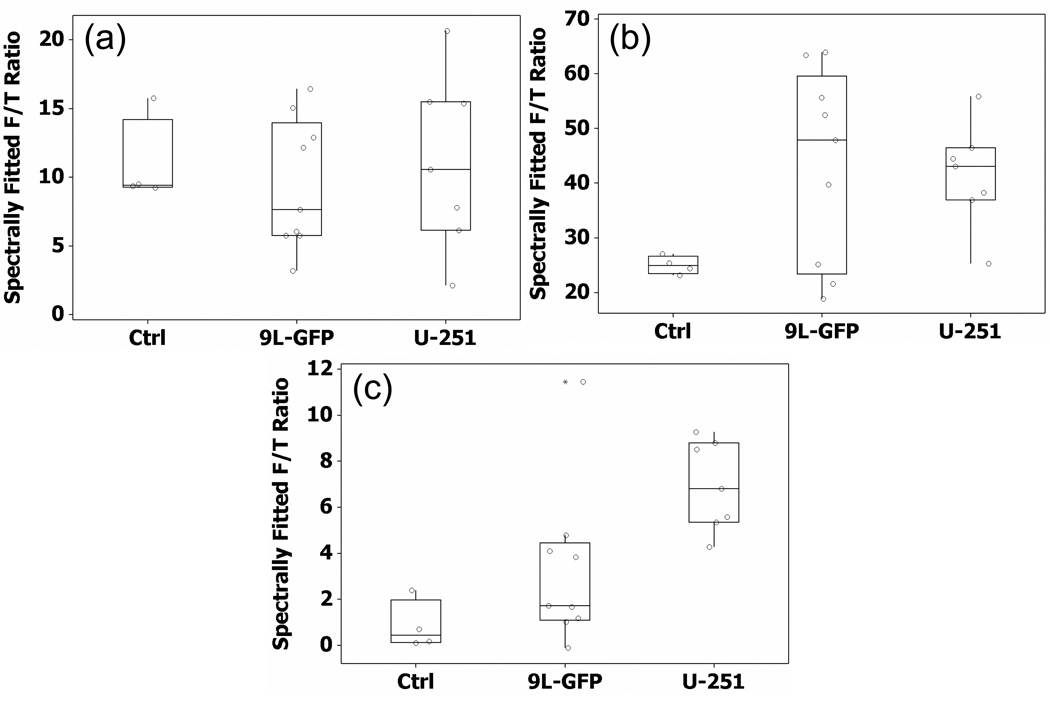
At 18 – 22 days following tumor implantation the mice were measured in the single channel spectroscopy system prior to ALA administration, 2 hours after ALA administration and ex vivo following brain extraction. As can be seen in Figure 4(a), prior to the administration of ALA the mean PpIX fluorescence of the control mice, 9L-GFP and U251 tumor-bearing mice were very similar. However, 2 hours after the administration of ALA there was a significant difference between the means of the control group and the 9L-GFP tumor-bearing group (p-value = 0.016) as well as between the means of the control group and the U251 tumor-bearing group (p-value = 0.004). The mean PpIX fluorescence of the 9L-GFP and U251 groups were similar 2 hours after ALA administration, however the variance of the 9L-GFP group was large compared to that of the U251 group (Figure 4(b)). Following sacrifice, ex vivo measurements were collected on the bulk brain tissue. As can be seen in figure 4(c), the mean fluorescence of the 9L-GFP group was slightly higher than that of the control group while the mean fluorescence of the U251 group was significantly higher than either the control group (p-value < 0.0001) or the 9L-GFP group (p-value = 0.021).
Figure 4.
The PpIX fluorescence to transmittance signal ratio (F/T Ratio) (a) prior to ALA administration in vivo, (b) 2 hours after ALA administration in vivo, and (c) 2 hours after ALA administration, measured ex vivo on the extracted whole brain.
