Abstract
In the setting of renal ischemia-reperfusion injury (IRI), the effect and mechanism of action of glucocorticoids are not well understood. In rat renal IRI, a single dose of dexamethasone administered before ischemia, or at the onset of reperfusion, ameliorated biochemical and histologic acute kidney injury after 24 h. Dexamethasone upregulated Bcl-xL, downregulated ischemia-induced Bax, inhibited caspase-9 and caspase-3 activation, and reduced apoptosis and necrosis of proximal tubular cells. In addition, dexamethasone decreased the number of infiltrating neutrophils and ICAM-1. We observed the protective effect of dexamethasone in neutrophil-depleted mice, suggesting a neutrophil-independent mechanism. In vitro, dexamethasone protected human kidney proximal tubular (HK-2) cells during serum starvation and IRI-induced apoptosis, but inhibition of MEK 1/2 abolished its anti-apoptotic effects in these conditions. Dexamethasone stimulated rapid and transient phosphorylation of ERK 1/2, which required the presence of the glucocorticoid receptor and was independent of transcriptional activity. In summary, in the setting of renal ischemia-reperfusion injury, dexamethasone directly protects against kidney injury by a receptor-dependent, nongenomic mechanism.
Renal ischemia-reperfusion injury (IRI) causes acute renal failure in varied clinical settings including kidney transplantation1 and cardiopulmonary2 and aortic bypass surgery3 and is associated with high mortality and morbidity.4–6 For decades, glucocorticoids (GCs) have been successfully used in the treatment of numerous clinical conditions for their anti–inflammatory and immunosuppressive effects. GCs have been studied as renoprotective agents in a few small postvascular surgical studies and in varied experimental settings7–10 associated with renal IRI. However, the effects of GCs on renal IRI have never been thoroughly studied. Various anti-apoptotic modalities ameliorate renal dysfunction secondary to renal IRI.11 GCs have anti-apoptotic effects on other cell lines12–14; however, it is unknown whether GCs have such effects on the proximal tubular cell, the cell that is most vulnerable to renal IRI. Moreover, the renal proximal tubular epithelial cells are “steroid insensitive” with respect to its direct anti–inflammatory effects.15–17
Classically, GCs enter target cells and bind to the inactive cytoplasmic glucocorticoid receptor (GR);the activated ligand–receptor complex translocates into the nucleus to regulate gene transcription directly (transactivation) by binding as a homodimer to the glucocorticoid response elements (GREs) or indirectly (transrepression).18,19 Transrepression is considered to be the key mechanism for the anti-inflammatory activity of GCs, whereas, in contrast, several side effects are thought to be predominantly mediated via transactivation. There is increasing evidence that steroid hormones regulate physiologically important processes via nongenomic mechanisms.20 Recently, GCs have been shown to exert cardio- and neuroprotective effects against their respective ischemic injury primarily through augmentation of blood flow.21,22 This was caused by nongenomic activation of endothelial nitric oxide synthase mediated by phosphatidylinositol 3-kinase (PI3K) and Akt phosphorylation. Dexamethasone, a synthetic GC, has been shown to induce rapid phosphorylation of extracellular signal–regulated kinase 1/2 (ERK 1/2) in ovarian follicular cells23,24 that has been suggested as one of the possible mechanisms responsible for its anti-apoptotic effects. Sex steroids, estrogens, and androgens exert bone-protective effects by attenuating the apoptosis of osteoblasts/osteocytes via rapid, nongenomic activation of the Src/Shc/ERK signaling pathway.25,26
Indeed, studying these nongenomic effects of glucocorticoids and determining their therapeutic relevance is crucial because they may provide novel therapeutic options.27 Accordingly, in this study, the role of nongenomic signaling by dexamethasone in the renoprotection against rat renal IRI is examined.
Results
Dexamethasone Ameliorates Renal Dysfunction and Attenuates the Histologic Damage of Rat Renal IRI
In comparison with sham animals, control animals that were subjected to renal IRI (I/R+V, V is vehicle) caused significant renal dysfunction at 24 h as suggested by significant increase in serum creatinine (Figure 1A). However, the rats that were administered a single intraperitoneal bolus of dexamethasone (Dex, 3 mg/kg) 45 min before ischemia (I/R+Dex) had a marked reduction in serum creatinine (324 ± 18 to 153 ± 17 μM; I/R+V versus I/R+Dex, P < 0.01, respectively). Similarly, a significant protection was noted when dexamethasone was administered at the onset of reperfusion (324 ± 18 to 240 ± 18 μM; I/R+V versus I/R+Dex, P < 0.05, respectively; Figure 1A).
Figure 1.
Dexamethasone ameliorates renal dysfunction and attenuates the histologic damage of rat renal IRI. (A) The increase in serum creatinine at 24 h after reperfusion after renal IRI is abrogated by dexamethasone (Dex, 3 mg/kg, intraperitoneal) administered either preischemia or on release of clamps. n = 12 per group. (B) Renal histopathology. Hematoxylin and eosin–stained sections are from the outer medulla (representative images of at least three experiments). (C) Quantitative scores of tubular injury in the outer medulla. The analysis was performed under the same magnification for respective groups. (D) Neutrophil accumulation within the interstitium of the kidney was significantly less in I/R+Dex rats versus I/R+V (V, vehicle) rats at 24 h after reperfusion. (numbers/10 high-power fields). *P < 0.05, ** P < 0.01.
Histology indicated the kidneys from the I/R+V group had severe tubular damage in the outer medulla, as evidenced by widespread tubular necrosis, luminal congestion, and significant infiltration of neutrophils at 24-h reperfusion. In contrast, all renal sections obtained from animals that were treated with dexamethasone, either preischemia or at the onset of reperfusion, showed significantly less tubular damage at the same time point (Figure 1B). Quantification of the tubular damage and infiltrating neutrophils in the outer medulla showed a markedly lower mean tubular injury score and neutrophil infiltration from the kidneys of rats treated with dexamethasone (Figure 1, C and D).
Dexamethasone Increases the Expression of Anti-Apoptotic Bcl-xL and Decreases Proapoptotic Bax, Inhibits Renal IRI-Induced Caspase-3 and -9 Activation, and Exerts Anti-Apoptotic Effects
Kidneys from control animals (I/R+V) showed extensive nuclear changes consistent with apoptotic cell death as shown by intense widespread terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL)-positive cells. In comparison, kidney sections from sham animals had no TUNEL-positive cells. Dexamethasone administration before ischemia had a marked reduction in the percentage of TUNEL-positive cells, suggesting a profound anti-apoptotic effect in vivo (Figure 2A).
Figure 2.
Dexamethasone has profound anti-apoptotic effects against renal IRI. Apoptosis was studied by (A) TUNEL staining, (B) Western blots for Bcl-xL and Bax expression in whole tissue lysates, and (C) caspase-9 and -3 activity (nmol AMC/min per mg protein, measured using the fluorometric substrate Ac-IETD-AMC and Ac-DEVD-AMC, respectively) in homogenates of kidneys after 24 h of reperfusion. (A) TUNEL staining showed strikingly fewer apoptotic nuclei in rats treated with dexamethasone (I/R+Dex, 3 mg/kg) compared with the control (I/R+V) group. The number of TUNEL-positive cells per high-power field was counted in 5 to 10 fields for each coded slide. Arrows showing TUNEL-stained apoptotic nuclei of the tubular epithelial cells. (B) Dexamethasone prevents the postischemic activation of proapoptotic Bax and significantly increases anti-apoptotic Bcl-xL. Kidneys from sham-operated rats, saline-treated rats (I/R+V), and dexamethasone-treated rats (I/R+Dex, 3 mg/kg, intraperitoneal) were harvested after 24 h of reperfusion. Equal protein loading was confirmed by β-actin. The blots and densitometric quanti-fication are representative of the results obtained from three animals. The same blot was probed for Bcl-xL and Bax, respectively. (C) Dexamethasone pretreatment markedly inhibited the activation of I/R induced caspase-9 and caspase-3 activity, respectively. **P < 0.01, *P < 0.05.
