Abstract
The kidney papilla contains a population of cells with several characteristics of adult stem cells, including the retention of proliferation markers during long chase periods (i.e., they are label-retaining cells [LRCs]). To determine whether the papillary LRCs generate new cells in the normal adult kidney, we examined cell proliferation throughout the kidney and found that the upper papilla is a site of enhanced cell cycling. Using genetically modified mice that conditionally expressed green fluorescence protein fused to histone 2B, we observed that the LRCs of the papilla proliferated only in its upper part, where they associate with “chains” of cycling cells. The papillary LRCs decreased in number with age, suggesting that the cells migrated to the upper papilla before entering the cell cycle. To test this directly, we marked papillary cells with vital dyes in vivo and found that some cells in the kidney papilla, including LRCs, migrated toward other parts of the kidney. Acute kidney injury enhanced both cell migration and proliferation. These results suggest that during normal homeostasis, LRCs of the kidney papilla (or their immediate progeny) migrate to the upper papilla and form a compartment of rapidly proliferating cells, which may play a role in repair after ischemic injury.
Adult stem cells contribute to organ repair after injury1–5; however, their contribution to normal tissue homeostasis by the generation of a continuous supply of new cells has not been readily apparent, except in tissues with relatively simple architecture and high rate of cell turnover, such as the skin and the intestinal epithelia.1,6 In addition, recent studies have shown that in some organs, precursor cells responsible for homeostatic cell turnover are different from those responsible for cell replacement after injury. For example, stem cells in the bulge of the hair follicle contribute to wound repair,5 but normal cell turnover of the epidermis is maintained by a distinct progenitor population located in the interfollicular epidermis.7 In the olfactory neuroepithelium, a site of continuous neurogenesis under normal conditions in the adult, new neurons are generated by the globose basal cells that reside in the basal germinal zone of the pseudostratified olfactory neuroepithelium; however, after extensive tissue damage, another population of cells (horizontal basal cells) starts proliferating and generates several cell types.8 Finally, β cells of the pancreas are maintained by proliferation of terminally differentiated β cells,9,10 but in injured pancreas, multipotent progenitor cells can give rise to β cells.11
The normal adult kidney has a very low rate of cell turnover,12 but it is capable of responding quickly to injury by generating new cells to replace dying ones. In a recent study using genetic fate mapping techniques, Humphreys et al.13 found that after transient kidney ischemic injury, most if not all of the newly generated tubular epithelial cells derived from surviving differentiated epithelial cells. Whether other cell types in the adult kidney can generate new cells is unknown.
We previously found that the papilla contains a population of cells with several characteristics of adult, organ-specific stem cells.14 The cells were identified by retention of an S-phase label (bromodeoxyuridine [BrdU]) given as a short pulse to newborn rats and mice followed by several months of nucleotide-free chase. We found that like other adult organ-specific stem cells,1,15,16 these papillary cells were “label-retaining” cells (LRCs); however, although they were quiescent for long periods under normal conditions, they entered the cell cycle shortly after transient ischemic kidney injury, and 3 wk afterwards, because cell proliferation will dilute the label, we found that the number of papillary LRCs had dramatically decreased after kidney injury.14
Because low cycling history (i.e., “label retention”) and proliferation (causes loss of label in the cell progeny) are opposite properties, it is likely that the number of proliferating LRCs that can be detected under normal conditions is very small. Hence, to determine whether papillary LRCs generate new cells in the adult kidney in the absence of injury, we first examined for proliferating cells in different areas of the kidney and found that the upper papilla is a site of enhanced cellular proliferation. By using genetically modified mice that conditionally express a fusion protein of histone 2B–green fluorescence protein (H2B-GFP) under the control of doxycycline,16 we found that LRCs of the papilla that proliferate are located in the upper papilla, where they form “chains” of proliferating cells. We also found that the population of papillary LRCs decreases as the animals age, suggesting that the cells migrate to the upper papilla before entering the cell cycle. By labeling a restricted area of the kidney papilla with vital dyes in vivo, we found that there is an upward migration of papillary cells, some of which are LRCs. In the aggregate, the data suggest that during normal homeostasis, papillary LRCs (or their immediate progeny) migrate to the upper part of the papilla, where they generate a “transit amplifying”1,6 compartment of rapidly proliferating cells. Transient ischemic injury seemed to enhance both processes.
Results
Cellular Proliferation in Adult Kidney
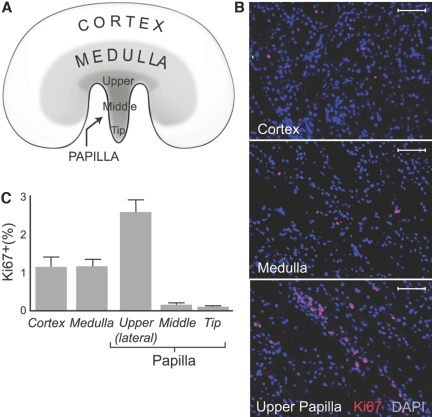
To determine the location of new cells in adult kidney, we stained tissue sections for Ki67, a marker of cellular proliferation,17 and examined their various regions (Figure 1A). Representative microphotographs of cortex, medulla, and upper papilla are shown in Figure 1B, where cell nuclei were labeled with DAPI (blue) and Ki67 immunoreactivity with rhodamine (red); thus, Ki67-positive nuclei are pink. In these adult, 1-yr-old rats, Ki67-positive cells were rare in the cortex and medulla and were solitary and of similar frequency (1.1 ± 0.1 and 1.1 ± 0.2% of the total nuclei, respectively; Figure 1C). In contrast, the papilla showed marked heterogeneity in its abundance of Ki67-positive cells; they were extremely rare in its tip and middle part (0.1 ± 0.1% of the total cells for both; P < 0.01 versus all other parts of the kidney; Figure 1C) but were readily apparent in the upper papilla, especially in its lateral areas, adjacent to the urinary space, where the kidney parenchyma forms a narrow angle that provides an easily identifiable landmark. In this region and unlike other areas of the kidney, Ki67-positive cells frequently formed chain-like structures (Figure 1B) and accounted for 2.6 ± 0.4% of the total cells (P < 0.01 versus other parts of the kidney; Figure 1C). Similar results were obtained in 6-mo-old rats (data not shown). Finally, when cellular proliferation was examined in adult rats 24 h after labeling cells in S phase by administration of a single dose of BrdU, proliferating cells were very rarely detected in the cortex and medulla, but the upper papilla consistently contained BrdU-labeled cells (data not shown).
Figure 1.
