Abstract
During embryonic development, the mesenchyme of the lungs, gut, kidneys, and other tissues expresses Trps1, an atypical member of the GATA-type family of transcription factors. Our previous work suggested the possibility that Trps1 acts downstream of bone morphogenic protein 7 (Bmp7), which is essential for normal renal development. To examine the role of Trps1 during early renal development, we generated Trps1-deficient mice and examined their renal histology. Compared with wild-type mice, Trps1-deficient newborn mice had fewer tubules and glomeruli, an expanded renal interstitium, and numerous uninduced metanephric mesenchymal cells, which resulted in fewer nephrons. In wild-type kidneys, Trps1 expression was present in ureteric buds, cap mesenchyme, and renal vesicles, whereas Trps1 was virtually absent in Bmp7-deficient kidneys. Furthermore, Trps1-deficient kidneys had low levels of Pax2 and Wt1, which are markers of condensed mesenchymal cells, suggesting that a lack of Trps1 affects the differentiation of cap mesenchyme to renal vesicles. In cultured metanephric mesenchymal cells, Bmp7 induced Trps1 and E-cadherin and downregulated vimentin. Knockdown of Trps1 with small interference RNA inhibited this Bmp7-induced mesenchymal-to-epithelial transition. Last, whole-mount in situ hybridization of Wnt9b and Wnt4 demonstrated prolonged branching of ureteric buds and sparse cap mesenchyme in the kidneys of Trps1-deficient mice. Taken together, these findings suggest that normal formation of nephrons requires Trps1, which mediates mesenchymal-to-epithelial transition and ureteric bud branching during early renal development.
Mutation or deletion of the TRPS1 gene causes the tricho-rhino-phalangeal syndrome (TRPS), which is characterized by craniofacial and skeletal malformations.1 This gene encodes a novel transcription factor with nine zinc-finger domains, including a single GATA-type DNA-binding domain.1 We generated Trps1-deficient (knockout [KO]) mice and demonstrated that Trps1 regulates the proliferation and apoptosis of chondrocytes by binding directly to GATA sites in the promoter region of the Stat-3 gene.2
Although the phenotypic expression of TRPS in human is limited to skeletal tissues and hair follicles, Trps1 is expressed not only in cartilage, joints, and hair follicles but also in the mesenchyme of the developing lungs, gut, kidneys, and other tissues during murine embryonic development.3 Trps1 expression in the lungs corresponds with morphologic defects in the distal respiratory epithelium of TRPS1 Δgt/Δgt mice.4 During analysis of KO mice, we have observed substantial morphologic abnormalities of the kidneys in newborn homozygous animals.
Metanephric kidneys develop via a program of reciprocal inductive interactions between the ureteric bud and nephrogenic mesenchyme.5 Briefly, signals from the ureteric bud promote the survival of nephrogenic mesenchymal cells, leading to renal tubular formation. A member of the Wnt family, Wnt9b, is expressed in the ureteric epithelium and plays a central role in the induction of epithelial renal vesicles.6 Concomitantly, signals from the mesenchymal cells stimulate growth and branching of the ureteric bud that ultimately forms the collecting duct system. During this process, several growth factors and their receptors promote metanephric differentiation.7–9 One group of such growth factors is the bone morphogenic proteins (BMPs), which belong to the TGF-β superfamily. BMPs have been widely recognized as having an important role in renal development.10,11
On the basis of current evidence, it seems that Bmp7 plays a more important role in renal tubule formation than the other members of this family. In the developing kidney, Bmp7 is initially expressed by the ureteric epithelium. As development proceeds, early expression is observed in the condensed mesenchyme and later in the pretubules.12–14 During the process of kidney development, Bmp7 prevents metanephric mesenchymal cells from undergoing apoptosis,12,15 promotes metanephric differentiation,16 and controls the proliferation and apoptosis of epithelial cells in the collecting tubules.17
In a previous study, we demonstrated that Trps1 is regulated by growth and differentiation factor 5 (Gdf5), another BMP that shares the same receptor with Bmp7, in ATDC5 chondrogenic cells.18 This finding immediately raised the possibility that Trps1 may also act downstream of Bmp7 on metanephric mesenchymal cells during early kidney development.
In this study, to elucidate the role of Trps1 in nephron formation, we examined the functional interaction between Bmp7 and Trps1 in metanephric mesenchymal cells during early renal development. We also analyzed the expression of Wnt9b and Wnt4 by whole-mount in situ hybridization. Our results indicate that Trps1 acts on both metanephric mesenchymal cells and ureteric buds, being required for the mesenchymal-to epithelial transition (MET) and for regulation of ureteric bud branching.