PpIX Tumor Tissue Production Heterogeneity
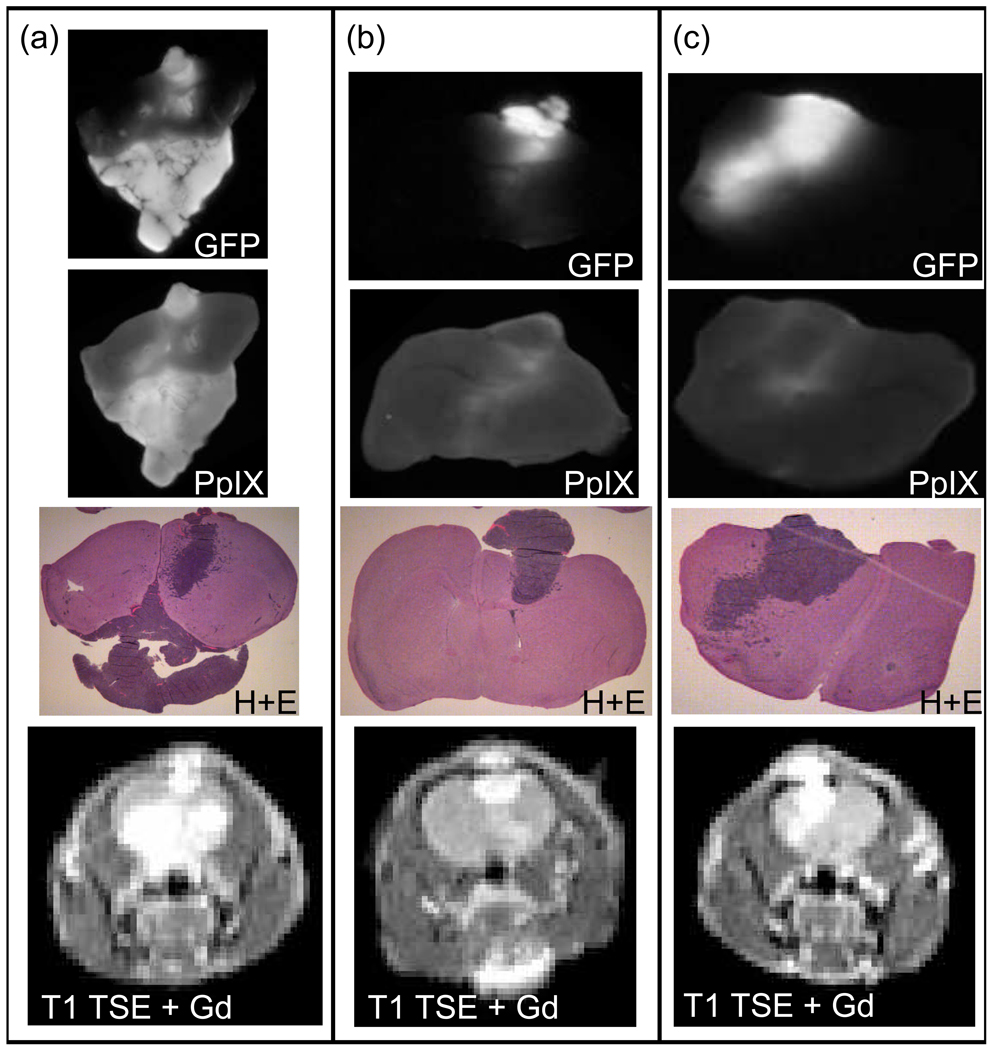
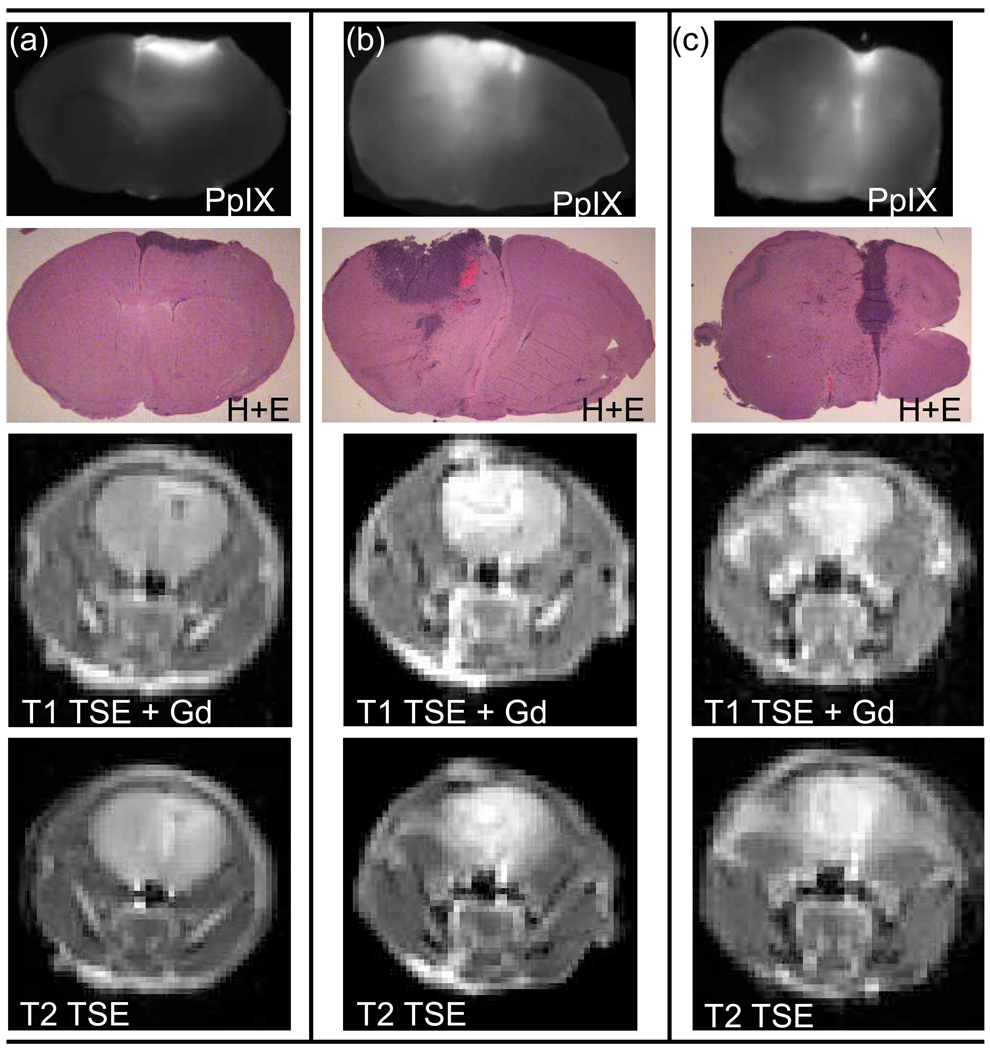
PpIX production following ALA administration was heterogeneous between mice as can be seen in figure 5, which shows three sample 9L-GFP tumor-bearing mice. For each mouse the same brain section is shown in a PpIX fluorescence image, a GFP fluorescence image and an H+E stained image. Approximately the same section can be viewed in vivo via the T1 turbo spin echo (TSE) Gadolinium contrast enhanced MR image. Figure 5 illustrates that while some of the 9L-GFP mice showed PpIX production in the bulk tumor, as can be seen in Figure 5(a), most of the 9L-GFP mice only had PpIX fluorescence on the periphery of the tumor tissue and virtually no increase in PpIX fluorescence in the bulk tumor tissue over the surrounding normal brain tissue (Figure 5(b) & 5(c)). The pattern of tumor tissue PpIX production in the 9L-GFP gliosarcoma model was very different from that seen in the U251 glioma model. Figure 6 shows that the PpIX production in the U251 tumors was confined to the bulk tumor when compared to the H+E stained section in all three examples. It can also be seen that the T1 TSE Gadolinium enhanced MR images and T2 TSE MR images allowed for in vivo visualization of approximately the same tumor tissue section shown ex vivo.
Figure 5.
Images of mouse brain tissue with 9L-GFP tumors, showing GFP (1st row), PpIX (2nd row) and H+E (3rd row) of the same sections of the brain. Also, in the bottom row, in vivo T1 turbo spin echo contrast enhanced (TSE-CE) MRI of approximately the same section. In (a) the PpIX fluorescence can be seen in bulk tumor as compared to the H+E stained image and the GFP fluorescence image. In (b) and (c) the PpIX fluorescence was observed only at the periphery of the tumor tissue, not in bulk tumor as can be seen by comparing the PpIX fluorescence image with the corresponding H+E stained images and GFP fluorescence images.
Figure 6.