Cellular proliferation in adult kidney is shown. (A) Kidney regions were examined for cellular proliferation. (B) Ki67-positive cells in representative sections of kidney cortex, medulla, and upper papilla are shown. Cell nuclei stained with DAPI (blue) and Ki67 stained with rhodamine (red) are shown. Bars = 50 μm. (C) Fraction of Ki67-positive cells is shown. Cortex and medulla had a similar number of Ki67-positive cells, but the lateral side of the upper papilla had significantly more Ki67-positive cells than both the cortex and the medulla as well as other parts of the papilla (P < 0.01). The tip and middle part of the papilla had significantly fewer Ki67-positive cells than all other areas of the kidney (P < 0.01).
Proliferation of Papillary LRCs
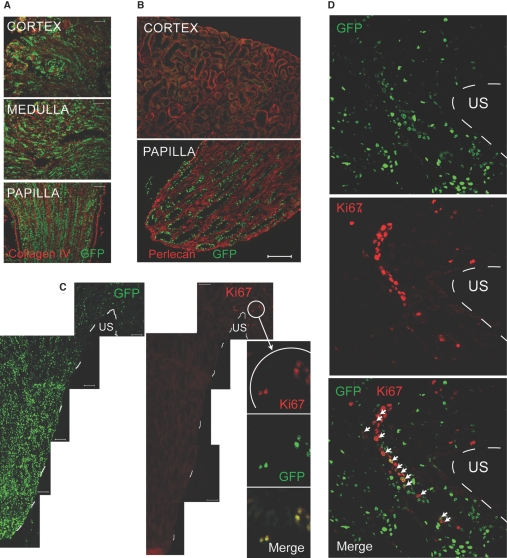
To examine whether the proliferating cells in the upper papilla related to the papillary LRCs that we previously described,14 we used transgenic mice that express hybrid histone 2B-GFP molecules (H2B-GFP) under the control of tetracycline regulatory element (i.e., when the animals are given doxycycline, dividing cells express H2B-GFP, as previously described16). The mice received doxycycline during embryonic life, and when examined shortly after birth, most if not all cells in the kidney were labeled with GFP (Figure 2A), indicating ubiquitous expression of the transgene. In addition, in the absence of doxycycline, kidneys of mice with the tetracycline-regulated H2B-GFP allele contained only an extremely rare GFP weakly positive cell (n = 2), indicating a very low level of transgene leaky expression in the absence of doxycycline. In the mice given doxycycline during embryogenesis, withdrawal of the drug at birth allowed these rapidly growing pups to dilute the H2B-GFP in the progeny of all dividing cells. In low-cycling cells, however, the H2B-GFP, being a very stable molecule, was retained for long periods after withdrawal of doxycycline.18 In the kidneys of these adult mice, we found that the papilla but not the cortex (Figure 2B) or medulla (data not shown) had many GFP+ cells, thus confirming our previous studies in the rat using BrdU as the marker for low-cycling cells.14 A similar strategy was used to identify stem cells in the skin by Tumbar et al.16 Because these mice allow identification of LRCs without the denaturing conditions needed to detect BrdU, they are ideally suited for detection of the very rare LRCs (i.e., low cycling) that at any given time may be dividing.
Figure 2.
Papillary LRCs in mice expressing histone 2B-GFP are shown. (A) Cortex, medulla, and papilla from a 10-d-old mouse pulsed with doxycycline during embryonic life are shown. (B) Low-cycling cells in kidney papilla are shown. A mouse was pulsed with doxycycline during embryonic life and chased for 11 wk after birth; shown are kidney cortex and papilla. Papillary LRCs retain GFP. Extracellular matrix labeled with an antibody to perlecan is shown. Bar = 50 μm. (C) Papillary LRCs proliferate in the upper papilla. Shown is kidney papilla with multiple LRCs; broken white line depicts papillary edge. Proliferating cells (Ki67 positive) were restricted to the upper papilla, and high-magnification views of the Ki67-positive cells showed them also to be GFP+ (i.e., low-cycling cells). Bars = 50 μm. (D) Chains of proliferating cells in upper papilla contain LRCs: Papillary LRCs (GFP+, top) and proliferating cells (Ki67-positive, middle). The merged picture shows that several cells are positive for both Ki67 and GFP (arrows). Broken while line depicts papillary edge. US, urinary space.
When we stained the papilla of these mice with Ki67 (Figure 2C), we found that whereas GFP-retaining cells were abundant throughout its body, Ki67-positive cells were detected only in the upper part of the papilla, particularly next to the urinary space. Important, as shown in the high-magnification inset, some of these Ki67-positive cells were also GFP retaining, indicating that papillary LRCs proliferate in the upper part of the papilla. Detailed examination of this region (Figure 2D) showed that, similar to the results obtained in rat kidney shown in Figure 1B, Ki67-positive cells (red) occasionally formed chain-like structures. Note in the merged image that several cells that were proliferating also contained GFP (highlighted by arrows), indicating that they were papillary LRCs.
Age-Dependent Depletion of Papillary LRCs
If under normal conditions papillary LRCs provide a steady supply of new cells in the upper part of the papilla, then it would be expected that these cells will become depleted as the animal ages. To test this, we examined papillae of rats pulsed with BrdU as pups and chased for 3 mo (n = 4) or 12 mo (n = 4), at which times their kidneys were examined. As shown in Figure 3A, along the length of the papilla, the number of BrdU-retaining cells was significantly lower in the older rats (P < 0.001). This difference was more pronounced in the most distal parts of the papilla, where the number of BrdU-retaining cells in the 12-mo-old rats was very low. Remarkably, in younger animals, the BrdU-retaining cells were uniformly distributed along the medioperipheral axis of the parenchyma of the papilla, whereas the LRCs in 12-mo-old rats were preferentially found in the periphery beneath the urothelium lining the urinary space (Figure 3B) with the center of the papilla containing few BrdU-retaining cells.
Figure 3.
Papillary LRCs decrease with age. (A) Shown is the frequency of BrdU-retaining cells in papillae of 3- and 12-mo-old rats (n = 4 for each age). There was a marked decrease in the number of BrdU-retaining cells in the 12-mo-old rats (P < 0.001). (B) Shown is the location of BrdU-retaining cells in papilla of a 3-mo-old rat. (C) Shown is the location of BrdU-retaining cells in papilla of a 12-mo-old rat. Cross-sectional views were taken of mid-papilla labeled with Dolichos biflorus agglutinin-Rhodamine (identifies collecting ducts) and BrdU-antibody (FITC) of papillae from 3- and 12-mo-old rats. In the 3-mo-old rat, BrdU-retaining cells were abundant at both the center and the periphery of the papilla, whereas in the 12-mo-old rat, few BrdU-retaining cells remained at the center of the papilla. US, urinary space.