In the control (I/R+V) kidneys, the immunoreactivity of Bax protein was significantly increased at 24 h after reperfusion. The ischemia-induced changes in Bax and Bcl-xL expression were prevented by dexamethasone pretreatment, thereby restoring the ratio of anti-apoptotic Bcl-xL to proapoptotic Bax protein in the favor of former. In particular, a striking reduction in Bax expression was detected in the dexamethasone-treated kidneys (Figure 2B). Furthermore, the increase in IRI induced caspase-3 and caspase-9 activity in the homogenates of the kidneys harvested at 24 h after reperfusion was also significantly attenuated in the dexamethasone group compared with the control group (Figure 2C; P < 0.05).
Dexamethasone Attenuates Intercellular Adhesion Molecule-1 Expression After Renal IRI
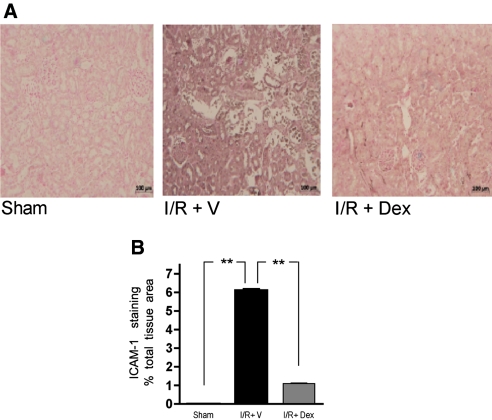
The kidneys from the I/R+V group showed an increased intensity of staining for intercellular adhesion molecule-1 (ICAM-1) that was markedly attenuated in the kidneys of animals treated with dexamethasone (Figure 3A). Quantitative assessment showed a significant reduction in ICAM-1 staining in the I/R+Dex group (Figure 3B; P < 0.01).
Figure 3.
Reduction in ICAM-1 expression in the ischemic kidneys from dexamethasone-treated rats. (A) Immunostaining for ICAM-1 in kidney tissue at 24 h after reperfusion from sham-operated rats, saline-pretreated rats (I/R+V), and dexamethasone-treated rats (I/R+Dex, 3 mg/kg, intraperitoneal, preischemia). (B) Quantitative evaluation of ICAM-1 expression. Significant reduction in the ICAM-1 staining in the kidneys from dexamethasone-treated rats. **P < 0.01 versus other groups.
Dexamethasone Protects Neutrophil-Depleted Mice Against Ischemic AKI
To determine the relative contribution of the direct anti-apoptotic effects of dexamethasone versus the reduction in the inflammatory cells to the overall renoprotection, the effects of dexamethasone on ischemic AKI in neutrophil-depleted mice were studied. The neutrophil count immediately before renal IRI (Figure 4A) was 5.6 ± 0.6% in the RB6–8C5 group versus 50 ± 5% in the IgG κ isotype antibody (control) group (P < 0.0001, n = 12).
Figure 4.
Role of neutrophils in the protective effects of dexamethasone against ischemic acute kidney injury. (A) RB6–8C5 mAb (anti-Ly-6G and Ly-6C monoclonal antibody, 0.08 mg/mice, intraperitoneal) administered 24 h earlier caused profound neutropenia compared with mice that received the κ isotype control antibody. Blood neutrophil count (% of neutrophils, after red cell lysis) was determined immediately before the induction of renal IRI by flow cytometry. Representative picture from FACS analysis with the gated area containing neutrophils. (B) Renoprotective effects of dexamethasone against mice ischemic AKI are neutrophil independent. Neutrophil-depleted mice were not protected against renal IRI compared with the group that received the κ isotype antibody. Serum creatinine was similar in the group that received the κ isotype control antibody compared with the group that was administered normal saline as vehicle (I/R+V+). Serum creatinine after renal IRI was significantly reduced in mice with normal neutrophil count (I/R+Dex+) and in the neutrophil-depleted mice that received dexamethasone (I/R+Neutro-Dex+, 8 mg/kg, intraperitoneal) 1 h before ischemia. **P < 0.01, sham versus I/R+V+ and sham versus I/R+κ isotype; *P < 0.05, I/R+V versus I/R+Dex and I/R+κ isotype versus I/R+Neutro−Dex+. All serum creatinine measurements were performed 24 h after reperfusion. n = 6–8 mice/group. (C) Renal histopathology. Hematoxylin and eosin–stained sections are from the outer medulla (representative images of at least three experiments). Infiltrating neutrophils after renal IRI in the kidney parenchyma were strikingly reduced in the neutrophil-depleted mice.
In comparison with sham animals, control animals that were subjected to renal IRI (I/R+V, V is vehicle) had significant renal dysfunction at 24 h as suggested by a significant increase in serum creatinine (Figure 4B). However, the mice with normal neutrophil count (n = 8) that were administered a single intraperitoneal bolus of dexamethasone (Dex, 8 mg/kg) 45 min before ischemia (I/R+Dex) had a significant reduction in mean serum creatinine (141.5 ± 13 to 90 ± 13 μM; I/R+V versus I/R+Dex, P < 0.05, respectively). In contrast, neutrophil-depleted animals were not protected against renal IRI compared with the group that received the κ isotype antibody. Moreover, dexamethasone maintained its renoprotective effects in the neutrophil-depleted mice.
To gain further insight into the mechanism through which dexamethasone prevents renal IRI-induced proximal tubular cell apoptosis, in vitro experiments were performed. Serum starvation of human kidney-2 (HK-2) cells and in vitro IRI were performed as an in vitro model to mimic renal ischemia induced apoptosis. We have previously shown that serum starvation of HK-2 cells is associated with significant apoptosis as assessed by DNA fragmentation and caspase-3 activation.28
Dexamethasone Protects HK-2 cells Against Serum Starvation and In Vitro IRI-Induced Apoptosis
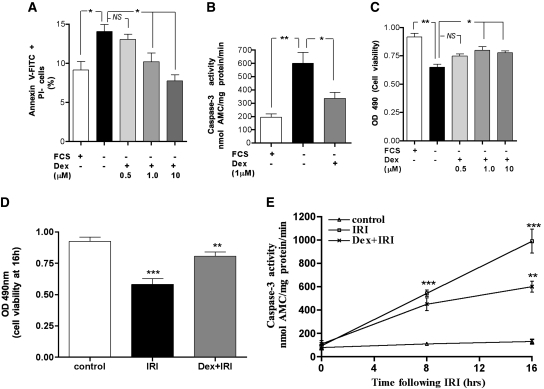
Figure 5A shows that serum starvation (FCS−) for 24 h caused significant apoptosis as assessed by percentage of annexin V-fluorescein isothiocyanate (annexin V-FITC %)–positive, propidium iodide (PI)-negative cells (P < 0.05). Preincubating the cells with dexamethasone (FCS−Dex+) for 1 h before serum starvation protected the cells from apoptosis in a dose-dependent manner at 24 h (P < 0.05). The minimal dose of dexamethasone found to have anti-apoptotic effects was 1 μM. Therefore, for all subsequent in vitro experiments, the 1 μM dose was used. Furthermore, dexamethasone significantly prevented the serum starvation–induced activation of caspase-3 at 24 h (P < 0.01; Figure 5B). This anti-apoptotic effect translated into preserved cell viability in a dose-dependent manner (Figure 5C). Moreover, dexamethasone (1 μM) also significantly preserved cell viability in cells subjected to IRI (Figure 5D) and alleviated the IRI-induced caspase-3 activation at 16 h (Figure 5E).