Results
Trps1 Is Required for Normal Nephrogenesis
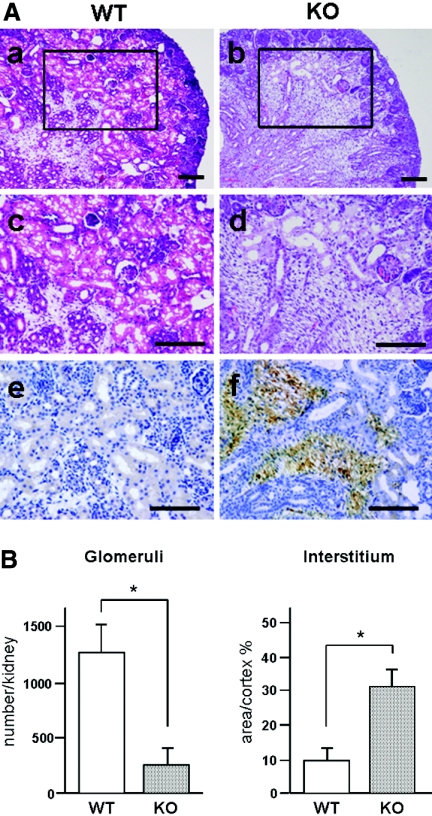
Histologic examination of newborn Trps1 null (KO) mice revealed that the number of tubules was obviously smaller in KO kidneys compared with wild-type (WT) kidneys (Figure 1A, a through d, and B). In fact, glomeruli were significantly reduced to approximately one fifth of the levels in WT kidneys (Figure 1B). In contrast, the interstitium of the renal cortex (stained with anti-vimentin antibody) was wider in KO kidneys than in WT kidneys (Figure 1A, e and f). The cells in this region were not stained with α-smooth muscle actin antibodies (data not shown), so they seemed to be metanephric mesenchymal cells rather than myofibroblasts. From these observations, we hypothesized that metanephric mesenchymal cells failed to form epithelial renal vesicles during early renal development with the MET as a result of lack of Trps1 in KO kidneys, suggesting that Trps1 could be involved in the MET process.
Figure 1.
Differences of nephrogenesis between newborn WT and Trps1 KO mice. (A) Morphologic differences of the kidneys between newborn WT and KO mice. Paraffin sections of kidneys from newborn WT mice (a, c, and e) and KO mice (b, d, and f) were stained with hematoxylin and eosin (H&E; a through d) and then immunostained with an antibody for vimentin (e and f). c and d are higher magnification images of the boxed areas in a and b, respectively. Bars = 100 μm. (B) Comparison of the number of glomeruli and interstitial area in the kidneys of newborn WT and KO mice. Data are means ± SD for five samples. *P < 0.01.
Differentiation of Metanephric Mesenchymal Cells Is Perturbed in KO Kidneys
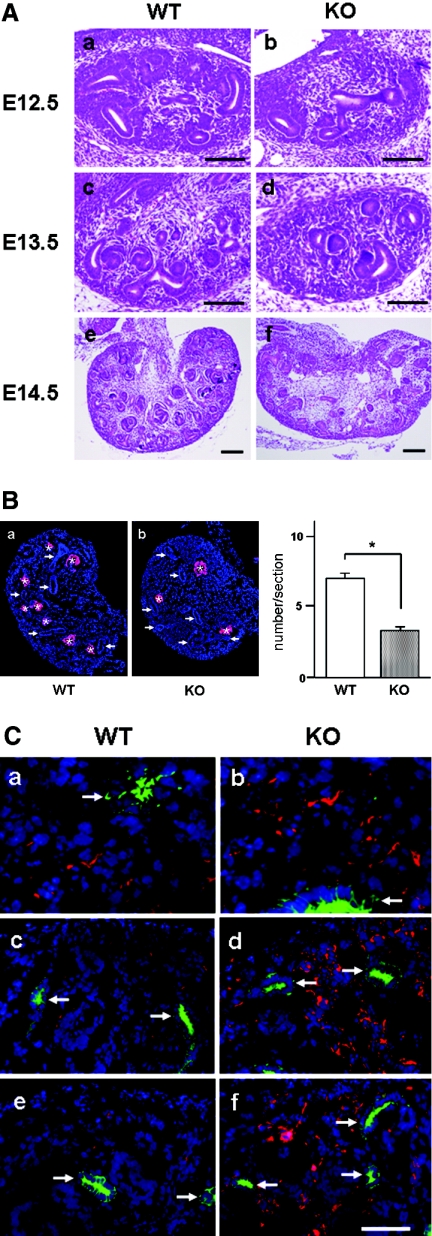
To address this hypothesis, we examined the process of nephron formation during early renal development from embryonic day 12.5 (E12.5) to E14.5. In E12.5 embryos, ureteric buds penetrate metanephric tissue and the distal parts of these buds branch several times. At this stage, there were no significant histologic differences between WT and KO kidneys (Figure 2A, a and b). At E13.5, metanephric mesenchymal cells underwent differentiation to condensed mesenchymal cells (cap mesenchyme), and such differentiation of metanephric mesenchymal cells was less prominent in KO kidneys compared with WT kidneys (Figure 2A, c and d). The paucity of renal vesicles in KO kidneys was clearly shown by immunostaining with an antibody directed against Pax8,19 a specific marker for renal vesicles (Figure 2B). After E13.5, renal vesicles such as comma-shaped or S-shaped bodies were well formed and were observed more frequently in WT kidneys than in KO kidneys (Figure 2A, c through f).
Figure 2.