Migration of Papillary Cells
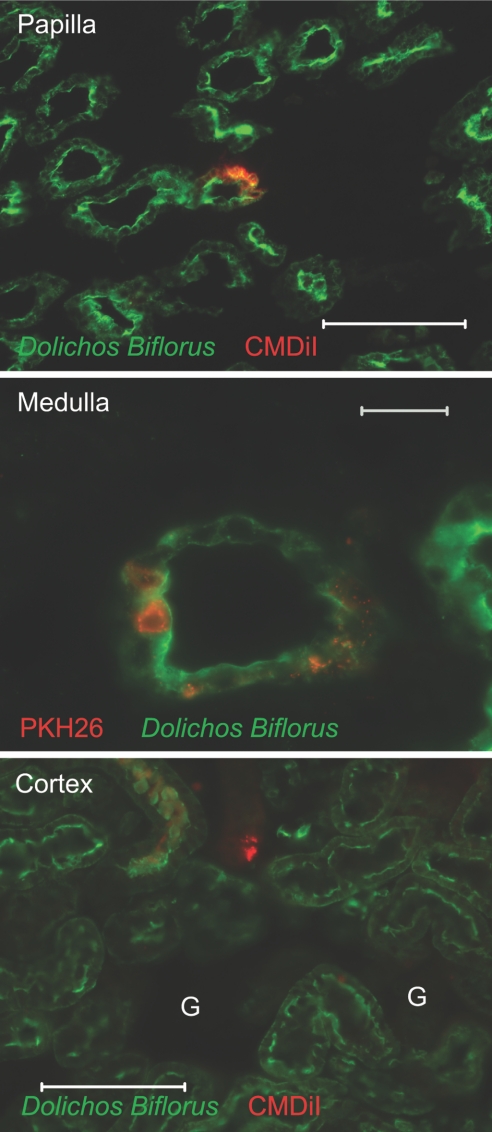
Patschan et al.19 reported a population of nestin-positive cells in the kidney papilla that migrates to other parts of the kidney after injury. Because papillary LRCs are present over the whole length of the papilla but proliferate in its upper region and their abundance in the papilla decreases with age, it suggests that the cells upwardly migrate to provide a steady supply of proliferating cells. To examine this cell migration, we developed an in vivo assay whereby a fluorescence vital dye was injected into the urinary space of the urinary pelvis of rat kidneys, and the fate of cells labeled with the dye followed (see the Concise Methods section). When papillae were examined 1 h after introduction of the dye (n = 3), cells in the superficial papilla were efficiently labeled (with PKH26 in the example shown in Figure 4A) to a depth of a few cell diameters beneath the urothelium, but no cells with dye were found in the internal parenchyma of the papilla (labeled here with Dolichos biflorus agglutinin–FITC which identifies collecting ducts); however, when kidneys (n = 8) were examined 3 d after labeling (Figure 4B), we found that whereas the superficial papilla was still the site of most labeled cells (data not shown), some labeled cells were deeper in the renal parenchyma, particularly in the papilla (top) and occasionally in the medulla (bottom, identified here by the presence of Tamm-Horsfall protein). To rule out the possibility that some of the labeled cells that were present in the kidney parenchyma may be macrophages, we co-stained sections with a CD68 antibody. Analysis of 50 PKH26-labeled cells from three experiments, which were present deep in the kidney parenchyma, revealed that none of them co-labeled with the CD68 antibody, although many CD68+ cells were detected (data not shown).
Figure 4.
In vivo labeling and migration of renal papillary cells are shown. (A) Cells in the superficial papilla of rat were labeled. Rat kidney papilla were labeled with KPH26 and isolated 1 h later. Collecting ducts were labeled with Dolichos biflorus agglutinin-FITC (photomicrograph of 25-μm section). Bar = 200 μm. (B) Papillary cells labeled with dye show upward migration; these are representative sections of normal kidneys 3 d after introduction of dye into the urinary space. (Top) Upper papilla shows CMDiI-containing cells; collecting ducts were labeled with Dolichos biflorus agglutinin-FITC. Bar = 20 μm. (Bottom) Kidney medulla shows cells containing PKH26, and thick ascending limbs were labeled with antibody to Tamm-Horsfall protein–FITC (photomicrographs of 25-μm sections). Bar = 100 μm. (C) Cells in the superficial papilla of H2B-GFP mouse were labeled. Note GFP+ cells in the body of the papilla. (D) Papillary LRCs labeled with dye show upward migration. Note that some cells in the upper papilla (top) and medulla (bottom) that contain injected CMDiI (red) were also LRCs (had a GFP+ nucleus). Bars = 20 μm
We next performed similar studies on H2B-GFP mice (n = 2), in which the superficial papilla was also successfully stained with dye (Figure 4C). Similar to studies of the rat, 3 d after injection of the dye into the urinary space, some cells with dye were found high up in the kidney parenchyma, some of which were LRCs (Figure 4D). These studies suggest that papillary cells (including LRCs) are capable of leaving the region of labeling (i.e., that region nearest the urinary space).
Papillary Cell Proliferation after Transient Ischemic Injury
We previously found that papillary LRCs were proliferating 36 h after transient ischemic injury.14 To test whether the LRCs in the upper papilla also participate in the response to injury, we first examined whether cell proliferation in this region was selectively enhanced after transient ischemic injury. To do this, we developed an assay of cell proliferation in which BrdU was administered for a short time so as to label the fewest number of cells in a normal kidney. Adult normal rats (n = 2) were given a single dose of BrdU, and their kidneys were harvested 1 h later (control rats) whereas other rats (n = 3) were subjected to 45 min of unilateral kidney ischemia, after which they were given a single dose of BrdU and 1 h later killed. Thus, only cells that were in S phase during this hour could be labeled. Using 5-μm kidney sections, we saw only rare labeled cells in kidneys subjected to ischemia, but with 100-μm vibratome sections, we consistently found abundant cells in S phase in the upper papilla (Figure 5A), next to the urinary space. In contrast, only an occasional labeled cell was detected in the cortex and medulla, where their abundance was not greater than that in the contralateral, nonischemic kidneys (data not shown). As expected, the upper papilla of the control kidneys not subjected to ischemia had no or only extremely rare cells in S phase (see Figure 5A).
Figure 5.