Figure 5.
Dexamethasone protects HK-2 cells against serum starvation and cellular IRI-induced apoptosis. HK-2 cells were incubated with indicated doses of dexamethasone for 1 h. (A) HK-2 cells were significantly protected from serum starvation–induced apoptosis by dexamethasone. Apoptosis was ascertained after 24 h of serum starvation by measuring the percentage of early apoptotic cells (annexin V-FITC staining positive and PI-negative cells) by flow cytometry. *P < 0.05, **P < 0.01. (B) Serum starvation of HK-2 cells for 24 h caused significant activation of caspase-3 activity that was attenuated by dexamethasone (1 μM) *P < 0.05, **P < 0.01. (C) Dexamethasone significantly preserved viability of HK-2 cells on serum starvation for 24 h. (D) Dexamethasone (1 μM) significantly preserved cell viability in cells subjected to IRI. Control versus IRI, ***P < 0.001; IRI versus Dex+IRI, **P < 0.01. (E) Dexamethasone (1 μM) alleviated the IRI-induced caspase-3 activation at 16 h. At 8 h, a significant increase in caspase-3 activation in the cells subjected to ischemic insult was detected (control versus IR, ***P < 0.001). There was a trend (although not statistical significant) of lower caspase-3 activity in the dexamethasone-pretreated group at 8 h. By 16 h, the caspase-3 activity was significantly lower in the dexamethasone-treated cells (I/R versus Dex+I/R, **P < 0.01). HK-2 cells were incubated with 100 μM antimycin A plus 10 mM 2-deoxyglucose for 1 h to induce ischemic injury in vitro. The in vitro reperfusion was achieved by incubating cells in glucose-replete complete growth medium. Cells were subjected to IRI for 8 and 16 h. Caspase-3 activity (nmol AMC/min per mg protein) was quantified using the fluorometric substrate Ac-DEVD-AMC. The cell viability was determined by methylthiazoletetrazolium assay, and absorbance, proportional to the number of viable cells, was measured at 490 nm.
Anti-Apoptotic Effects of Dexamethasone Are Not via the PI3K/Akt Pathway
Having shown that dexamethasone is anti-apoptotic in vivo and in vitro, we next sought to determine the possible mechanism for this. The role of the PI3K–Akt pathway was investigated by using a specific PI3K inhibitor, LY294002.
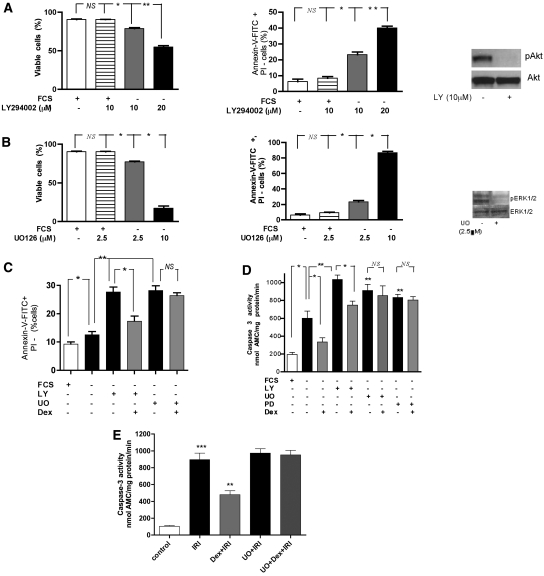
Serum starvation after 1-h incubation with LY294002 caused significant apoptosis without direct cell toxicity (proportion of PI-positive cells or dual positive cells) at 10 μM concentration, however, caused significant direct cell toxicity at higher dose (i.e., 20 μM). Hence, the 10-μM dose was chosen, and the same concentration under the same conditions inhibited phosphorylation of Akt (Western blot; Figure 6A). Incubating the cells with LY294002 for 1 h and subsequent serum starvation for 24 h caused a significant increase in the percentage of cells undergoing apoptosis (P < 0.01; Figure 6C) and also a significant augmentation of caspase-3 activity (P < 0.01; Figure 6D). However, dexamethasone maintained its anti-apoptotic effects despite the presence of a PI3K inhibitor (P < 0.05; Figure 6C) and attenuated the increase in caspase-3 activity (P < 0.05; Figure 6D). Moreover, stimulation of HK-2 cells with dexamethasone caused no increase in expression of phosphorylated Akt (data not shown).
Figure 6.
Anti-apoptotic effects of dexamethasone are mediated via MEK-ERK 1/2. HK-2 cells were pretreated with the PI3 kinase inhibitor, LY294002 (LY, 10 μM), or with the MEK1/2 inhibitors, UO126 (UO, 2.5 μM) or PD980059 (PD, 50 μM), for 1 h or in addition, followed by dexamethasone (1 μM, 1 h) as indicated and subsequently serum starved (FCS−) for 24 h. (A) LY294002 (LY, 10 μM) and (B) UO126 (UO, 2.5 μM) inhibited phospho-Akt and phospho-ERK 1/2, respectively, at 24 h and caused significant apoptosis without causing direct cell toxicity under the above-mentioned conditions. The blots represent three independent experiments. *P < 0.05, **P < 0.01. (C) Dexamethasone rescued the HK-2 cells from combined proapoptotic effects of PI3 kinase inhibition and serum starvation; however, the anti-apoptotic effects were completely abolished by UO126 (2.5 μM). Apoptosis was ascertained by measuring the percentage of early apoptotic cells (annexin V-FITC staining–positive and PI-negative cells) by flow cytometry. (D) Dexamethasone (1 μM) prevented the activation of caspase-3 (nmol AMC/min per mg protein) caused by LY 294002 and subsequent serum starvation but failed to prevent caspase-3 activation by UO 126 (2.5 μM) or PD980059 (50 μM). *P < 0.05, **P < 0.01, FCS– versus FCS-LY+ or FCS-UO+ or FCS-PD+. (E) In the in vitro IRI model, pretreatment with UO126 (5 μM for 1 h, in the presence of serum) blocked the Dex-mediated suppression of caspase-3 activation induced by in vitro IRI. HK-2 cells were incubated with 100 μM antimycin A plus 10 mM 2-deoxyglucose for 1 h to induce ischemic injury in vitro. The in vitro reperfusion was achieved by incubating cells in glucose-replete complete growth medium. Caspase-3 activity (nmol AMC/min per mg protein) was quantified using the fluorometric substrate Ac-DEVD-AMC at 16 h after IRI. **P < 0.01, ***P < 0.001.