Comparison of mesenchymal cell differentiation during early renal development between WT and Trps1 null mice. (A) H&E-stained sections of kidneys obtained at E12.5 (a and b), E13.5 (c and d), and E14.5 (e and f) from WT mice (a, c, and e) and Trps1 KO mice (b, d, and f). Bar = 200 μm. (B) Immunofluorescence staining of kidneys obtained at E13.5 from WT mice (a) and Trps1 KO mice (b) with an antibody for Pax8 (red, left). Asterisks and arrows indicate renal vesicles and ureteric buds, respectively. Nuclei are stained with DAPI. (Right) Comparison of the number of renal vesicles in kidneys from WT and KO mice. Data are means ± SD for four samples. *P < 0.01 (C) Double immunofluorescence staining of kidneys obtained at E12.5 (a and b), E13.5 (c and d), and E14.5 (e and f) from WT mice (a, c, and e) and Trps1 KO mice (b, d, and f) with antibodies for vimentin (red) and cytokeratin (green). The ureteric buds (arrows) are positive for cytokeratin. Nuclei are stained with DAPI.
This reduction in the number of renal vesicles in KO kidneys was associated with a large number of undifferentiated metanephric mesenchymal cells stained with vimentin (Figure 2C). In WT kidneys, vimentin-positive cells showed a dramatic decrease after E13.5 when metanephric mesenchymal cells differentiated into epithelial renal vesicles, whereas there was no obvious change of vimentin immunostaining in the KO kidneys from E12.5 to E14.5. From these findings, it is possible to conclude that loss of Trps1 impaired the differentiation of cap mesenchyme into epithelial renal vesicles at the initial step of nephrogenesis.
Trps1 Is Expressed in Ureteric Buds as Well as Cap Mesenchyme and Renal Vesicles
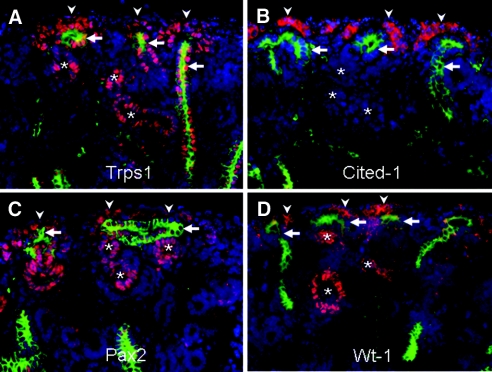
To examine Trps1 expression in developing kidneys, we performed immunohistochemistry on E15.5 WT kidneys with antibodies directed against Trps1, Cited1 (a marker of cap mesenchyme), Pax2, and Wt1 (Figure 3). Trps1 was strongly positive in the nuclei of ureteric bud cells, as well as in the nuclei of renal vesicle cells and cap mesenchyme cells, which are specifically stained by Cited1 antibody (Figure 3, A and B). The pattern of Trps1 expression largely corresponded with that of Pax2 and Wt1, except in the ureteric bud (Figure 3, C and D).
Figure 3.
Co-localization of Trps1, Cited1, Pax2, and Wt1 in WT kidneys on E15.5. (A) Double immunofluorescence staining of kidneys obtained from E15.5 WT mice using antibodies for Trps1 (red) and cytokeratin (green), (B) Cited1 (red) and cytokeratin (green), (C) Pax2 (red) and cytokeratin (green), or (D) Wt1 (red) and cytokeratin (green). The ureteric buds (arrows) are positive for cytokeratin. Arrowheads and asterisks indicate cap mesenchyme and renal vesicles, respectively. Nuclei are stained with DAPI.
Trps1 Is Coexpressed with Bmp7 during Nephrogenesis
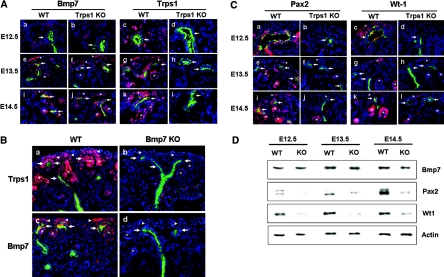
To examine Trps1 and Bmp7 expression during early renal development, we performed immunohistochemistry. Consistent with previous reports,12–14 on E12.5, Bmp7 was expressed in epithelial cells of the ureteric buds and the surrounding condensed mesenchymal cells in both WT and KO kidneys (Figure 4A, a and b). On E13.5 and E14.5, Bmp7 expression was detected in ureteric buds, cap mesenchyme, and renal vesicles, where Trps1 was also expressed. Bmp7 was also detected in KO kidneys from E12.5 to E14.5, and there was no difference in expression between WT and KO kidneys by Western blot analysis (Figure 4D). In WT kidneys, Trps1 expression was restricted to ureteric buds, cap mesenchyme, and renal vesicles throughout this period (Figure 4A, c, g, and k); however, it was virtually absent in the ureteric buds, cap mesenchyme, and renal vesicles of Bmp7 null kidneys,12 suggesting that Trps1 is a molecule located downstream of Bmp7 (Figure 4B).
Figure 4.