Cellular proliferation occurred in upper papilla after ischemic injury. (A) There was a selective increase of cells in S phase in the rat upper papilla during the first hour after injury. Shown is the lateral part of the upper papilla from a rat subjected to 45 min of unilateral renal ischemia (left) and from a control normal rat (right) both given BrdU for 1 h. Note abundant BrdU-labeled cells in the postischemic papilla, whereas the cortex and medulla of the same kidney revealed very rare BrdU-labeled cells. US, urinary space. Photomicrographs were done in 100-μm tissue sections obtained with a vibratome. Bars = 100 μm. (B) Papillary LRCs in the upper papilla proliferate after ischemic injury. Rat upper papilla have BrdU-retaining cells 12 h after injury; Ki67-positive cells (i.e., proliferating) formed chain-like structures, and several of these cells contained BrdU, indicative of low-cycling history (n = 2). (C) There is a selective increase of proliferating cells in the upper papilla of an H2B-GFP mouse subjected to 20 min of unilateral renal ischemia. Twenty-four hours later, there were abundant Ki67-positive cells in the postischemic upper papilla, whereas cycling cells were very rare in the contralateral organ (right). Bars = 50 μm. (D) Some proliferating cells in the upper papilla of H2B-GFP mouse were LRCs. Confocal microphotograph with ortho projections show that several LRCs (GFP+) from the postischemia section in C also contained Ki67. (E) Decrease in LRCs occurs 2 wk after ischemia. LRCs (GFP+) are seen in the upper papillae from an H2B-GFP mouse subjected to unilateral transient ischemia. (Left) Papilla of the kidneys were subjected to ischemia. (Right) The contralateral, control side is shown. Bars = 50 μm.
To examine whether papillary LRCs contributed to this proliferative response, we induced transient renal ischemia in rats given BrdU as newborns and chased for 3 mo (n = 2). Twelve hours after transient ischemia, kidneys were harvested and stained for Ki67 (a marker of proliferation) and for BrdU (in these rats, a marker of low-cycling activity). As shown in Figure 5B, chains of proliferating cells could be easily detected in the upper papilla, with many of the proliferating cells also positive for BrdU. Second, we induced transient unilateral kidney ischemia in H2B-GFP mice (n = 2) and examined for Ki67-positive (i.e., proliferating) cells 24 h later. As shown in Figure 5C, the upper part of the papilla of the postischemic kidney contained many cycling cells, which were very rare in the contralateral, control kidney. In addition, high-power magnification showed that many of the cycling cells (Ki67 positive) were LRCs (GFP+), as shown in Figure 5D. Thus, as in normal conditions, LRCs in the base of the papilla generate new cells shortly after transient ischemic kidney injury but do so at a much higher frequency.
Because of the higher cycling frequency by LRCs after transient ischemic injury, we next examined whether this resulted in a decrease in the number of papillary LRCs in H2B-GFP mice. Confirming previous results in the rat,1 as shown in Figure 5E, 2 wk after transient ischemic injury, when compared with the control papilla, the papilla of the injured kidney had substantially fewer LRCs as a result of their proliferation, thereby losing the label as well as their migration out of the papilla.
Papillary Cell Migration In Vivo after Transient Ischemic Injury
To test whether papillary cell migration may be enhanced after injury, we labeled cells of the superficial papilla, as described previously, with the kidneys being subjected to transient ischemia shortly before the introduction of the dye (n = 8). Compared with the kidneys of rats not subjected to ischemia, labeled cells were found not only in the papilla and medulla (Figure 6) but also occasionally in the cortex (identified here by the presence of glomeruli), a distant location from the site of dye introduction; however, the small number of observations prevented formal comparisons, and it needs to be established that ischemic injury enhanced migration. Remarkably, some of the images show that the labeled cells became incorporated into renal tubules (medulla, Figure 6). To rule out the possibility that some of the labeled cells that were located in the kidney parenchyma could be macrophages, we again co-stained tissue sections with a CD68 antibody. Although most of the cells with vital dye did not label with CD68, some did, and their frequency was quantified. In kidney sections from four experiments, of 100 cells that contained vital dyes, 10 cells were also labeled with CD68 (data not shown), indicating that, unlike normal conditions, after ischemic injury, 10% of the cells with dye were macrophages.
Figure 6.
Papillary cells labeled with dye after injury show upward migration. Labeled cells with dye were easily detected in the parenchyma of the papilla; collecting ducts were labeled with Dolichos biflorus agglutinin–FITC (top; bar = 100 μm). The kidney medulla also contained cells with dye, some of which integrated into collecting ducts (bar = 20 μm). Only in kidneys subjected to ischemic injury were cells with dye occasionally detected in the kidney cortex. G, glomerulus. Bar = 100 μm (photomicrographs of 25-μm sections).
Characterization of Papillary LRCs
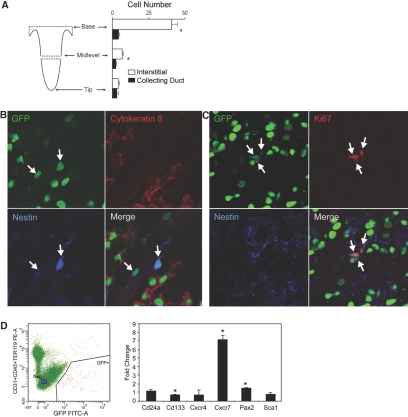
To determine the identity of the papillary LRCs, we isolated from the rest of the kidney at their base and further sectioned at midlevel renal papillae from adult rats that had been pulse-chased with BrdU (Figure 7A, left). Tissue blocks containing papillary tips, midlevel areas, and papillary bases were obtained, and sections along the axial plane were used to detect LRCs. Identification of the LRCs was done by identifying BrdU-positive nuclei in cells of the following compartments: Collecting duct (labeled with Dolichos biflorus agglutinin, as shown in Figure 3, B and C), thin descending limbs (labeled with an antibody to aquaporin 1), thin ascending limbs (labeled with an antibody to chloride channel CLC-K), and interstitial space (outside these structures). Analysis of three noncontinuous sections from each block showed that except for extremely rare exceptions, BrdU-positive cells were either collecting duct cells or interstitial cells. No BrdU-positive nuclei were identified in cells positive for the chloride channel CLC-K, and on an extremely rare occasion, a BrdU-positive nucleus was identified in a cell positive for aquaporin 1 (data not shown); these cells were excluded from analysis. Figure 7A (right) shows that in the tip of the papilla, LRCs were similarly located in the collecting duct (3 ± 1 cells per field) and in the interstitial space (4 ± 3 cells per /field; NS). In the midlevel of the papilla, there was a slight but statistically significantly greater number of BrdU-positive interstitial cells than collecting duct cells (7 ± 1 versus 3 ± 1 cells per field; P < 0.05). This difference was markedly accentuated at the base of the papilla, where most of the BrdU-positive cells were interstitial cells and only 14% of the total number of positive cells were located in the collecting ducts (44 ± 5 versus 7 ± 2 cells per field; P < 0.001).