Mitogen-Activated Protein Kinase/ERK Kinase–ERK 1/2 Pathway Mediates Anti-Apoptotic Effects of Dexamethasone
Mitogen-activated protein kinase/ERK kinase (MEK) is a specific activator of ERK, a dual specificity protein kinase that phosphorylates both threonine and tyrosine regulatory sites in ERK. The specific MEK 1/2 inhibitors, UO126 and PD980059, were used. UO126 at the 2.5-μM dose was noted to inhibit phosphorylation of ERK 1/2 and cause significant apoptosis without significant direct toxicity (Figure 6B). Therefore, in subsequent serum starvation experiments, UO126 (2.5 μM) was used. Incubating HK-2 cells with UO126 (2.5 μM) for 1 h and subsequent serum starvation for 24 h caused significant increase in apoptosis (P < 0.01; Figure 6C). UO126 completely abolished the anti-apoptotic effects of Dex and Dex treatment failed to prevent caspase-3 activation in the presence of the MEK inhibitor (P > 0.05; Figure 6D). The same effect on caspase-3 activity was detected using another MEK inhibitor, PD980059 (50μM). In an in vitro IRI model, we first showed that UO126 (5 μM) alone had no effect on HK-2 cell viability (data not shown). However, pretreatment with UO126 (5 μM for 1 h) blocked the Dex-mediated suppression of caspase-3 activation in the in vitro IRI model also (Figure 6E).
Dexamethasone Causes Rapid and Transient Phosphorylation of ERK1/2 In Vitro and In Vivo
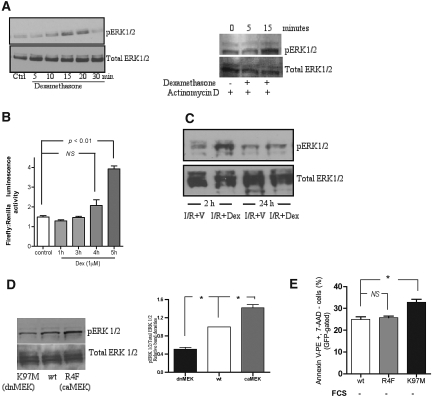
To confirm the involvement of the MEK–ERK 1/2 pathway, whether dexamethasone could phosphorylate ERK1/2 was studied. Stimulation of HK-2 cells after 24 h of serum starvation with dexamethasone (1 μM) resulted in a time-dependent increase in phosphorylation of ERK 1/2 (pERK 1/2; Figure 7A) with peak phosphorylation at 15 min of incubation that returned to baseline by 30 min. To establish whether similar rapid phosphorylation of ERK 1/2 occurs in vivo; Western blots on the whole kidney homogenates harvested 2 h after reperfusion were performed. Western blot showed significant expression of pERK in rat kidneys treated with dexamethasone compared with vehicle-treated IRI rat kidneys (Figure 7B). The two features of pERK 1/2 noted on the above Western blots were rapidity and transient nature.
Figure 7.
Dexamethasone stimulates nontranscriptional, rapid, and transient phosphorylation of ERK 1/2 in vitro and in vivo. (A) HK-2 cells were serum-starved for 24 h and incubated with dexamethasone (1 μM) for various periods of time as indicated and subjected to Western blotting. Dexamethasone-triggered rapid activation of ERK 1/2 was also preserved in the cells that were pretreated with actinomycin D (0.5 μg/ml), a transcriptional inhibitor, for 2 h. Blots are representative of three different experiments. (B) The time-dependent effect of dexamethasone (1 μM) on GRE promoter activity was studied in HK-2 cells. Significant luciferase activity measured as luminescence activity (corrected for Renilla luciferase, internal control reporter) was detected after 4 h of incubation. (C) Kidneys harvested after 2-h reperfusion showed markedly increased expression of pERK 1/2 in the dexamethasone-treated rats (I/R+Dex, 3 mg/kg, intraperitoneal) compared with the saline-treated rats (I/R+V). The protein expression of pERK 1/2 and total ERK was determined by Western blot in whole tissue lysates. The blots are representative of the results obtained from six animals. (D) Representative Western blot showing the effect of MEK plasmid transfection on ERK activation with quanti-tative analysis of Western blots relative to the wt plasmid. The blot represents three independent experiments. (E) Constitutive activation of the MEK-ERK1/2 pathway is not associated with anti-apoptotic effects in HK-2 cells. Serum starvation for 24 h of transfected HK-2 cells with dnMEK (K97M) plasmid caused a significant increase in early apoptotic cells compared with the cells transfected with wtMEK plasmid. No difference in the apoptosis was detected between the cells expressing caMEK (R4F) and wtMEK. Transfection was performed in the presence of serum. Apoptosis was ascertained after 24 h of serum starvation after transfection by measuring the percentage of early apoptotic cells (GFP-gated, annexin V-PE staining–positive and PI-negative cells) by flow cytometry. *P < 0.05.
Rapid Phosphorylation of ERK 1/2 by Dexamethasone Is Nongenomic
The rapidity of phosphorylation of ERK 1/2 is suggestive of a nongenomic mechanism. To confirm this, the effect of actinomycin D, a transcription inhibitor, in a nontoxic dose that has been previously used in HK-2 cells was studied. Dexamethasone rapidly phosphorylated ERK 1/2 in the cells that were incubated for 2 h with actinomycin D, before stimulation by dexamethasone (Figure 7C). In addition, significant (4-fold, P < 0.01) luciferase activity was detected after 4 h of incubation (Figure 7D), thereby suggesting that it is highly likely that transcriptional activity of dexamethasone in HK-2 cells starts between 4 and 5 h after stimulation. The above two approaches confirm that phosphorylation of ERK 1/2 detected within 15 min is nongenomic.
Transient Activation of the MEK–ERK1/2 Pathway Is Associated With Anti-Apoptotic Effects in HK-2 Cells
To characterize the significance of the transient nature of pERK 1/2, cells were cotransfected with a green fluorescent protein (GFP) reporter plasmid and (1) wild-type MEK (wtMEK); (2) constitutively active MEK (caMEK; R4F); or (3) dominant negative MEK (dnMEK; K97M) plasmids. Figure 7D shows that transfection with dnMEK and caMEK plasmids caused a significant 42 ± 7% decrease and 51 ± 4% increase, respectively, in the expression of pERK 1/2 compared with wtMEK (P < 0.01). Serum starvation for 24 h of dnMEK (K97M)–transfected cells led to a significant increase in the number of GFP-positive apoptotic cells (P < 0.05; Figure 7E), whereas there was no difference between the percentage of GFP-positive apoptotic cells between cells expressing wtMEK or R4F plasmids (P > 0.05; Figure 7E).
Glucocorticoid Receptor Mediates Dexamethasone-Triggered ERK Activation and Its Anti-Apoptotic Effects
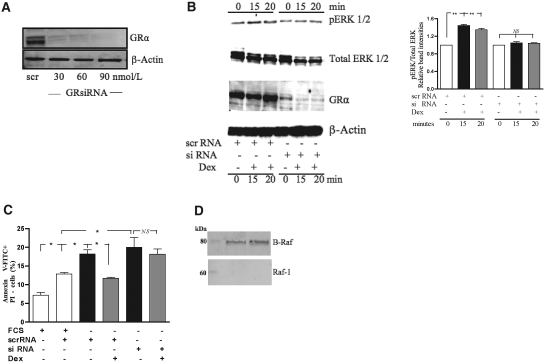
To ascertain the role of the GR in GC-mediated prosurvival effects, GR was knocked down using small interfering RNA (siRNA) specific to the GR before experimental protocols were conducted. Western blot analysis confirmed significant reduction in GR expression (Figure 8A). Figure 8B shows that the GR knockdown prevented GC-mediated ERK activation. Furthermore, serum starvation of control cells for 24 h caused a significant increase in apoptosis (P < 0.05; Figure 8C). Preincubation with Dex (1 μM) for 1 h of the control cells caused a marked reduction in the number of apoptotic cells (P < 0.05; Figure 8C) that was abolished in cells transfected with GR-specific siRNA GR (P > 0.05; Figure 8C). The control cells were treated with nonspecific mRNA sequences and subjected to the same experimental protocols.