Images of mouse brain tissue with U251 tumors shown with ex vivo PpIX fluorescence images (1st row) and corresponding H+E stained images (2nd row) of the same sections of the brain. In vivo T1 turbo spin echo contrast enhanced (TSE-CE) MRI (3rd row) and T2 TSE MR images (4th row) of approximately the same section shown in ex vivo images. In (a) – (c) all mice exhibit increased PpIX fluorescence corresponding to the bulk tumor area as compared to the PpIX fluorescence of the surrounding normal brain tissues.
Red Light Time Course Photobleaching
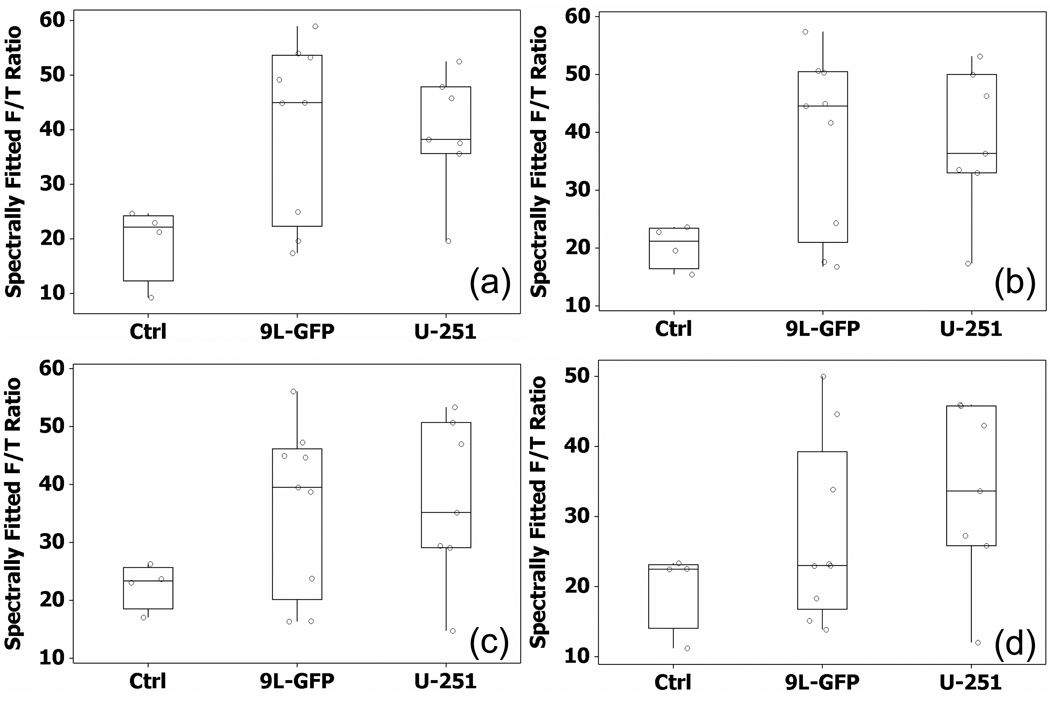
Due to high PpIX production in skin tissue, selective photobleaching of skin PpIX fluorescence should improve noninvasive tumor tissue detection. The mean PpIX fluorescence measured 2 hours after ALA administration showed a different relationship between the control group and the two tumor bearing groups than that seen by ex vivo PpIX measurements. Specifically, the 2 hour in vivo measurements showed the 9L-GFP group with slightly higher mean PpIX fluorescence than the U251 group (mean PpIX fluorescence: control = 25.02, 9L-GFP = 43.20, U251 = 41.50) as can be seen in Figure 4(b), while the ex vivo measurements illustrated that the mean PpIX fluorescence of the U251 group was more than twice that of the 9L-GFP group (mean PpIX fluorescence: control = 0.85, 9L-GFP = 3.3, U251 = 6.9) as reported in Figure 4(c). The time course photobleaching measurements were examined to determine if the red light photobleaching facilitated in vivo visualization of the same PpIX fluorescence contrast pattern seen in ex vivo measurements.