Figure 7.
(A) LRCs were identified in rat kidney papilla. Kidney papillae from 1-yr-old rats (n = 5) that had been pulse-chased with BrdU were isolated from the rest of the kidney and sectioned as shown (left). (Right) LRCs in the tip of the papilla were similarly located in the collecting duct and in the interstitial space, whereas in the middle level of the papilla, there was a slight but statistically significant greater number of LRC interstitial cells than collecting duct cells. This difference was markedly accentuated at the base of the papilla. (B) LRCs were identified in H2B-GFP mouse. Confocal image of a papillary tip of a H2B-GFP mouse show LRCs (GFP) in the collecting ducts (cytokeratin 8 positive; Rhodamine) and interstitium. Note in the merged image that two LRCs that are outside the collecting ducts (marked with arrows) are nestin positive (blue). (C) LRCs in H2B-GFP mouse proliferate. Confocal image of the upper part of the papilla shows three LRCs (arrows) that are cycling (Ki67 positive; Rhodamine) and nestin (blue) positive and hence are interstitial cells. When the same area was stained with cytokeratin 8 to identify LRCs that were also proliferating (Ki67 positive), none was found. (D) FACS isolation and relative overexpression of mRNA in GFP+ versus GFP− cells are shown. Cells from kidney papilla that were negative for DAPI (i.e., alive cells) and PE-labeled antibodies to CD45, TER119-PE, and CD31 were sorted to GFP+ and GFP− populations. Of the genes tested, Cd24a, Cd133, Cxcr7, and Pax2 were significantly overexpressed (fold change) in the papillary LRCs, whereas the expression of Cxcr4 and of Sca1 was no different between the two cell populations. Expression of Oct4 was not detected in either cell population. *P < 0.05.
The two populations of LRCs were also found in the H2B-GFP mouse (see Figure 7B), where the collecting ducts were identified here by cytokeratin-8 immunolabeling.20 Similarly, as in the rat, interstitial cells were markedly more abundant in the base of the papilla than in its tip (data not shown). The H2B-GFP mouse further allowed us to examine several potential markers of the interstitial LRCs, and, stimulated by recent findings,19 we examined for the presence of nestin; as shown in Figure 7, B and C, we found that the interstitial LRCs were nestin positive.
To determine which of the two types of LRCs proliferate in the upper part of the papilla, we examined tissue sections from H2B-GFP mice for LRCs that were also Ki67 positive and stained them with either cytokeratin-8 (to identify collecting duct LRCs) or nestin (to identify interstitial LRCs). As described already, we found only rare LRCs that were also Ki67 positive, but all of these cells were also nestin positive (i.e., interstitial), as illustrated in Figure 7C; no LRCs from the collecting duct was found to be proliferating in the base of the papilla (data not shown).
To determine whether the LRCs of the papilla shared some of the molecular markers described in a variety of precursor/stem cells in the adult kidney,21–25 we isolated LRCs from the H2B-GFP mouse as described previously,14 and after incubation with antibodies for hematopoietic and endothelial lineages (CD45, TER119, and CD31, all coupled to PE), cells that were PE negative were sorted into GFP+ and GFP− populations (see Figure 7D, left). Real-time PCR on the GFP+ and GFP− cells was then conducted, and as shown in Figure 7D (right) relative to the GFP− cell population, papillary LRCs overexpressed Cd133, Pax2, and particularly Cxcr7; expression of Oct4 was not detected in either cell population.
Discussion
The data indicate that the low-cycling papillary LRCs of the adult normal kidney are a steady source of new cells and that they likely contribute to the kidney's homeostasis and function. The data also show that the renal papilla has two domains with strikingly different effects on the cell cycle of papillary LRCs. When located more distally in the papilla's parenchyma, the cells remain quiescent for long times, but in a restricted area of the base of the papilla, they enter the cell cycle. Low cycling history (i.e., label retention) and proliferation are mutually exclusive, because cell cycling leads to dilution and loss of the label into the LRC progeny. Hence, the number of LRCs that are proliferating under normal conditions is likely to be very small, and it is not surprising that their detection is difficult. We were greatly aided by the initial observation that the base of the papilla is a site of enhanced cell proliferation (Figure 1C). Interestingly, in the base of the papilla, proliferating LRCs were occasionally found in “chain-like structures” of cycling cells Figure 2D), suggesting that, in this region, papillary LRCs generate a population of quickly dividing daughter cells, a similar arrangement to that found in other organs with adult, organ-specific stem cells such as the skin,1 brain,26 prostate,27 and gonads28; however, whether the cycling LRCs of the upper papilla generate a true “transit amplifying” cell compartment that is capable of differentiating into mature kidney cells will require lineage tracing experiments. Interestingly, the upper papilla seems to be a site of privileged cellular proliferation only in adult animals. Although we found that the upper papilla contained the greatest number of cycling cells in adult rats, Vogetseder et al.29 found that cell proliferation in growing rats (4 to 5 wk old) was most apparent in the medullary rays of the cortex and the outer stripe of the medulla. The reason(s) for these differences is at present unknown, but it is to be expected that growing animals would have different rates of cell proliferation than adult ones.
Transient renal ischemia rapidly and selectively enhanced cellular proliferation in the upper papilla, and at least some of these proliferating cells were papillary LRCs (Figure 5), suggesting that in response to ischemic injury, LRCs of the upper papilla also contribute to the initial generation of new cells in response to injury. We also found that the number of papillary LRCs decreased with age (Figure 4), and although several possibilities such as cell death or slow cell division in situ could account for this decrease, when combined with the finding that LRCs proliferate in the upper papilla, it suggests that papillary LRCs or their immediate progeny (and still label retaining) migrate upward in the papilla. Results of in vivo experiments with vital dyes support this hypothesis. Three days after labeling a narrow region of the superficial papilla in vivo with vital dyes, labeled cells were detected deep in the kidney parenchyma, some of which were LRCs (Figure 4D). Interestingly, transient kidney ischemia seemed to increase upward migration of papillary cells; unlike in normal kidneys, in kidneys subjected to ischemia, labeled cells were occasionally found in the cortex (Table 1).