Figure 8.
The glucocorticoid receptor mediates the phosphorylation of ERK 1/2 and anti-apoptotic effects of dexamethasone. HK-2 cells were transfected with either control scrRNA or GR-specific siRNA. (A) Protein lysates were assessed for GR expression using Western blot analysis, and results shown are representative of three individual experiments. (B) Representative Western blot and the quantitative analysis (from three independent experiments) shows that dexamethasone was unable to significantly phosphorylate ERK 1/2 in the GR knockdown cells; in contrast, dexamethasone rapidly phosphorylated ERK 1/2 in the cells transfected with scrRNA. The same membrane was probed for pERK 1/2, total ERK 1/2, GR, and β-actin, respectively. (C) Knockdown of GR abolished the cytoprotective effect of dexamethasone. HK-2 cells were transfected with either control siRNA or GR-specific siRNA, followed by serum starvation and/or dexamethasone as indicated. (D) Representative Western blots showing a striking absence of expression of Raf-1 in contrast to B-Raf in HK-2 cells. The same blot was probe for Raf-1 and B-Raf, respectively. *P < 0.05, **P < 0.01.
To elucidate which Raf is involved in GR-mediated ERK 1/2 activation, immunoblots of C-Raf and B-Raf were performed. To our surprise, we found that Raf-1 (C-Raf) expression on Western blotting was strikingly absent; in contrast, there was significantly greater abundance of B-Raf (Figure 8D). Indeed, this finding suggests a C-Raf–independent activation of the MAP kinase cascade and identifies B-Raf as the most likely candidate involved in GR-mediated MEK/ERK 1/2 activation in HK-2 cells.
Discussion
The main findings of this study are that (1) dexamethasone is renoprotective against IRI; (2) the direct anti-apoptotic effects of dexamethasone on human renal proximal tubular cells contribute to the observed protection; and (3) prosurvival effects of dexamethasone are mediated via cytosolic GR–dependent nongenomic signaling involving the MEK–ERK 1/2 pathway.
Several observations led to the examination of the role of GCs in renal IRI. First, it has been assumed thus far that GCs protect the kidney against ischemic insults by virtue of their anti-inflammatory properties and membrane stabilization properties.29–32 Second, few recent studies have shown pleiotropic effects of the GR on signaling pathways including activation of the MEK–ERK 1/2 pathway in human ovarian follicular cells24 and the PI3K/Akt pathway in human endothelial cells.21 Third, Dex was noted to have a direct protective effect on podocytes against puromycin-induced apoptosis.12 However, it is well known that effects of GCs are cell specific, and one cannot extrapolate data from one cell type to another. These observations help to formulate a hypothesis that GCs might have a direct protective effect on renal proximal tubular cells via nongenomic activation of a survival pathway that could contribute to renoprotection against renal IRI.
In the 1980s, methylprednisolone was shown to protect the kidneys against warm ischemia.29–32 It was speculated that steroid protective effects were caused by its anti-inflammatory and lysosomal-membrane stabilizing properties. Subsequently, Takahira et al.33 noted that dexamethasone attenuated the neutrophil infiltration and ICAM-1 expression in kidneys; however, it failed to ameliorate the renal dysfunction secondary to renal IRI. However, the degree of IRI was perhaps too severe. To the best of our knowledge, this is the first study to show the direct cytoprotective properties of dexamethasone in vitro and in vivo for proximal tubular cells.
ERK 1/2 has been implicated in postischemia/reperfusion cell survival and apoptosis.34–36 Although GR knockdown experiments suggest GR-dependent phosphorylation of ERK 1/2, no GRE-mediated gene transcription is necessary for its activation. The rapid phosphorylation of ERK 1/2 noted is most likely nongenomic in nature because of its rapidity and preservation of this phenomenon in the cells that were pretreated with a transcriptional inhibitor.
Importantly, the concentration of Dex (i.e., 1 μM) is clinically relevant. Indeed, it is significantly less than those found after intravenous pulse glucocorticoid therapy used in a few major vascular surgical studies7–10 to protect against renal IRI, where serum concentrations can reach 60 to 200 M.37 The plausible mechanism of GR-mediated activation of the MEK/ERK 1/2 module in proximal tubular cells is partly speculative. The cytosolic nonliganded GR is a part of a multiprotein complex in which, among other proteins, two molecules of heat shock protein 90 (hsp90), as well as one molecule each of hsp70 and hsp56 are known to be included. Widen et al.38 identified that Raf-1 copurified with GR with 14-3-3 proteins by immunoaffinity chromatography purification from liver cytosol. Interestingly, hsp90 has been shown to mediate intercellular signal transduction events including B-Raf–mediated activation of mitogen-activated protein kinase cascade by nerve growth factor.39 It is indeed tempting to speculate that hsp90 or 14-3-3 that dissociate from the ligand–GR complex on ligand–GR binding may provide the crosstalk with the coresident B-Raf or Raf-1. We detected high expression of B-Raf in HK-2 cells on Western blots, in contrast to C-Raf. This finding suggests a C-Raf–independent activation of mitogen-activated protein kinase cascade and identifies B-Raf as the most likely candidate involved in GR-mediated MEK/ERK 1/2 activation in HK-2 cells. Indeed, MEK1 and MEK2 are the only known substrates for B-Raf, and it is 50-fold more potent at phosphorylating MEK1 and MEK2 than either A-Raf or C-Raf.40
In this study, significant reduction in neutrophil infiltration and ICAM-1 staining in the kidneys of dexamethasone-treated rats was also detected. It has been shown that abrogation of early renal IRI-induced apoptosis prevents the development of subsequent inflammation and organ dysfunction.41 It is possible that the early direct cytoprotective effects of dexamethasone on IRI-induced renal injury could have contributed to the attenuation of the post-IRI inflammation. This is supported by the finding that renal proximal tubular epithelial cells including HK-2 cells are “steroid insensitive” with respect to its direct anti-inflammatory effects,15–17 possibly because of the lack of interference with the NF-κB signaling pathway.15,16 However, the role of neutrophils in renal IRI is controversial. The renoprotection observed in ICAM-1 knockout mice is neutrophil dependent and is similar to that afforded by severe neutrophil depletion.42 Therefore, we used neutrophil-depleted mice to determine the contribution of neutrophils to overall renoprotection. The neutrophil-depleted mice were not protected against ischemic injury. This is in agreement with other studies.43–45 The preservation of Dex's beneficial effects in neutrophil-depleted mice suggests that perhaps direct cytoprotective effects do play a role in vivo also. However, our study cannot unequivocally prove that activation of ERK in proximal tubular cells after IRI in vivo is responsible for the overall direct cytoprotective effects.
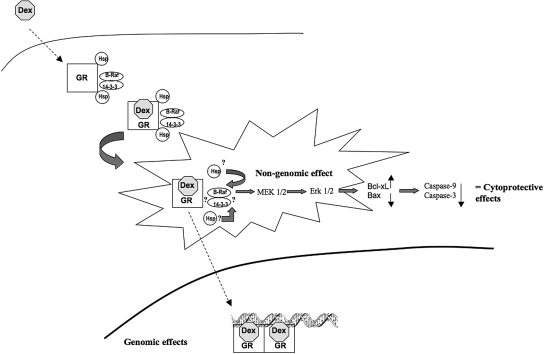
The clinical relevance of GC-induced nongenomic activation of this prosurvival pathway can be potentially very important. The nongenomic GC effects are currently the focus of research20,46,47 because they promise the possibility for the generation of novel selective GR agonists, which might have significantly superior benefit to risk ratio by reducing the adverse effects. The results of this study may provide impetus to the development of novel selective GR agonists with predominant anti-apoptotic properties. Moreover, this pleiotropic effect of dexamethasone could form the basis of clinical trials in patient groups at high risk of developing acute kidney injury. In conclusion, this study shows for the first time that nongenomic signaling of dexamethasone in the proximal tubules may contribute to the direct renoprotective effects against renal IRI (Figure 9).