A similar relationship to that seen 2 hours after the administration of ALA was seen after 1, 2 and 4 minutes of red light photobleaching where the mean PpIX fluorescence of the 9L-GFP group was slightly higher than that of the U251 group. The measurement obtained after 8 minutes of red light photobleaching showed the mean PpIX fluorescence of the U251 group was higher than in the 9L-GFP group, which was the same relationship that was seen in the ex vivo measurements. Box and whisker plots that showed the individual mice in each group as well as the interquartile range can be seen in Figure 7 for the mice after 1, 2, 4 and 8 minutes of red light photobleaching. The measured PpIX fluorescence of the 9L-GFP group was considerably reduced following 8 minutes of red light photobleaching, the mean of which was lower than the U251 group (mean PpIX fluorescence: control = 19.92, 9L-GFP = 27.24, U251 = 33.38).
Figure 7.
The PpIX fluorescence to transmittance ratio (F/T Ratio) of the mice in the control group and each tumor-bearing group after (a) 1 minute, (b) 2 minutes, (c) 4 minutes and (d) 8 minutes of red light photobleaching. The mean of the U251 group was higher than the mean of the 9L-GFP group for the first time following ALA administration in the photobleaching time course after 8 minutes of red light photobleaching.
Receiver Operator Characteristic (ROC) Analysis
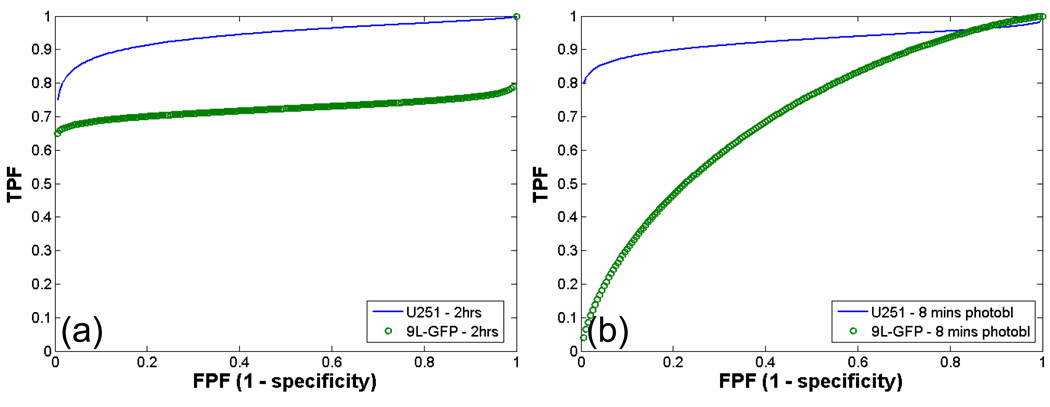
Detection of tumor-bearing animals over non-tumor-bearing control animals via ALA-induced PpIX fluorescence spectroscopy before and after red light photobleaching was assessed by ROC analysis. ROC curves were plotted for each tumor type relative to the control animals 2 hours after ALA administration, but prior to any photobleaching (Figure 8(a)) and after 8 minutes of red light photobleaching (Figure 8(b)). Two hours after ALA administration, prior to any photobleaching, the U251 tumor-bearing group could be detected with higher sensitivity and specificity than the 9L-GFP tumor-bearing group as compared to the control group (U251 AUC = 0.94, 9L-GFP AUC = 0.72). Following the 8 minutes of red light photobleaching, detection sensitivity and specificity were decreased in both tumor-bearing groups over the control group, however the decrease was more significant in the 9L-GFP tumor-bearing group than in the U251 tumor-bearing group. This decrease in detection sensitivity could be attributed to increased overlap between the PpIX fluorescence to transmittance ratio of the tumor-bearing group and the control group (U251 AUC = 0.92, 9L-GFP AUC = 0.69).
Figure 8.
Receiver operator characteristic (ROC) curve for PpIX fluorescence to transmittance ratio of U251 tumor-bearing group vs. control non-tumor-bearing group and 9L-GFP tumor-bearing group vs. control group. The area under the curve (AUC) was calculated as a metric to compare tumor detection sensitivity and specificity for the two tumor types over control, non-tumor-bearing animals. The ROC curve for PpIX fluorescence tumor detection (a) 2 hours after ALA administration where the U251 AUC = 0.94 and the 9L-GFP AUC = 0.72 and (b) after 8 minutes of red light photobleaching where the U251 AUC = 0.92 and the 9L-GFP AUC = 0.69.