Comparison of Bmp-7, Trps1, Pax2, and Wt1 expression during early renal development. (A) Double immunofluorescence staining of kidneys obtained at E12.5 (a through d), E13.5 (e through h), and E14.5 (i through l) from WT mice (a, e, i, c, g, and k) and Trps1 KO mice (b, f, j, d, h, and l) with antibodies for Bmp7 (red) and cytokeratin (green) (a, b, e, f, i, and j) or Trps1 (red) and cytokeratin (green) (e, d, g, h, k, and l). (B) Double immnofluorescent staining of kidneys obtained at E14.5 from WT mice (a and c) and Bmp7 null KO mice (b and d) with antibodies for Trps1(red) and cytokeratin (green) (a and b) or antibodies for Bmp7 (red) and cytokeratin (green) (c and d). (C) Double immunofluorescence staining of kidneys obtained at E12.5 (a through d), E13.5 (e through h), and E14.5 (i through l) from WT mice (a, e, i, c, g, and k) and Trps1 KO mice (b, f, j, d, h, and l) with antibodies for Pax2 (red) and cytokeratin (green) (a, b, e, f, i, and j) or Wt1 (red) and cytokeratin (green) (e, d, g, h, k, and l). The ureteric buds (arrows) are positive for cytokeratin. Arrowheads and asterisks indicate cap mesenchyme and renal vesicles, respectively. Nuclei are stained with DAPI. (D) Western blot analysis of Bmp7, Pax2, and Wt1 in protein extracts from E12.5, E13.5, and E14.5 kidneys of WT mice and Trps1 KO mice.
Reduced Expression of Pax2 and Wt1 in KO Kidneys
Pax2 is a member of the Pax family of transcription factors that regulate epithelial conversion of the metanephric mesenchyme.20 On E12.5, Pax2 was expressed in the ureteric bud and the surrounding condensed mesenchymal cells of WT kidneys, whereas it was virtually absent in KO kidneys (Figure 4C, a and b). On E13.5 and E14.5, there was moderate Pax2 expression in the ureteric buds and cap mesenchyme of WT kidneys, along with higher expression in the renal vesicles, whereas Pax2 expression was low in the ureteric buds and renal vesicles of KO kidneys (Figure 4C, e, f, i, and j). Western blotting also showed low levels of Pax2 expression in KO kidneys compared with WT kidneys (Figure 4D).
In agreement with previous reports,21,22 Wt1 expression was detected in the cap mesenchyme and renal vesicles of WT kidneys, whereas virtually no staining was seen in KO kidneys (Figure 4C, c, d, g, h, k, and l). Wt1 showed almost exactly the same localization as Pax2 from E12.5 to E14.5 in WT and KO kidneys (Figure 4C). Western blotting also demonstrated low expression of Wt1 from E12.5 to E14.5 in KO kidneys compared with WT kidneys (Figure 4D). From these results, it was concluded that absence of Trps1 affects the expression of Pax2 and Wt1, suggesting that the differentiation of mesenchymal cells to epithelial renal vesicles was disturbed by lack of Trps1.
Trps1 Expression Is Regulated by Bmp7 via the p38 Mitogen-Activated Protein Kinase Pathway
We have demonstrated that Trps1 is regulated by Gdf5 in ATDC5 cells.18 Because Bmp7 and Gdf5 share the same receptor (Alk-6) for signaling,23–25 we assumed that Trps1 would be regulated by Bmp7 in metanephric mesenchymal cells during renal development. Loss of Bmp7 results in renal hypoplasia with development of few nephrons and accumulation of loose interstitial mesenchyme,12,15 similar to the phenotype of Trps1 null kidneys. Consistent with this, Trps1 expression was virtually absent in Bmp7 null kidneys, as shown in Figure 4B.
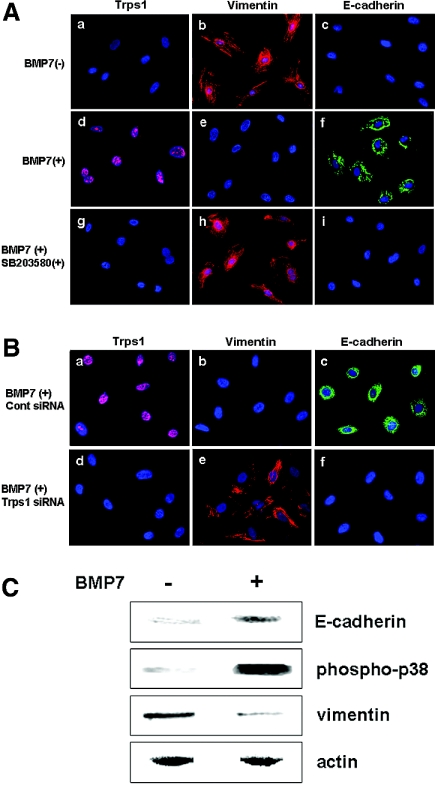
To investigate further whether Trps1 acts downstream of Bmp7 on metanephric mesenchymal cells, we examined Trps1 expression by cultured rat metanephric mesenchymal cells (RMMs) in the presence or absence of Bmp7 protein. Nuclear staining for Trps1 was detected in RMMs after treatment with Bmp7, whereas no staining was seen in the absence of Bmp7 (Figure 5A, a and b).
Figure 5.
RMMs are transformed into epithelial cells through Bmp7/p38 MAPK/Trps1 signaling. (A) Expression of Trps1 (pink; a, d, and g), vimentin (red; b, e, and h), and E-cadherin (green; c, f, and j) by metanephric mesenchymal cells without treatment (a through c), with Bmp7 treatment (d through f), and with treatment by Bmp7 plus SB203580 (g through i). Nuclei were stained with DAPI (blue). (B) Expression of Trps1 (pink; a and d), vimentin (red; b and e), and E-cadherin (green; c and f) by metanephric mesenchymal cells with or without treatment by Bmp7 and control siRNA (a through c), or with treatment by Bmp7 and Trps1 siRNA (d through f). Nuclei were stained with DAPI (blue). (C) Western blot analysis of E-cadherin, phosphorylated p38 MAPK, and vimentin in extracts of cultured primary metanephric mesenchymal cells from rat E13.5 kidneys.