Figure 9.
Schematic illustration of the cytoprotective effects of dexamethasone in HK-2 cells. Raf-1 and 14-3-3 proteins have been shown to colocalize with the GR multiprotein complex. Binding of the dexamethasone to the cytosolic GR causes release of heat shock proteins, activates the ligand–receptor complex, and triggers the translocation of the ligand receptor to the nucleus to subsequently elicit traditional genomic effects. In addition, the ligand–receptor interaction also rapidly activates the B-Raf/MEK/ERK 1/2 pathway, thereby exerting direct cytoprotective effects. It is possible that heat shock proteins or 14-3-3 proteins, both known mediators of Raf/MEK/ERK 1/2 signaling, provide the crosstalk. Dex, dexamethasone; GR, glucocorticoid receptor; hsp, heat shock protein. Block arrows denotes activation; dashed arrows suggest movement. ?? denotes possible role in activating the kinase cascade.
Concise Methods
Rat Renal IRI
The model of renal IRI in the anesthetized rat and the surgical procedures involved was similar to those described previously.48,49 Briefly, this study was carried out using male Wistar rats (Charles River Ltd., Margate, UK) that weighed 220 to 300 g and received standard diet and water ad libitum. Animals were cared for in accordance with the Home Office Guidance in the Operation of the Animals (Scientific Procedures) Act 1986 (HMSO, London, UK). All rats were anesthetized with sodium thiopentone (intraval sodium, 85 mg/kg intraperitoneally; Rhone Merieux, Essex, UK) and subjected to bilateral renal ischemia for 60 min, followed by reperfusion for 2 or 24 h.
Rats were randomly allocated into the following groups: (1) sham + saline group (n = 12); (2) I/R+saline (I/R+V, n = 12); (3) I/R+Dex (3 mg/kg, intraperitoneal) 30 min before onset of ischemia (n = 12); and (4) I/R+Dex (3 mg/kg, intraperitoneal) at the onset of reperfusion (n = 12). The volume of saline (V) administered was equal to the volume of dexamethasone administered. Sham-operated rats underwent identical surgical procedures but without bilateral renal pedicle clamping and were subjected to all other procedures that the other three groups underwent.
Mice Renal IRI
For all of the mouse studies, C57BL/6 male mice (Charles River Ltd.) were used. Age- and weight-matched mice (8 to 10 wk old, 22 to 25 g) were anesthetized with an intraperitoneal injection of an anesthetic cocktail consisting of ketamine and xylazine in a 2:1 ratio. Surgery was performed on a preheated mat to maintain body temperature at 37°C. A midline incision was made, and the renal pedicles were bilaterally clamped for 27 min with microaneurysm clamps. The time of ischemia was chosen to obtain a reversible model of ischemic acute renal failure and to avoid animal mortality. After 27 min, the clamps were removed. The kidneys were observed for restoration of blood flow, as shown by a return to their original color. The abdomen was closed in two layers. Sham surgery consisted of the same surgical procedure except that clamps were not applied. Immediately after closure of the abdomen, all mice were administered 500 μl of normal saline (preheated to 37°C) intraperitoneally. At 24 h, animals were killed, blood samples were obtained via cardiac puncture, and kidneys were harvested for further experiments. The veterinary clinical pathologists, based in Vetlab (www.vetlab.co.uk), a division of Idexx laboratories (Sussex, UK), blinded to the treatment given, performed serum creatinine measurements.
Mice were randomly allocated into the following groups: (1) sham + saline group (n = 8); (2) I/R + saline (I/R+V, n = 8); and (3) I/R+Dex (8 mg/kg, intraperitoneal) 1 h before the onset of ischemia (n = 8). The volume of saline (V) administered was equal to the volume of dexamethasone administered.
The dose of 8 mg/kg body weight was chosen because it gave the best consistent, functional protection in mice compared with 3 mg/kg body weight in rats.
Neutrophil Depletion Model
Twenty-four hours before the ischemia-reperfusion surgery, mice were injected with 0.08 mg of the anti-mouse rat IgG2b mAb RB6–8C5 (BD Pharmingen) intraperitoneally. The RB6–8C5 monoclonal antibody (mAb) has been used extensively to assess the relative contribution of neutrophils in various murine disease models including renal IRI.44 It reacts with a common epitope on Ly-6G and Ly-6C and is known to deplete not only neutrophils but also Ly6C+ blood “inflamed monocytes” that are precursors of inflammatory macrophages.50 Immediately before the surgery, 20 μl of blood was subjected to red cell lysis by using ammonium-chloride-potassium buffer and analyzed by flow cytometry. Mice were randomly allocated into the following groups: (1) sham (n = 8); (2) I/R + anti-mouse IgG κ isotype anti-body (I/R+κ isotype, n = 8); (3) I/R + RB6–8C5 mAb (I/R+Neutro−, n = 8); and (4) I/R + RB6–8C5 mAb + Dex (I/R+Neutro−Dex+, 8 mg/kg, intraperitoneal) 1 h before the onset of ischemia (n = 8). The volume of κ isotype administered was equal to the volume of RB6–8C5 administered.
Histologic Evaluation
A renal pathologist, who was blinded to the treatment given, performed morphologic assessment. Kidney sections were stained with hematoxylin and eosin. A hundred intersections were examined for each kidney, and a score from 0 to 3 was given for each tubular profile. The scoring method has been described previously by our group28: 0, normal histology; 1, tubular cell swelling, brush border loss, nuclear condensation, with up to one third of tubular profile showing nuclear loss; 2, as for score 1 but greater than one third and less than two thirds of tubular profile shows nuclear loss; and 3, greater than two thirds of tubular profile shows nuclear loss. The total score for each kidney was calculated by addition of all 100 scores with a maximum score of 300.
TUNEL Assay
In the in vivo model, a TUNEL assay was performed by the pathologist who was blinded to the treatment animals had received. The TUNEL assay was conducted using a TUNEL detection kit according to the manufacturer's instruction (HRP kit DBA; Apotag, Milan, Italy).
Cell Culture and Reagents
All reagents including Dex were obtained from Sigma-Aldrich (Poole, Dorset, UK) unless otherwise stated. UO126, a specific MEK1 inhibitor, and LY294002, a PI3K inhibitor, were from Merck Biosciences. HK-2 cells (European Collection of Cell Cultures, Salisbury, UK), an immortalized proximal tubular epithelial cell line from normal adult human kidney, were grown in media comprising DMEM Ham's F12 media (1:1) supplemented with 10% FCS and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin). Cells were seeded in a 6-well tissue culture plate and allowed to adhere for 18 h in an incubator at 37°C with 5% CO2 in 95% air and were subsequently serum starved. Cells were preincubated with dexamethasone for 60 min, before serum starvation, unless otherwise stated. Thereafter, during serum starvation, cells were incubated without dexamethasone.
Caspase Activity
The activity of caspase-3 was measured using the fluorometric substrate Ac-DEVD-AMC and Ac-DEVD-CHO as inhibitors, as described previously by our laboratory.51 The activity of caspase-9 was determined using Ac-IETD-AMC and Ac-IETD-CHO, as substrate and inhibitor, respectively. Fluorescence readings from wells that contained inhibitor were subtracted from total fluorescence, and results were calculated (nmol AMC/min per mg protein).