DISCUSSION
Two brain tumor lines which had very different tissue PpIX production patterns were studied in vivo. The 9L-GFP and U251 tumor lines were examined for ability to detect brain tumor presence noninvasively via spectroscopic measurements of PpIX fluorescence. Prior to the administration of ALA, tumor-bearing mice could not be distinguished from control mice via in vivo PpIX spectroscopy measurements (Figure 4(a)). At 2 hours after ALA administration, the average PpIX fluorescence in both tumor-bearing groups was significantly higher than in the control group (p-value 9L-GFP = 0.016, p-value U251 = 0.004 relative to control). Thus, on average a tumor-bearing mouse could be detected over a normal mouse by noninvasive in vivo PpIX spectroscopy measurements (Figure 4(b)). ROC analysis revealed detection sensitivity and specificity were significantly higher for the U251 group (AUC = 0.94) than for the 9L-GFP group (AUC = 0.72) (Figure 8(a)). This difference in PpIX fluorescence between the tumor-bearing groups and the control group was confirmed through ex vivo PpIX spectroscopy measurements. Both tumor bearing-groups had higher mean PpIX fluorescence than the control group as can be seen in Figure 4(c), although the 9L-GFP group was only slightly higher than the control group, explaining its lower sensitivity and specificity of detection in vivo as compared to the U251 group (Figure 8(a)).
The variance in PpIX fluorescence following the administration of ALA seen in the 9L-GFP tumor-bearing group via in vivo spectroscopy measurements was quite large in comparison to the U251 tumor-bearing group or the control group (PpIX fluorescence standard deviation (SD): control SD = 1.64, 9L-GFP SD = 17.7, U251 SD = 9.45). A similar pattern of variance was seen in the ex vivo spectroscopy measurements where the 9L-GFP group had a larger standard deviation than the U251 group, which was slightly larger than the standard deviation of the control group (PpIX fluorescence SD: control SD = 1.1, 9L-GFP SD = 3.5, U251 SD = 1.9). The varied ability of the 9L-GFP tumors to produce PpIX fluorescence could be explained when the PpIX production pattern of the tissue was examined in comparison to the GFP fluorescence and H+E stained section. Figure 5(a) showed the pattern of PpIX fluorescence which was expected prior to examination of the 9L-GFP tumor-bearing group ex vivo. The PpIX fluorescence was confined to the bulk tumor tissue, which could be seen when the PpIX fluorescence images were compared to both the GFP fluorescence images and the corresponding H+E images. However, many of the mice in the 9L-GFP group had a PpIX production pattern more like that illustrated in Figure 5(b) and (c), where the PpIX fluorescence in the bulk tumor was similar to that in surrounding normal brain, and increased production of PpIX was only visible in the periphery of the tumor. These two different production patterns would lead to high variability of PpIX fluorescence signal in vivo, as some tumor-bearing mice in the 9L-GFP group would appear to have high PpIX fluorescence, when the entire tumor produced PpIX (figure 5(a)), while other mice in the 9L-GFP group would appear to have relatively low PpIX fluorescence, when only the periphery of the tumor produced PpIX (figure 5(b) and (c)).
The PpIX fluorescence standard deviation in the U251 tumor bearing group was considerably smaller than in the 9L-GFP group by both in vivo and ex vivo spectroscopy measurements. As can be seen in Figure 6, the PpIX production pattern in the U251 group was significantly different from than in the 9L-GFP group. All of the mice in the U251 group showed extensive PpIX production in the bulk tumor tissue and much higher PpIX signal in the tumor tissue over the surrounding normal brain tissue. Thus, PpIX fluorescence signal detected via spectroscopy was not as variable as that of the 9L-GFP group since all the U251 mice had PpIX production which corresponded well to content of the brain tumor tissue. This decrease in PpIX production heterogeneity of the U251 tumor model as compared to the 9L-GFP tumor model translated to increased sensitivity and specificity of detection over the control animals (U251 AUC = 0.94, 9L-GFP AUC = 0.72).