It has been reported that BMPs transmit signals via p38 mitogen-activated protein kinase (MAPK) in various cells.17,26,27 To test whether Trps1 was induced by Bmp7 via p38 MAPK signaling, we pretreated RMMs with SB203580, a p38 MAPK inhibitor, before exposure to Bmp7. In this case, nuclear staining for Trps1 was not seen in RMMs after Bmp7 treatment (Figure 5A, g), suggesting that induction of Trps1 by Bmp7 occurred via p38 MAPK.
MET Is Mediated via Bmp7/Trps1 Signaling in RMMs
To investigate whether RMMs differentiated to the epithelial phenotype in response to Bmp7, we stained RMMs treated with Bmp7 with antibodies directed against vimentin and E-cadherin, markers of uninduced mesenchymal and epithelial cells, respectively.28 As displayed in Figure 5A, b and c, RMMs showed strong positive staining for vimentin and negative staining for E-cadherin in the absence of Bmp7. After treatment with Bmp7, vimentin expression disappeared and E-cadherin expression was induced within 48 h along with concomitant phosphorylation of p38 MAPK and Trps1 expression (Figure 5A, d through f, and C); however, no significant changes of vimentin or E-cadherin were observed after Bmp7 treatment when cells were pretreated with SB203580 or Trps1 small interference RNA (siRNA), both of which virtually abolished the expression of Trps1 (Figure 5A, g through i, and B, d through f). These data suggest that Bmp7 induces the differentiation of mesenchymal cells to epithelial cells via p38 MAPK and Trps1.
Branching Growth of Ureteric Bud Is Elongated by Loss of Trps1
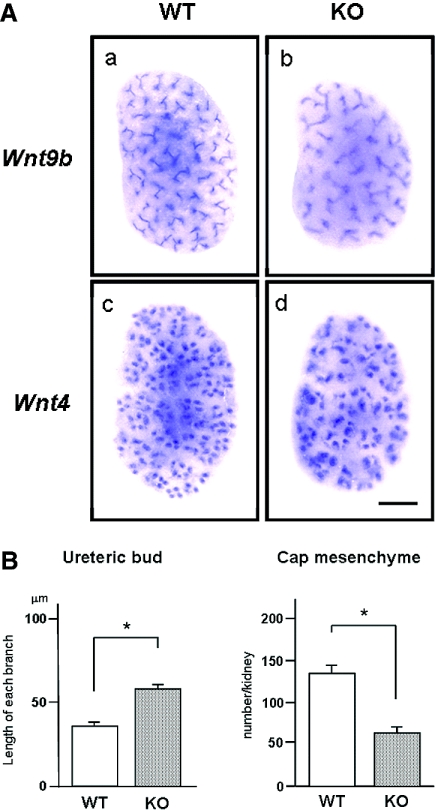
Wnt/β-catenin signaling has been reported to be crucial for the cellular transition to form renal vesicles.29 Two members of the Wnt family, Wnt9b and Wnt4, play a central role during the initial stages of tubulogenesis.6 To examine whether Trps1 affects the expression of Wnt9b and Wnt4, we performed whole-mount in situ hybridization using kidneys harvested from WT and KO embryos at E14.5 (Figure 6). Wnt9b and Wnt4 mRNA expression was seen within tissues corresponding to the ureteric bud and cap mesenchyme, respectively, and the intensity of staining was similar in both WT and KO kidneys (Figure 6A). In fact, real-time quantitative PCR did not show any significant difference of Wnt9b and Wnt4 mRNA levels between WT and KO kidneys (data not shown); however, there were significant differences in the branching of the ureteric bud and the density of cap mesenchyme between WT and KO kidneys. Quantification of the length of ureteric bud and the number of cap mesenchyme made it clear that each branch of the ureteric bud was longer in KO compared with WT kidneys, and the density of each cap mesenchyme formed beneath the tips of the ureteric buds was significantly reduced (Figure 6B). From these results, it is concluded that Trps1 does not affect the expression of Wnt9b and Wnt4 mRNA but still regulates ureteric branching.
Figure 6.
Branching of ureteric buds is affected by loss of Trps1. (A) Whole-mount in situ hybridization of Wnt9b and Wnt4 was performed using kidneys from E14.5 WT mice (a and c) and Trps1 KO mice (b and d). Bar = 200 μm. (B) Comparison of the length of ureteric bud and the number of cap mesenchyme in the kidneys of E14.5 WT and KO embryos. Data are means ± SD for four samples. *P < 0.01.