Viability Assay
Cell viability was determined by methylthiazoletetrazolium assay using the CellTiter 96 Non-Radioactive Cell Proliferation Assay kit (Promega, Southampton, UK) according to the manufacturer's instructions. Absorbance, proportional to the number of viable cells, was measured at 490 nm.
Induction of IRI in Cell Culture
IRI was induced in vitro according to the well-studied and extensively characterized model52,53 using a combination of antimycin A (a complex III inhibitor of mitochondrial electron transport) and 2-deoxyglucose (a nonmetabolizable isomer of l-glucose). In brief, when HK-2 cells were 80% confluent, the culture medium was replaced with Hank's balanced salt solution (containing 1.3 mM Ca2+ and 0.8 mM Mg2+) and were incubated with 100 μM antimycin A plus 10 mM 2-deoxyglucose for 1 h to induce ischemic injury in vitro. The in vitro reperfusion was achieved by incubating cells in glucose-replete complete growth medium.
Flow Cytometry
Fluorochrome-conjugated annexin V (annexin V-FITC) was used to identify apoptotic cells, following the manufacturer's instructions (BD Pharmingen). In brief, cells were harvested, washed, and incubated for 15 min with annexin V-FITC and PI. The cells were washed and transferred into 12 × 75-mm Falcon 2052 FACS tubes (Becton Dickinson, San Jose, CA), and data from 10,000 HK-2 cells were collected on FACS with a Becton Dickinson Biosciences FACScan and CellQuest software version 3.3. This combination allows for the differentiation between early apoptotic cells (annexin V-FITC positive, PI negative), late apoptotic and/or necrotic cells (annexin V and PI positive), and viable cells (unstained). To identify successfully transfected apoptotic cells (gating only on GFP-positive cells), annexin V-phycoerythrin and 7-amino-actinomycin stain was used.
Western Blot Analysis
Cells were washed with ice-cold PBS and lysed in ice-cold modified RIPA lysis buffer (50 mM Tris HCl, pH 7.5, 150 mM NaCl, 50 mM NaF, 0.5% deoxycholic acid, 1% NP-40, 1 mM sodium orthovanadate, 0.1% SDS). Insoluble material was removed by centrifugation at 12,000 × g for 15 min at 4°C. The protein concentrations were measured using a bicinchoninic acid assay (Perbio Science, Cramlington, UK) following the manufacturer's instructions. Lysates were matched for protein, separated by SDS-PAGE, and transferred to a polyvinylidene difluoride microporous membrane (Millipore, Bedford, MA). Membranes were blocked at room temperature for at least 1 h in blocking buffer before being probed with the primary antibody. The primary antibodies were detected using horseradish peroxidase–conjugated IgG (Santa Cruz Biotechnology, Autogen Bioclear, Calne, UK) and visualized with an enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, UK). All of the primary antibodies except GR antibody were obtained from Cell Signaling Technology. GR antibody was obtained from Santa Cruz Biotechnology. The densitometric quantifications of relative band intensities was performed using NIH Image J software.
Transient Transfection
Transient transfection of HK-2 cells was performed using Lipofectamine LTX according to the manufacturer's instructions (Invitrogen, Paisley, UK). These cells were cotransfected with GFP plasmid, kindly provided by Dr. S. Cooray (Queen Mary University of London, UK). Wild-type, constitutive active MEK 1/2 and dominant-negative MEK 1/2 were kindly provided by Professor Natalie Ahn (Department of Chemistry and Biochemistry, University of Colorado). Cotransfection with GFP was performed (1) to assess the transfection efficiency as determined by cytofluorimetry measurement of the GFP protein fluorescence and (2) to identify transfected cells, thereby permitting us to precisely assess the effect of MEK 1/2 plasmids on apoptosis (only GFP-gated cells were assessed for apoptosis). Briefly, 1 d before transfection, cells were plated in a 6-well plate so that the cells will be 60% to 70% confluent at the time of transfection. Cells were transfected with 2 μg of plasmid DNA (1.8 μg of MEK plasmid and 0.2 μg of GFP) and 5 μl of lipfectamine LTX reagent in the presence of serum but without antibiotics. After transfection, the cells were incubated overnight at 37°C. The media were changed the next day, and transfected cells were incubated for 4 h with fresh media and 10% FCS. Subsequently, transfected cells were serum starved for 24 h to assess apoptosis as described above. Transfection of HK-2 cells under these conditions yielded 70% to 80% positively transfected cells.
To detect the induction of the GRE by dexamethasone, HK-2 cells were transiently cotransfected with the pGRE-Luc vector containing the firefly luciferase reporter construct (Promega, Southampton, UK) and pRL-TK vector (Promega) containing Renilla luciferase reporter construct. The latter was used as an internal control. Transfection was performed according to the above protocol.
Luciferase Assay
Luciferase assays were performed using a Dual-Glo luciferase assay system according to the manufacturer's instructions (Promega). Briefly, 24 h after transfection, cells were incubated with Dex for a varying time period (30 min to 6 h) and subsequently harvested into Glo lysis buffer. The lysates were transferred to a 96-well luminometer plate. An equal volume of Dual-Glo luciferase substrate was added, and firefly luciferase activities produced by the pGRE-Luc were measured using a multiplate reader (Wallac Victor 2; PerkinElmer, Bar Hill, UK).
Renilla luciferase activities produced by the pRL-TK transfection efficiency control plasmid were assayed by adding an equal volume of Dual-Glo Stop and Glo substrate (comprising the stop solution for firefly luciferase and substate for Renilla luciferase) and remeasuring in the multiplate reader. Values were normalized to the pRL-CMV Renilla luciferase activity.
RNA Interference for GR Knockdown
Cells were transfected with four-pooled siRNA (siRNA duplexes, 30 nM) directed against separate GR mRNA target sequences or with four-pooled siRNA duplexes directed against nonspecific mRNA sequences (SMARTPool; Dharmacon) before experimental protocols. Transfection was conducted using RNAiMax (Invitrogen Life Technologies) using protocols specific for siRNA transfection according to the manufacturer's instructions.