Skin photobleaching was examined for its ability to increase PpIX contrast between tumor-bearing mice and control mice. Due to the high PpIX production of the skin, the in vivo spectroscopic measurements included PpIX produced in the brain tissue as well as PpIX produced in the skin. Theoretically, photobleaching of skin PpIX fluorescence could increase the difference in PpIX signal between control mice and tumor-bearing mice even though the overall measured signal would be decreased. In the red light photobleaching experiment, the average PpIX fluorescence following the administration of ALA, but prior to any skin photobleaching was higher in the 9L-GFP tumor-bearing group than in the U251 tumor-bearing group. However, the ex vivo spectroscopy measurements illustrated that the average PpIX fluorescence of the U251 group was more than twice that of the 9L-GFP group. The PpIX fluorescence measurements obtained during the time course of red light photobleaching were considered to determine if the ability to quantify the average PpIX contained in the brain tumor tissue was improved. As shown in Figure 7(d), after 8 minutes of red light photobleaching, a similar relationship to that seen in the ex vivo data (Figure 4(c)) was visible by in vivo spectroscopy measurements where the highest mean PpIX fluorescence was seen in the U251 group, followed by the 9L-GFP group and then the control group.
The sensitivity and specificity of detection as measured by AUC through ROC analysis were decreased by the red light photobleaching as compared to 2 hours after ALA administration but prior to any photobleaching. This decrease in sensitivity and specificity was due to increased overlap of the PpIX fluorescence to transmittance ratio of the tumor-bearing animals and the control animals (U251 AUC = 0.92, 9L-GFP AUC = 0.69) (Figure 8(b)). Following photobleaching the shape of the ROC curve for the 9L-GFP tumor-bearing animals was significantly different from that prior to photobleaching (Figure 8). The sensitivity of detection could be increased to 100% after photobleaching although; at this level of sensitivity to tumor presence the specificity of detection was significantly decreased and caused detection of false positives. Skin photobleaching was advantageous in the detection of the 9L-GFP tumors because due to the 9L-GFP tumor PpIX production heterogeneity, 100% sensitivity in detection of these tumors was not possible without photobleaching. In the case where tumor PpIX production was heterogenous and tumor detection was of most importance, skin photobleaching would be advantageous due to its ability to increase sensitivity to tumor presence. In contrast, when specificity was most important skin photobleaching would not be advantageous as it significantly decreased the specificity of detection for the 9L-GFP tumor-bearing group.
CONCLUSIONS
In conclusion, noninvasive PpIX spectroscopy measurements were able to detect the presence of both the 9L-GFP tumors and U251 tumors over non-tumor-bearing mice using the average PpIX fluorescence of each group as the metric. The variance in PpIX production was quite large in the 9L-GFP tumor-bearing group when compared to the U251 group or the control group. This PpIX production difference can be explained through tumor tissue PpIX production patterns, where some of the 9L-GFP tumors had high PpIX fluorescence in the bulk tumor while other had increased PpIX fluorescence only in the periphery of the tumor with little increase in PpIX fluorescence in the bulk tumor tissue. Red light skin photobleaching decreased the contrast between the tumor-bearing groups and the control group but increased the ability to quantify the PpIX fluorescence contained within the brain tissue over the PpIX fluorescence in the skin. A noninvasive, low cost fluorescence based detection system has been demonstrated to be useful for detection of brain tumor models although, sensitivity and specificity of noninvasive detection of tumors is influenced by the PpIX production pattern of the model tumor tissue. This system could potentially be extended to other laboratory tumor models following ex vivo studies to confirm tumor burden corresponded to PpIX fluorescence intensity, which appears to be model dependent.
ACKNOWLEDGEMENTS
The authors are grateful to Nathan Watson and Mark Israel for use of the U251 tumor cell line and for informative discussions about the glioma lines and data. This work has been funded by NCI grants RO1CA109558 and PO1CA84203 as well as the Norris Cotton Cancer Center Shared Resources.