Discussion
In this study, we found that nephrogenesis was disturbed in the kidneys of newborn Trps1 null mice, and a large number of stromal mesenchymal cells remained in the interstitium. We hypothesized that this phenotype of KO kidneys resulted from defects in the MET of metanephric mesenchymal cells during early kidney development. We showed that Trps1 was expressed by the ureteric buds, as well as by cap mesenchyme and renal vesicles. In KO kidneys, cap mesenchyme and renal vesicles were clearly reduced in number from E12.5 to E14.5 when immunostained with antibodies for Pax2 and Wt1, which show increased expression in these cells.30 It is possible that KO kidneys showed defective MET because inhibition of Pax2 expression prevents the condensation and epithelial transformation of metanephric mesenchymal cells,31 although the defective MET did not cause the total loss of nephron because there is still a cap mesenchyme and nephrons are forming. We suppose that the reduced number of nephrons would be led by less branching of the ureteric bud as well as by the defective MET. An alternative explanation is that a lack of Trps1 affects the recruitment of the metanephric mesenchyme to the cap, such that a larger number of cells take on a noncap fate. This would explain the expanded interstitium.
Using cultured RMMs, we demonstrated that these cells lost vimentin expression when treated with Bmp7 and gained Trps1 and E-cadherin expression instead. These results indicate that the RMMs were transformed from mesenchymal cells to epithelial cells by Bmp7/Trps1 signaling. In addition, this phenotypic transformation was almost completely blocked by pretreatment with a p38 MAPK inhibitor or Trps1 siRNA. Accordingly, we conclude that the transdifferentiation of RMMs to epithelial cells is mediated by Bmp7/p38 MAPK/Trps1 signaling, although it still remains possible that canonical Smad signaling would have a similar effect in this assay. This assumption is in good agreement with a report by Zeisberg et al.32 that Bmp7 induces the MET in adult renal fibroblasts.
Although Bmp7-deficient mice12,15 have a similar renal phenotype to that of Trps1 null mice, the newborn kidneys differ in size between Bmp7 mutant and Trps1 null mice. Bmp7-deficient newborn mice were reported to have small dysgenic kidneys, whereas Trps1 null newborn kidneys are the same size as those of wild-type mice. Considering that Bmp7 lies upstream of Trps1 and has two distinct BMP type I receptors, Alk3 and Alk6,33 that activate several intracellular signaling pathways (including Smads, p38 MAPK, extracellular signal–regulated kinase, and JNK), it is reasonable for Bmp7 mutant mice to have a more severe phenotype than Trps1 null mice.
Also, we reported previously that Trps1 directly binds to the GATA sites of the promoters of the Stat3 and Pthrp genes, thereby regulating the proliferation or apoptosis of chondrocytes and the length of the epiphyseal cartilage, respectively2,34; however, the downstream target genes of Trps1 involved in renal development have not yet been examined. Given that Trps1 is a transcriptional repressor,35 it seems possible that it negatively regulates a transcription factor that suppresses E-cadherin expression, such as Snail. It has been reported that Snail controls the epithelial-to mesenchymal transition by repressing E-cadherin expression.36 Another possibility is that Trps1 suppresses vimentin expression by directly binding to the GATA sites of the promoter. It would be interesting to determine whether MET of metanephric mesenchymal cells were induced after suppression of vimentin expression by Trps1.
Finally, we used whole-mount in situ hybridization to demonstrate that the branching of ureteric buds was affected by loss of Trps1. Because Trps1 is expressed in the epithelial cells of ureteric buds as well as in cap mesenchyme and renal vesicles, it is possible that Trps1 modulates the expression of genes that in turn regulate ureteric bud branching. It has been reported that a number of factors can stimulate or inhibit the process of ureteric bud branching.37,38 It would be possible for Trps1, directly or indirectly, to regulate negatively the expression of genes such as Bmp4, activin A, and semaphorin.38–41
In conclusion, Trps1 is expressed not only in the cap mesenchyme and renal vesicles but also in ureteric buds, and it plays a crucial role in regulating both the MET and ureteric bud branching. Further studies will be needed to elucidate the mechanism by which Trps1 regulates the MET in cap mesenchyme and the branching of ureteric buds during early renal development.
Concise Methods
All of the animal experiments in this study were performed according to the guidelines of the Animal Studies Committee of Wakayama Medical University.
Tissue Preparation and Histology
Heterozygous Trps1 null mice were mated, and the date of detecting a vaginal plug was defined as E0.5. Kidneys were removed from E12.5, E13.5, and E14.5 embryos and also from newborn mice; the tails were used for genotyping. The kidneys were fixed in 4% paraformaldehyde for 1 h at 4°C, soaked in 30% sucrose overnight at 4°C, and embedded in OCT compound (Sakura Finetechnical) at −80°C for cryosections or were fixed in 4% paraformaldehyde in PBS at 4°C overnight to make paraffin blocks. Sections (5 μm thick) were cut and stained with hematoxylin/eosin or were used for immunohistochemistry.