Statistical Analyses
All values are reported as mean ± SEM. Flow cytometry, caspase assays, and MTS assays were performed a minimum of three times. All multiple group comparisons were performed using ANOVA with a post test according to Bonferroni. We used an unpaired t test to compare the means of two different groups. All statistical analysis was performed using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA). P < 0.05 was considered significant.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Aydin Z, van Zonneveld AJ, de Fijter JW, Rabelink TJ: New horizons in prevention and treatment of ischaemic injury to kidney transplants. Nephrol Dial Transplant 22: 342–346, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT: Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 128: 194–203, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Kazmers A, Jacobs L, Perkins A: The impact of complications after vascular surgery in Veterans Affairs Medical Centers. J Surg Res 67: 62–66, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Levy EM, Viscoli CM, Horwitz RI: The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494, 1996 [PubMed] [Google Scholar]
- 5.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Schiffl H, Lang SM, Fischer R: Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 346: 305–310, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Morariu AM, Loef BG, Aarts LP, Rietman GW, Rakhorst G, van Oeveren W, Epema AH: Dexamethasone: Benefit and prejudice for patients undergoing on-pump coronary artery bypass grafting: a study on myocardial, pulmonary, renal, intestinal, and hepatic injury. Chest 128: 2677–2687, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Turner S, Dhamarajah S, Bosomworth M, Bellamy MC: Effect of perioperative steroids on renal function after liver transplantation. Anaesthesia 61: 253–259, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Baker RC, Armstrong MA, Young IS, McClean E, O'Rourke D, Campbell FC, D'Sa AA, McBride WT: Methylprednisolone increases urinary nitrate concentrations and reduces subclinical renal injury during infrarenal aortic ischemia reperfusion. Ann Surg 244: 821–826, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner S, Derham C, Orsi NM, Bosomworth M, Bellamy MC, Howell SJ: Randomized clinical trial of the effects of methylprednisolone on renal function after major vascular surgery. Br J Surg 95: 50–56, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ: Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: Role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Oh HY, Namkoong S, Lee SJ, Por E, Kim CK, Billiar TR, Han JA, Ha KS, Chung HT, Kwon YG, Lee H, Kim YM: Dexamethasone protects primary cultured hepatocytes from death receptor-mediated apoptosis by upregulation of cFLIP. Cell Death Differ 13: 512–523, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Messmer UK, Winkel G, Briner VA, Pfeilschifter J: Glucocorticoids potently block tumour necrosis factor-alpha- and lipopolysaccharide-induced apoptotic cell death in bovine glomerular endothelial cells upstream of caspase 3 activation. Br J Pharmacol 127: 1633–1640, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Haij S, Adcock IM, Bakker AC, Gobin SJ, Daha MR, van Kooten C: Steroid responsiveness of renal epithelial cells. Dissociation of transrepression and transactivation. J Biol Chem 278: 5091–5098, 2003 [DOI] [PubMed] [Google Scholar]
- 16.de Haij S, Daha MR, van Kooten C: Mechanism of steroid action in renal epithelial cells. Kidney Int 65: 1577–1588, 2004 [DOI] [PubMed] [Google Scholar]
- 17.de Haij S, Woltman AM, Bakker AC, Daha MR, van Kooten C: Production of inflammatory mediators by renal epithelial cells is insensitive to glucocorticoids. Br J Pharmacol 137: 197–204, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahn C, Lowenberg M, Hommes DW, Buttgereit F: Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol 275: 71–78, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Schacke H, Schottelius A, Docke WD, Strehlke P, Jaroch S, Schmees N, Rehwinkel H, Hennekes H, Asadullah K: Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci U S A 101: 227–232, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haller J, Mikics E, Makara GB: The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front Neuroendocrinol 29: 273–291, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Limbourg FP, Huang Z, Plumier JC, Simoncini T, Fujioka M, Tuckermann J, Schutz G, Moskowitz MA, Liao JK: Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J Clin Invest 110: 1729–1738, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK: Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med 8: 473–479, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasson R, Amsterdam A: Pleiotropic anti–apoptotic activity of glucocorticoids in ovarian follicular cells. Biochem Pharmacol 66: 1393–1401, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Sasson R, Shinder V, Dantes A, Land A, Amsterdam A: Activation of multiple signal transduction pathways by glucocorticoids: Protection of ovarian follicular cells against apoptosis. Biochem Biophys Res Commun 311: 1047–1056, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC: Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell 104: 719–730, 2001 [PubMed] [Google Scholar]
- 26.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC: Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298: 843–846, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Kleiman A, Tuckermann JP: Glucocorticoid receptor action in beneficial and side effects of steroid therapy: Lessons from conditional knockout mice. Mol Cell Endocrinol 275: 98–108, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM: Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol 15: 2115–2124, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Toledo-Pereyra LH, Ramakrishnan VR, Zammit M: Study of the protective effect of methylprednisolone, furosemide, and mannitol on ischemically damaged kidneys. Eur Surg Res 11: 179–184, 1979 [DOI] [PubMed] [Google Scholar]
- 30.Green RD, Boyer D, Halasz NA, Collins GM: Pharmacological protection of rabbit kidneys from normothermic ischemia. Transplantation 28: 131–134, 1979 [DOI] [PubMed] [Google Scholar]
- 31.Aydin G, Okiye SE, Zincke H: A comparative study of several agents alone and combined in protection of the rodent kidney from warm ischaemia: methylprednisolone, propranolol, furosemide, mannitol, and adenosine triphosphate-magnesium chloride. Urol Res 11: 105–109, 1983 [DOI] [PubMed] [Google Scholar]
- 32.Smeesters C, Corman J, Fassi JC, Giroux L, St-Louis G, Jean G, Daloze P: Beneficial effects of methylprednisolone on urinary excretion of lysosomal enzymes in acute renal ischemia. Can J Surg 26: 175- 177: 180, 1983 [PubMed] [Google Scholar]
- 33.Takahira R, Yonemura K, Fujise Y, Hishida A: Dexamethasone attenuates neutrophil infiltration in the rat kidney in ischemia/reperfusion injury: The possible role of nitroxyl. Free Radic Biol Med 31: 809–815, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Pombo CM, Bonventre JV, Avruch J, Woodgett JR, Kyriakis JM, Force T: The stress-activated protein kinases are major c-Jun amino-terminal kinases activated by ischemia and reperfusion. J Biol Chem 269: 26546–26551, 1994 [PubMed] [Google Scholar]
- 35.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331, 1995 [DOI] [PubMed] [Google Scholar]
- 36.di Mari JF, Davis R, Safirstein RL: MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol 277: F195–F203, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Searle M, Lawson G, Chakraborty J, Baylis EM, Lee HA, Marks V: High-dose methylprednisolone sodium succinate (pulse therapy) in the treatment of renal disease: plasma and urine concentrations. Eur J Clin Pharmacol 28: 245–248, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Widen C, Zilliacus J, Gustafsson JA, Wikstrom AC: Glucocorticoid receptor interaction with 14–3-3 and Raf-1, a proposed mechanism for cross-talk of two signal transduction pathways. J Biol Chem 275: 39296–39301, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Jaiswal RK, Weissinger E, Kolch W, Landreth GE: Nerve growth factor-mediated activation of the mitogen-activated protein (MAP) kinase cascade involves a signaling complex containing B-Raf and HSP90. J Biol Chem 271: 23626–23629, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Beeram M, Patnaik A, Rowinsky EK: Raf: A strategic target for therapeutic development against cancer. J Clin Oncol 23: 6771–6790, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Daemen MA, van 't Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA: Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest 104: 541–549, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV: Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paller MS: Effect of neutrophil depletion on ischemic renal injury in the rat. J Lab Clin Med 113: 379–386, 1989 [PubMed] [Google Scholar]
- 44.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL: Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110: 1083–1091, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries B, Kohl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA: Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol 170: 3883–3889, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Losel R, Wehling M: Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4: 46–56, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Song IH, Buttgereit F: Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol 246: 142–146, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C: Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 58: 658–673, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee PK, Zacharowski K, Cuzzocrea S, Otto M, Thiemermann C: Inhibitors of poly (ADP-ribose) synthetase reduce renal ischemia-reperfusion injury in the anesthetized rat in vivo. FASEB J 14: 641–651, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE: Use of Ly6G-specific monoclonal anti-body to deplete neutrophils in mice. J Leukoc Biol 83: 64–70, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM: High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J 17: 908–910, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Lee HT, Emala CW: Preconditioning and adenosine protect human proximal tubule cells in an in vitro model of ischemic injury. J Am Soc Nephrol 13: 2753–2761, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Xie J, Guo Q: Apoptosis antagonizing transcription factor protects renal tubule cells against oxidative damage and apoptosis induced by ischemia-reperfusion. J Am Soc Nephrol 17: 3336–3346, 2006 [DOI] [PubMed] [Google Scholar]