REFERENCES
- 1.Kennedy JC, Marcus SL, Pottier RH. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): mechanisms and clinical results. Journal of Clinical Laser Medicine & Surgery. 1996;14(5):289–304. doi: 10.1089/clm.1996.14.289. [DOI] [PubMed] [Google Scholar]
- 2.Bogaards A, et al. Increased brain tumor resection using fluorescence guidance in a preclinical model. Lasers in Surgery & Medicine. 2004;35(3):181–190. doi: 10.1002/lsm.20088. [DOI] [PubMed] [Google Scholar]
- 3.Stummer W, et al. Fluorescence Guided Resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. Journal of Neurosurgery. 2000;93(6):1003–1013. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 4.Stummer W, et al. Intraperative Detection of Malignant Gliomas by 5-Aminolevulinic Acid-induced Porphyrin Fluorescence. Neurosurgery. 1998;42(3):518–526. doi: 10.1097/00006123-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Collaud S, et al. On the selectivity of 5-aminolevulinic acid-induced protoporphyrin IX formation. Current Medicinal Chemistry Anti Cancer Agents. 2004;4(3):301–316. doi: 10.2174/1568011043352984. [DOI] [PubMed] [Google Scholar]
- 6.Peng Q, et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79(12):2282–2308. doi: 10.1002/(sici)1097-0142(19970615)79:12<2282::aid-cncr2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Friesen SA, et al. 5-Aminolevulinic acid-based photodynamic detection and therapy of brain tumors (review) International Journal of Oncology. 2002;21(3):577–582. [PubMed] [Google Scholar]
- 8.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncology. 2004;5(8):497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs SL, et al. Protoporphyrin IX Level Correlates with Number of Mitochondria, But Increase in Production Correlates with Tumor Cell Size. Photochemistry & Photobiology. 2006;82(5):1334–1341. doi: 10.1562/2006-03-11-RA-843. [DOI] [PubMed] [Google Scholar]
- 10.Wu SM, et al. Protoporphyrin IX production and its photodynamic effects on glioma cells, neuroblasomta cells and normal cerebellar granule cells in vitro with 5-aminolevulinic acid and its hexylester. Cancer Letters. 2003;200:123–131. doi: 10.1016/s0304-3835(03)00271-4. [DOI] [PubMed] [Google Scholar]
- 11.Duffner F, et al. Specific intensity imaging for glioblastoma and neural cell cultures with 5-aminolevulinic acid-derived protoporphyrin IX. Journal of Neuro-Oncology. 2005;71:107–111. doi: 10.1007/s11060-004-9603-2. [DOI] [PubMed] [Google Scholar]
- 12.Eleouet S, et al. Heterogeneity of delta-aminolevulinic acid-induced protoporphyrin IX fluorescence in human glioma cells and leukemic lymphocytes. Neurological Research. 2000;22:361–368. doi: 10.1080/01616412.2000.11740685. [DOI] [PubMed] [Google Scholar]
- 13.Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial.[see comment] Lancet Oncology. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 14.Ntziachristos V, et al. Planar fluorescence imaging using normalized data. Journal of Biomedical Optics. 2005;10(6):064007-1-8. doi: 10.1117/1.2136148. [DOI] [PubMed] [Google Scholar]
- 15.de Bruijn HS, et al. Fractionated illumination after topical application of 5-aminolevulinic acid on normal skin of hairless mice: The influence of the dark interval. Journal of Photochemistry & Photobiology. B Biology. 2006;85(3):184–190. doi: 10.1016/j.jphotobiol.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Johansson A, et al. In vivo measurement of parameters of dosimetric importance during interstitial photodynamic therapy of thick skin tumors. Journal of Biomedical Optics. 2006;11(3):May–Jun. doi: 10.1117/1.2204027. [DOI] [PubMed] [Google Scholar]
- 17.Nadeau V, et al. In vivo measurement of 5-aminolaevulinic acid-induced protoporphyrin IX photobleaching: a comparison of red and blue light of various intensities. Photodermatology, Photoimmunology & Photomedicine. 2004;20(4):170–174. doi: 10.1111/j.1600-0781.2004.00100.x. [DOI] [PubMed] [Google Scholar]
- 18.Moore A, et al. Novel Gliosarcoma Cell Line Expressing Green Fluorescent Protein: A Model for Quantitative Assessment of Angiogenesis. Microvascular Research. 1998;56:145–153. doi: 10.1006/mvre.1998.2102. [DOI] [PubMed] [Google Scholar]
- 19.Davis SC, et al. Magnetic resonance-coupled fluorescence tomography scanner for molecular imaging of tissue. Review of Scientific Instruments. 2008;79(6):064302. doi: 10.1063/1.2919131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pogue BW, et al. Image analysis methods for diffuse optical tomography. Journal of Biomedical Optics. 2006;11(3):033001-1–033001-16. doi: 10.1117/1.2209908. [DOI] [PubMed] [Google Scholar]