Glomerular Count and Estimation of Interstitial Area
Glomeruli were counted as described previously.42 Briefly, kidneys were isolated from newborn mice and incubated in 1 ml of 6 M HCl at 37°C for 90 min. Tissue was homogenized by several pipettings, and 25 ml of H2O was added. After incubation at 4°C, glomeruli were counted in a counting dish. The total number of glomeruli per kidney was calculated from the mean number of five counts in 1 ml. The volume density of the interstitium in the renal cortex of newborn kidneys was measured as described elsewhere.43
Immunohistochemistry
For immunostaining, frozen sections or cultured cells were incubated with antibodies directed against Trps1, Pax2 (Zymed Laboratories), Wt1 (Santa Cruz Biotechnology), Vimentin (Santa Cruz Biotechnology), cytokeratin (Sigma), E-cadherin (BD Transduction Laboratories), Pax8 (Cosmo Bio Co., Ltd.), and Cited1 (NeoMarkers). Then the sections or cells were washed in PBS, incubated with secondary antibodies, and finally mounted in VectaShield Mounting Medium (Vector Laboratories) with DAPI. Paraffin sections were deparaffinized, heated in sodium citrate for antigen retrieval, and incubated with an antibody directed against vimentin. Reaction products were with a Vectastain ABC kit (Vector Laboratories) according to the manufacturer's instructions. The anti-Trps1 antibody was generated as described previously.18 For quantification of renal vesicles stained with the anti-Pax8 antibody, the total number of renal vesicles in eight sections cut from the center of a kidney was counted and divided by 8. Four kidneys were examined.
Primary Culture of RMMs
Kidneys were dissected from E13.5 rat embryos at the stage when the ureteric buds had started to penetrate the mesenchyme. Primary culture of metanephric mesenchymal cells was performed as described elsewhere.44 Briefly, the ureteric buds were removed from isolated rudiments, and the remaining mesenchyme was incubated in 0.05% trypsin with 0.5 mM EDTA at 0°C for 5 min. Cells were mechanically dissociated by gentle aspiration, and the cell suspension was plated into six-well culture dishes (Costar) or Lab-Tek chamber slides (Nalge Nunc Int.) containing DMEM with 10% FCS, FGF2 (50 ng/ml), and TGF-α (10 ng/ml).45,46 No epithelial cell contamination was detected in the cultures. For the inhibition assay, cells were pretreated with SB203580 (30 μM) (Wako Pure Chemical Industries Ltd.) for 2 h or with Trps1 siRNA (5 nM) for 24 h before exposure to Bmp7.
Western Blot Analysis
Cultured cells were starved of serum overnight and then incubated with Bmp7 (60 ng/ml) for 48 h. After washing with cold PBS, cells were lysed in RIPA buffer on ice. Then the lysates were boiled, separated by SDS-PAGE, and blotted on nitrocellulose membranes (S&S) or polyvinylidene difluoride membranes (Millipore). The membranes were blocked with TBS containing 0.1% Tween20 and 3% BSA for 1 h at room temperature, and finally were incubated with antibodies for phospho-p38 MAPK (Cell Signaling Technology), E-cadherin (BD Transduction Laboratories), and vimentin (Santa Cruz Biotechnology) overnight at 4°C. Subsequently, the blots were washed with TBS containing 0.1% Tween20, treated with horseradish peroxidase–conjugated secondary antibodies at room temperature for 1 h, and developed with the ECL detection system (Amersham Bioscience).
Whole-Mount In Situ Hybridization
Whole-mount in situ hybridization was performed as described previously.47 Briefly, E14.5 kidneys were fixed overnight in 4% paraformaldehyde at 4°C and dehydrated in ethanol. Hybridized samples were developed with NBT/BCIP solution (Roche) and photographed under a KEYENCE VHX-900 digital microscope. For quantification of branching and nephron initiation, the length of each branch of ureteric bud was measured and the number of cap mesenchyme areas was counted. For determination of the length of the ureteric bud branches, 10 segments between branches were measured by using Image J software (NIH), and the average length was calculated. The number of colored dots in a whole kidney was counted to calculate the number of cap mesenchyme areas. Four kidneys were used for these analyses.
Statistical Analysis
All of the results are expressed as means ± SD. Unpaired t test and an analysis of multiple variance by Scheffe method were used for statistical comparison. P < 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
This work was supported in part by a research grant for priority areas from Wakayama Medical University (to Y.M.).