Table 1.
Area of kidney parenchyma with cells containing dye 3 d after its introduction in the urinary space
| Parameter | Papilla | Medulla | Cortex |
|---|---|---|---|
| Control (n = 8) | 8 | 2 | 0 |
| After ischemia (n = 8) | 8 | 4 | 3 |
Data derived from six sections of 25-μm thickness from each kidney. In each section, the total area of the kidney was examined for the presence of labeled cells, and detection of a single cell with dye was scored as positive.
Characterization of the papillary LRCs showed that both in rats pulse-chased with BrdU and in the H2B-GFP mouse, papillary LRCs were identified as collecting duct cells and interstitial cells. It is unresolved whether these two cell populations are related, but detailed morphologic observations of collecting duct cells found numerous projections extending from the basal part of the cell's cytoplasm through the duct's basement membrane into the papillary interstitium30 and suggest that collecting duct cells can migrate into the interstitium.31 Interestingly, we also found that interstitial LRC are nestin positive, a protein of the intermediate filament family originally found in neuroepithelial stem cells (see reference32 for a review). Nestin function is unknown, and although it can be found in terminally differentiated cells such as podocytes, it is also found in a variety of multilineage progenitor cells and injured tissues.32 Using a nestin-GFP transgenic mouse, Patschan et al.19 reported that the kidney papilla had abundant nestin-positive cells and that after ischemia, the cells migrated from the papilla and outer medulla toward the cortex. Their results are consistent with our findings and suggest that among the population of nestin-positive cells migrating out of the papilla are LRCs and their progeny. Unlike interstitial LRCs, we could not detect collecting duct LRCs that were proliferating. This could be due to several possibilities. First, because LRCs that are proliferating are difficult to find, it is possible that insufficient focus was applied to the proper site where the cells cycle. Second, collecting duct LRCs enter the cell cycle solely under conditions that we did not examine (e.g., acidosis33). Finally, it is also possible that these cells undergo epithelia-to-mesenchymal transformation31,34 and perhaps migrate out of the collecting duct before proliferation.30,31 Additional work is needed to distinguish among these alternatives.
The molecular cues that signal the papillary LRCs to be in cell-cycle arrest deep in the papilla and to proliferate in its upper part as well as to migrate remain obscure, but the unique environment of the kidney papilla, with its low oxygen tension and marked osmolar and oxygen gradients,35,36 is likely to be important in their regulation. A related question is the mechanism and migratory pathways used by renal papillary LRCs in their upward movement. The most urgent question that needs to be answered, however, is the identity of the papillary LRC progeny and what their function is; this is likely to require genetic labeling of the LRC progeny.
The origin of new cells in the adult kidney during normal conditions and after injury is beginning to be elucidated. Morphologic analysis had long suggested that terminally differentiated epithelial cells can generate new tubular cells,37 an observation supported by studies with timed administration of thymidine analogues29,38 and more elegantly confirmed recently by Humphreys et al.13 Others have also searched for LRCs in the kidney; for instance Maeshima et al.38 administered BrdU to adult rats for 1 wk and harvested the kidneys 2 wk after a period of nucleotide-free chase. They found scattered LRCs in the proximal, distal, and collecting tubules. To accumulate the label, these cells must have divided during the week of BrdU administration and either had not divided or had divided only rarely during the subsequent 2 wk of chase. These results are consistent with low abundance of cycling cells in the adult kidney (see Figure 1). It is not surprising that their LRCs differ from the ones we found in the papilla, because, unlike their long pulse/short chase method of identifying LRCs, we labeled cells during embryogenesis (with GFP) or immediately after birth (with BrdU) and using a short pulse and a very long chase, often several months in duration. Moreover, this chase period occurs during the transition from newborn to adulthood (i.e., a period of intensive cell cycling); hence, only slow-cycling cells will remain labeled in our method. Thus, the difference between the two protocols is that although both identify LRCs, the short pulse/long chase identifies slow-cycling cells, one of the defining characteristics of stem/precursor cells. Whether the LRCs identified by Maeshima et al.38 were labeled as a consequence of the normal turnover of renal epithelial cells are low-cycling cells or are true progenitor cells remains to be determined. Nonetheless, their finding that many of the epithelial BrdU-labeled cells in the tubules were cycling after kidney ischemic injury indicates that during organ repair, new renal cells generate from division of terminally differentiated cells.38 Indeed, Humphreys et al.13 genetically labeled newly generated cells and found that after kidney injury, most if not all of the new epithelial cells derived from differentiated surviving epithelial cells. Nonetheless, it is likely that both under homeostatic conditions and during repair from injury, several mechanisms and cell populations might contribute to new kidney cells and organ repair. Other organs such as the skin39 and brain,40 to cite the most prominent, contain several types of stem/precursor cells that provide new cells for specific locations and functions. Given the complex renal architecture and the multiple cell types present in the kidney, it is likely that this organ also possesses multiple mechanisms of cell renewal and regeneration. Indeed, recent studies of adult kidney reported the presence of several cells with variable properties of precursor/stem cells. Some of these cells are epithelial,24,38,41 others mesenchymal,22,23,42,43 and yet others of unclear origin.21,43 Analysis of these cells showed the frequent presence of the somatic stem cell marker CD13321,24 and the renal embryonic stem cell marker Pax2,21,22 which we also found in papillary LRCs (Figure 7D). Whether these findings suggest a common embryonic origin and/or function for all of these cells remains to be determined.
Concise Methods
BrdU Loading of Rats
Rats with BrdU-retaining cells were obtained as described previously.14 In the rats in which cellular proliferation was examined by the acute incorporation of BrdU, in both control rats and rats subjected to transient renal ischemia, BrdU at 100 mg/kg was administered intraperitoneally 1 h before killing. In the latter animals, BrdU was given immediately after releasing the renal artery clamp.
H2B-GFP Mice
For marking infrequently cycling cells (i.e., LRCs) with GFP, H2B-GFP mice under the control of tetracycline regulatory element16 were generated (A.K. and A.E., manuscript in preparation). Pregnant female mice were given drinking water containing 2 mg/dl doxycycline starting after plug detection and until day of delivery. Pups expressing H2B-GFP were identified by analysis of the tail tip with a fluorescence microscope (when given doxycycline during embryogenesis) or by PCR with appropriate GFP primers. After withdrawal of doxycycline (chase period; up to 20 wk of age), quiescent cells retain GFP because the fusion H2B-GFP protein is very stable,18 but cells that continue to divide lose the GFP signal because the H2B-GFP protein is diluted in their progeny.