We are grateful to Hiroko Tanaka and Shimpei Maruoka for technical assistance and thank Dr. Elizabeth J. Robertson for use of Bmp7 null mice.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Momeni P, Glockner G, Schmidt O, von Holtum D, Albrecht B, Gillessen-Kaesbach G, Hennekam R, Meinecke P, Zabel B, Rosenthal A, Horsthemke B, Ludecke HJ: Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat Genet 24: 71–74, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Suemoto H, Muragaki Y, Nishioka K, Sato M, Ooshima A, Itoh S, Hatamura I, Ozaki M, Braun A, Gustafsson E, Fassler R: Trps1 regulates proliferation and apoptosis of chondrocytes through Stat3 signaling. Dev Biol 312: 572–581, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kunath M, Ludecke HJ, Vortkamp A: Expression of Trps1 during mouse embryonic development. Mech Dev 119[Suppl 1]: S117–S120, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Malik TH, Von Stechow D, Bronson RT, Shivdasani RA: Deletion of the GATA domain of TRPS1 causes an absence of facial hair and provides new insights into the bone disorder in inherited tricho-rhino-phalangeal syndromes. Mol Cell Biol 22: 8592–8600, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxen L, Sariola H: Early organogenesis of the kidney. Pediatr Nephrol 1: 385–392, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP: Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier C, Birchmeier W: Molecular aspects of mesenchymal-epithelial interactions. Annu Rev Cell Biol 9: 511–540, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Mizuno T, Yasugi S: Susceptibility of epithelia to directive influences of mesenchymes during organogenesis: Uncoupling of morphogenesis and cytodifferentiation. Cell Differ Dev 31: 151–159, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Ott KM, Barasch J: WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int 74: 1004–1008, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez G, Bertram JF: Organisation of bone morphogenetic proteins in renal development. Nephron Exp Nephrol 93: e18–e22, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Simic P, Vukicevic S: Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev 16: 299–308, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Dudley AT, Lyons KM, Robertson EJ: A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9: 2795–2807, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Dudley AT, Robertson EJ: Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in Bmp7 deficient embryos. Dev Dyn 208: 349–362, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Lyons KM, Hogan BL, Robertson EJ: Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev 50: 71–83, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G: BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9: 2808–2820, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Vukicevic S, Kopp JB, Luyten FP, Sampath TK: Induction of nephrogenic mesenchyme by osteogenic protein 1 (bone morphogenetic protein 7). Proc Natl Acad Sci U S A 93: 9021–9026, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Wasserman D, Hartwig S, Rosenblum ND: p38MAPK acts in the Bmp7-dependent stimulatory pathway during epithelial cell morphogenesis and is regulated by Smad1. J Biol Chem 279: 12051–12059, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Itoh S, Kanno S, Gai Z, Suemoto H, Kawakatsu M, Tanishima H, Morimoto Y, Nishioka K, Hatamura I, Yoshida M, Muragaki Y: Trps1 plays a pivotal role downstream of Gdf5 signaling in promoting chondrogenesis and apoptosis of ATDC5 cells. Genes Cells 13: 355–363, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M: Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol 18: 1121–1129, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H: Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature 362: 65–67, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R: WT-1 is required for early kidney development. Cell 74: 679–691, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB: The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech Dev 40: 85–97, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, Miyazono K: Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci 114: 1483–1489, 2001 [DOI] [PubMed] [Google Scholar]
- 24.ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin CH: Characterization of type I receptors for transforming growth factor-beta and activin. Science 264: 101–104, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K: Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol 130: 217–226, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otani H, Otsuka F, Inagaki K, Takeda M, Miyoshi T, Suzuki J, Mukai T, Ogura T, Makino H: Antagonistic effects of bone morphogenetic protein-4 and -7 on renal mesangial cell proliferation induced by aldosterone through MAPK activation. Am J Physiol Renal Physiol 292: F1513–F1525, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Ott KM, Lan D, Hirsh BJ, Barasch J: Dissecting stages of mesenchymal-to-epithelial conversion during kidney development. Nephron Physiol 104: 56–60, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Klein G, Langegger M, Goridis C, Ekblom P: Neural cell adhesion molecules during embryonic induction and development of the kidney. Development 102: 749–761, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Park JS, Valerius MT, McMahon AP: Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 134: 2533–2539, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ribes D, Fischer E, Calmont A, Rossert J: Transcriptional control of epithelial differentiation during kidney development. J Am Soc Nephrol 14[Suppl 1]: S9–S15, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Rothenpieler UW, Dressler GR: Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development 119: 711–720, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Zeisberg M, Shah AA, Kalluri R: Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem 280: 8094–8100, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Patel SR, Dressler GR: Bmp7 signaling in renal development and disease. Trends Mol Med 11: 512–518, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Nishioka K, Itoh S, Suemoto H, Kanno S, Gai Z, Kawakatsu M, Tanishima H, Morimoto Y, Hatamura I, Yoshida M, Muragaki Y: Trps1 deficiency enlarges the proliferative zone of growth plate cartilage by upregulation of Pthrp. Bone 43: 64–71, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Malik TH, Shoichet SA, Latham P, Kroll TG, Peters LL, Shivdasani RA: Transcriptional repression and developmental functions of the atypical vertebrate GATA protein TRPS1. EMBO J 20: 1715–1725, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA: The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Ishibe S, Karihaloo A, Ma H, Zhang J, Marlier A, Mitobe M, Togawa A, Schmitt R, Czyczk J, Kashgarian M, Geller DS, Thorgeirsson SS, Cantley LG: Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development 136: 337–345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R: Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134: 2397–2405, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Tufro A, Teichman J, Woda C, Villegas G: Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev 125: 558–568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakurai H, Bush KT, Nigam SK: Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis. Development 128: 3283–3293, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Maeshima A, Vaughn DA, Choi Y, Nigam SK: Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct. Dev Biol 295: 473–485, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Godley LA, Kopp JB, Eckhaus M, Paglino JJ, Owens J, Varmus HE: Wild-type p53 transgenic mice exhibit altered differentiation of the ureteric bud and possess small kidneys. Genes Dev 10: 836–850, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Cochrane AL, Kett MM, Samuel CS, Campanale NV, Anderson WP, Hume DA, Little MH, Bertram JF, Ricardo SD: Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol 16: 3623–3630, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Herzlinger D, Koseki C, Mikawa T, al-Awqati Q: Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development 114: 565–572, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA: Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 99: 377–386, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO: TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development 128: 1045–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Nakazawa M, Matsunaga K, Asamura S, Kusuhara H, Isogai N, Muragaki Y: Molecular mechanisms of cleft lip formation in CL/Fr mice. Scand J Plast Reconstr Surg Hand Surg 42: 225–232, 2008 [DOI] [PubMed] [Google Scholar]