Transient Renal Ischemia
Renal injury was induced by clamping of the left renal artery as described previously.14 In rats, renal artery clamping was done for 45 min, whereas in H2B-GFP mice, clamping lasted 20 min. In addition, For ensuring that adequate urine flow was maintained after kidney injury, in the experiments in which vital dyes were injected into the urinary pelvis of rats (and are described next), the period of ischemia was only 30 min.
In Vivo Labeling of Renal Papillary Cells
To track movement of cells in the renal papilla, we developed a method to label a discrete layer of cells by the brief introduction of vital dyes directly into the urinary pelvis. Sprague-Dawley 3-mo-old male rats were anesthetized, and a posterior subcostal incision was made in their left side, as described previously.14 The ureter adjacent to the urinary pelvis was dissected and surrounded by a silk thread, which, upon gentle pooling, stopped the flow of urine. Within 2 to 3 min, the renal pelvis dilated, thereby allowing insertion into the pelvis of a 36-G needle and injection of the dye. The dye was maintained inside the renal pelvis by keeping the ureter occluded for an additional 5 min so that total occlusion time was <10 min. After removal of the occluding suture, the dilation of the upper ureter and renal pelvis as well as the dye quickly disappeared, indicating restoration of the flow of urine. In the kidneys examined 3 d after introduction of the dyes—eight control rats and eight rats subjected to 30 min of unilateral renal ischemia (done 3 h after introduction of the dye)—the following dyes were used: 50 μg of CMDiI (Invitrogen) dissolved in 50 μl of DMSO was injected into five control rats and five postischemic rats; 25 μl of 1 mM PKH26 Red (Sigma) was injected into two control and two postischemic rats, and 25 μg of FM1–43 (Invitrogen) dissolved in 30 μl of PBS was injected into one control and one postischemic rat. Because PKH26 is dissolved in ethanol and the final concentrations of both the dye and its solvent in the urinary space are unknown, it is possible that some cells in contact with the dye lost membrane integrity. Thus, it is conceivable that some of the PKH26-containing cells found deep in the kidney parenchyma may have obtained the label after phagocytosis of fragments from cells damaged by the ethanol or PKH26; however, analysis of the kidneys showed no detectable differences among the three dyes. For analysis of the control kidneys after dye introduction into the urinary space, three rats received one of the three dyes described and were killed 1 h later.
When vital dyes were administered into the renal pelvis of the H2B-GFP mice, 10 μg of CMDiI dissolved in 10 μl of DMSO was used. In addition, because of technical difficulties to canalize the ureter, instead of a subcostal approach, an anteriolaparotomy was used.
Immunodetection
Kidneys were isolated and sectioned along the coronal plane with care taken to ensure that papilla remained in one of the two half kidneys, which, after fixation, was used for analysis. The half kidney was fixed with 4% paraformaldehyde and after incubation with 30% sucrose was frozen in Tissue-Tek OTC compound (Sakura Finetek). For 100-μm vibratome sections, kidneys were fixed in −20°C methanol, embedded in a block of 3% agarose, and sectioned with a 1000 Plus Vibratome (Pelco). BrdU detection was done as described previously.14 Cell proliferation assay with Ki67 antibodies was always preceded by incubation of the section with DakoCytomation Target Retrieval Solution (DAKO) in the experiments with rats; for the experiments with mouse expressing H2B-GFP, the antigen retrieval incubation was omitted. Fluorescence signals were detected with a fluorescence microscope and an RT Slider SPOT digital camera (Diagnostic Instruments). Sections were also viewed with an Axiovert 100 laser-scanning confocal microscope (model LSM 410; Carl Zeiss).
Quantification of Ki67-Positive Cells
In sections of whole kidneys of 1-yr-old rats, the number of cells labeled by the antibody to Ki67 was quantified as a percentage of the total number of cells (stained with DAPI) in four ×200 random fields from each one of three noncontinuous 5-μm sections of one kidney from four different rats.
Quantification of BrdU-Positive Cells
Kidney papillae from rats pulsed with BrdU when pups as described previously14 and chased to 3 and 12 mo were mounted in blocks of Tissue-Tek OCT. Transversal sections of the papillae were obtained from the tip, middle part, and base. At each level, three nonconsecutive 5-μm sections were analyzed, and the BrdU-positive cells present in three ×200 random fields were quantified.
FACS Isolation of Papillary Cells
Adult mice expressing histone 2B-GFP were pulsed with doxycycline during embryonic age and chased to adulthood. Nine mice were killed, and their kidney papilla was isolated as described previously.14 Upon isolation, cells were incubated at 4°C (30 min) with anti-mouse antibodies to CD45-PE (clone 30F11), to TER119-PE (clone TER-119), and to CD31 (clone 390) all coupled to PE (BioLegend). After washing, a BD FACSria was used to sort live (DAPI-negative population) GFP+ and GFP− cells.
RNA Analysis of Isolated Cells
Total RNA was isolated from both GFP− and GFP+ cell populations using the Absolutely RNA Microprep Kit (Stratagene) with on-column DNase treatment. cDNA was synthesized using the MessageBOOSTER Whole-Transcriptome cDNA Synthesis Kit for qPCR according to the manufacturer's instructions (Epicenter Biotechnologies). Quantitative reverse transcriptase–PCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR system using Power SYBR Green PCR Master Mix (10 min at 95°C with 45 cycles of 15 s at 95°C, 30 s at 56°C, and 45 s at 72°C). Each experiment was performed in triplicate. Specificity of the amplification was verified by melting-curve analysis and agarose gel electrophoresis. Expression values were normalized against glyceraldehyde-3-phosphate dehydrogenase and relative fold increases of gene expression in GFP+ versus GFP− populations were calculated using the Pfaffl technique of relative quantification, which accounts for real-time efficiencies. Results were assessed by ANOVA. The primers for real-time quantitative PCR analysis are listed in Table 2.
Table 2.
Primers for real-time quantitative PCR analysis
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Cd24a | CTCCTACCCACGCAGATTTA | TGGTGGTAGCGTTACTTGGA |
| Cd133 | ACCAACACCAAGAACAAGGC | GGAGCTGACTTGAATTGAGG |
| Cxcr4 | AGGAAACTGCTGGCTGAAAAG | GATGCTCTCGAAGTCACATCCT |
| Cxcr7 | GGTCAGTCTCGTGCAGCATA | GTGCCGGTGAAGTAGGTGAT |
| Gapdh | AAGGTCATCCCAGAGCTGAA | CCTGCTTCACCACCTTCTTG |
| Oct4 | GTGTTCAGCCAGACCACCAT | GAACCATACTCGAACCACATCC |
| Pax2 | AAGCGACAGAACCCGACTATGT | CACTCCTGTCCCTGCCCCATC |
| Sca1 | CTCTGAGGATGGACACTTCT | GGTCTGCAGGAGGACTGAGC |
Antibodies
The following antibodies were used: Monoclonal against BrdU, clone BMC9318 (Roche); monoclonal against Ki67 antigen, clone MIB-5 (DAKO); rabbit polyclonal against Ki67 antigen (Novocastra and Abcam); rat monoclonal against perlecan, clone A7L6 (RDI); rabbit polyclonal to collagen IV (Abcam); goat polyclonal to nestin (Santa Cruz Biotechnology); rabbit polyclonal against aquaporin I (Abcam); rabbit polyclonal to CLC-K (Chemicon); and monoclonal to rat CD68 (Serotec); chicken polyclonal to GFP (Aves). FITC- or rhodamine-coupled Dolichos biflorus agglutinin was from Vector Laboratories. Nuclei were stained with DAPI (Invitrogen).
Statistical Analysis
Data are shown as means ± SEM and were analyzed by ANOVA with Bonferroni correction for multiple comparisons. When appropriate for transformed values, the Tukey-Kramer multiple comparison test was used.44
Disclosures
None.
Acknowledgments
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant DK-55388 (to J.O. and Q.A.) and by a gift from the Berrie Foundation (to A.E.) and by National Cancer Institute grant 1P01 CA97403 (Project2 to A.E.). A.K. was supported by a Fellowship from the Jane Coffin Childs Memorial Fund for Medical Research. R.V.S. was supported by a Research Career Award from the National Institute of Diabetes and Digestive and Kidney Diseases (K08-DK078014).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Slow-Cycling Cells in Renal Papilla: Stem Cells Awaken?” on pages 2277–2279.
References
- 1.Lavker RM, Sun TT: Epidermal stem cells: Properties, markers, and location. Proc Natl Acad Sci U S A 97: 13473–13475, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P: Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 658–659, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Petersen BE, Zajac VF, Michalopoulos GK: Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology 27: 1030–1038, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Mothe AJ, Tator CH: Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult bat. Neuroscience 131: 177–187, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM: Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102: 451–461, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Crosnier C, Stamataki D, Lewis J: Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat Med 7: 349–359, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G: Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Leung CT, Coulombe PA, Reed RR: Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci 10: 720–726, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA: Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12: 817–826, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Xu X, D'Hoker J, Stangé G Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H: Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197–207, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Ledda-Columbano GM, Columbano A, Coni P, Curto M, Faa G, Pani P: Cell proliferation in rat kidney induced by 1,2-dibromoethane. Toxicol Lett 37: 85–90, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q: The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A: Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E: Defining the epithelial stem cell niche in skin. Science 303: 359–363, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholzem T, Gerdes J: The Ki-67 protein: From the known and the unknown. J Cell Biol 182: 311–322, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Brennand K, Huangfu D, Melton D: All beta cells contribute equally to islet growth and maintenance. PLoS Bio 5: 1–10, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patschan D, Michurina T, Shi HK, Dolff S, Brodsky SV, Vasilieva T, Cohen-Gould L, Winaver J, Chander PN, Enikolopov G, Goligorsky MS: Normal distribution and medullary-to-cortical shift of Nestin-expressing cells in acute renal ischemia. Kidney Int 71: 744–754, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hemmi A, Mori Y: Immunohistochemical study of cytokeratin distribution in the collecting duct of the human kidney. Acta Pathol Jpn 41: 516–520, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G: Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166: 545–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME: Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol 17: 3028–3040, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, Reisner Y: Isolation and characterization of nontubular Sca-1+Lin multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol 17: 3300–3314, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P: Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med 205: 479–490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn S, Joyner AL: In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehod. Nature 437: 894–897, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, Goto K, Wilson EL: Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci U S A 102: 7180–7185, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa T, Nabeshima Y, Yoshida S: Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 12: 195–206, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M: Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol 292: C807–C813, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Bulger RE, Trump BF: Fine structure of the rat renal papilla. Am J Anat 118: 685–722, 1966 [DOI] [PubMed] [Google Scholar]
- 31.Butt MJ, Tarantal AF, Jimenez DF, Matsell DG: Collecting duct epithelial-mesenchymal transition in fetal urinary tract obstruction. Kidney Int 72: 936–944, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM: Nestin expression: A property of multi-lineage progenitor cells? Cell Mol Life Sci 61: 2510–2522, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huyen JP, Cheval L, Bloch-Faure M, Belair MF, Heudes D, Bruneval P, Doucet A: GDF15 triggers homeostatic proliferation of acid-secreting collecting duct cells. J Am Soc Nephrol 19: 1965–1974, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanova L, Butt MJ, Matsell DF: Mesenchymal transition in kidney collecting duct epithelial cells. Am J Physiol Renal Physiol 294: 1238–1248, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Edwards A: Oxygen transport across vasa recta in the renal medulla. Am J Physiol Heart Circ Physiol 283: H1042–H1055, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Sadowski J, Dobrowolski L: The renal medullary interstitium: Focus on osmotic hypertonicity. Clin Exp Pharmacol Physiol 30: 119–126, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Oliver J, MacDowell M, Tracy A: The pathogenesis of acute renal failure associated with traumatic and toxic injury: renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest 30: 1307–1439, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeshima A, Yamashita S, Nojima Y: Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 14: 3138–3146, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Levy V, Lindon C, Harfe BD, Morgan BA: Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 9: 855–861, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Alvarez-Buylla A, Lim DA: For the long run: Maintaining germinal niches in the adult brain. Neuron 41: 683–686, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Challen GA, Bertoncello I, Deane JA, Ricardo SD, Little MH: Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J Am Soc Nephrol 17: 1896–1912, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Meirelles L, Chagastelles PC, Nardi NB: Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119: 2204–2213, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Hishikawa K, Marumo T, Miura S, Nakanishi A, Matsuzaki Y, Shibata K, Ichiyanagi T, Kohike H, Komori T, Takahashi I, Takase O, Imai N, Yoshikawa M, Inowa T, Hayashi M, Nakaki T, Nakauchi H, Okano H, Fujita T: Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J Cell Biol 169: 921–928, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zar JH: Biostatistical Analysis, 2nd Ed., Upper Saddle River, NJ, Prentice-Hall, 1984, pp 239–241 [Google Scholar]