Abstract
Intestinal phosphate absorption occurs through both a paracellular mechanism involving tight junctions and an active transcellular mechanism involving the type II sodium-dependent phosphate cotransporter NPT2b (SLC34a2). To define the contribution of NPT2b to total intestinal phosphate absorption, we generated an inducible conditional knockout mouse, Npt2b−/− (Npt2bfl/fl:Cre+/−). Npt2b−/− animals had increased fecal phosphate excretion and hypophosphaturia, but serum phosphate remained unchanged. Decreased urinary phosphate excretion correlated with reduced serum levels of the phosphaturic hormone FGF23 and increased protein expression of the renal phosphate transporter Npt2a. These results demonstrate that the absence of Npt2b triggers compensatory renal mechanisms to maintain phosphate homeostasis. In animals fed a low phosphate diet followed by acute administration of a phosphate bolus, Npt2b−/− animals absorbed approximately 50% less phosphate than wild-type animals, confirming a major role of this transporter in phosphate regulation. In vitro analysis of active phosphate transport in ileum segments isolated from wild-type or Npt2b−/− mice demonstrated that Npt2b contributes to >90% of total active phosphate absorption. In summary, Npt2b is largely responsible for intestinal phosphate absorption and contributes to the maintenance of systemic phosphate homeostasis.
Inorganic phosphate is an essential mineral critical for cellular processes and bone mineralization. Severe disruptions in serum phosphate have pathologic consequences.1,2 Hypophosphatemic disorders are associated with rickets, osteomalacia, and a host of secondary dysfunctions.3 In contrast, hyperphosphatemia associated with chronic kidney disease (CKD) is linked tightly to increased risk of cardiovascular morbidity and mortality.4–6 Recent studies show that elevated phosphate concentrations within the high normal range in individuals with functional kidneys also are correlated with increased cardiovascular risk and mortality.7,8 Thus, an elevated serum phosphate level is an emerging health risk.
Despite the importance of maintaining a relatively narrow serum phosphate range, nearly 70% of dietary phosphate is absorbed, resulting in transient postprandial increases in serum phosphate concentrations.9 Normalization of serum phosphate appears to be managed primarily within the renal proximal tubule by the type II sodium-dependent phosphate cotransporters NPT2a (SLC34a1) and NPT2c (SLC34a3). Genetic knockout mouse models demonstrate that 80% and 20% of total urinary phosphorus are managed by the Npt2a and Npt2c transporters, respectively.10,11 Chronic and acute regulation of these renal transporters is modulated by changes in dietary and serum phosphate levels and by three major hormones: parathyroid hormone (PTH), 1,25-dihydroxy vitamin D3 (1,25(OH)2D3), and fibroblast growth factor 23 (FGF23).1
The emergence of data demonstrating that regulation of the renal phosphate transporters can adequately maintain systemic phosphate levels has reduced contemporary interest in intestinal phosphate regulation. Furthermore, early studies of intestinal transport suggest that paracellular transport driven by a passive diffusional process predominates under standard dietary conditions.12,13 An alternative transcellular mechanism in the small intestine is dependent on active transport through the sodium-dependent phosphate cotransporter NPT2b (SLC34a2).14 Npt2b has a relatively low Km for phosphate, suggesting that transport would be readily saturated under standard conditions and therefore may be important only under conditions of hypophosphatemia or low dietary phosphate intake.15 Furthermore, individuals with inactivating NPT2b mutations have pulmonary alveolar microlithiasis (PAM) but do not have serum or urinary phosphate abnormalities.16,17 Despite this cumulative evidence downplaying the relative importance of NPT2b, its expression is increased by either 1,25(OH)2D3 or low dietary phosphate and decreased by nicotinamide. Interestingly, nicotinamide treatment in late stage hyperphosphatemic CKD patients has been shown to lower serum phosphate concentrations,18 raising the possibility that phosphate transport in the intestine by Npt2b may be important under the pathologic conditions associated with loss of renal function.
To assess Npt2b's relative importance under defined conditions, we have generated a tamoxifen-inducible ubiquitous Npt2b deletion. Surprisingly, deletion of the intestinal transporter leads to altered compensatory hormonal responses that maintain serum phosphate levels within normal limits. These data demonstrate that Npt2b plays an active role in systemic phosphate regulation.
Results
Targeted Disruption of the Npt2b Gene
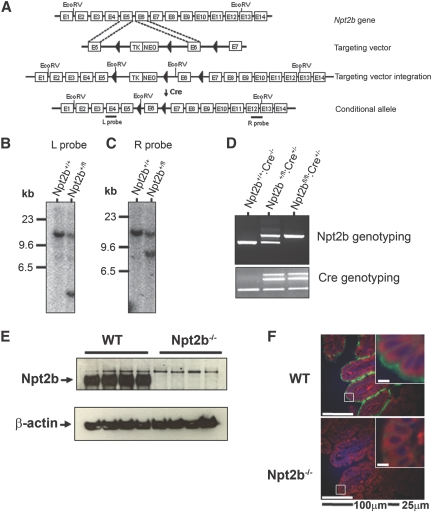
To generate the inducible Npt2b knockout mice, a conditional allele was produced by insertion of LoxP sites within introns 5 and 6 (Figure 1, A–C). Resulting agouti mice were genotyped to identify normal (Npt2b+/+), conditional heterozygous (Npt2b+/fl), or conditional homozygous alleles (Npt2bfl/fl) (Figure 1D). Mice were bred with CreERT2 mice19 under the control of the cytomegalovirus promoter, resulting in a tamoxifen-inducible Cre mouse, which was confirmed by PCR (Figure 1D). Mating to CreERT2 and induction by tamoxifen in conditional Npt2b mice generated the following genotypes: WT (Npt2bfl/fl:Cre−/−), Npt2b+/− (Npt2b+/fl:Cre+/−), and Npt2b−/− (Npt2bfl/fl:Cre+/−).
Figure 1.
Establishment of the Npt2b conditional mouse. (A) Generation of conditional allele and introduction into embryonic stem (ES) cells were done by standard methods. The ES cells were treated with Cre to remove the neomycin cassette, and clones containing the conditional Npt2b allele were selected. Clones were microinjected into C57Bl/6 mouse blastocysts to generate mice with the floxed allele (◀, LoxP sites). Npt2bfl/+ mice were mated with a mouse expressing a fusion protein consisting of Cre recombinase and a mutated ligand-binding domain of the estrogen receptor (CreERT2). The Cre recombinase was activated upon tamoxifen administration to the mouse, leading to recombination of the “lox” sequences and inactivation of the gene. (B and C) Genomic DNA isolated from ES cells after Cre recombination was digested with EcoRV, and a Southern blot analysis was performed. (B) Left arm internal probe corresponding to exon 4 was used, where the nonintegrated (WT) allele corresponds to 12.4 kb and the integrated floxed (conditional) allele corresponds to 4.1 kb. (C) Right arm external probe corresponding to exons 12 and 13 was used, where nonintegrated allele corresponds to 12.4 kb and the floxed allele corresponds to 8.2 kb. (D) Genotyping for the conditional allele and the Cre gene were performed on mouse tail genomic DNA. After induction of knockdown with tamoxifen, protein from intestinal mucosa was extracted. (E) Shown is a representative immunoblot for Npt2b protein. (F) Immunohistochemistry on a cross-sectional area of ileum showing Npt2b protein (green) in WT mice but absent in Npt2b−/− mice, Hoechst staining for nuclei (blue), and actin staining with phalloidin (red).
Knockdown of Npt2b Expression after Tamoxifen Administration
Npt2b mRNA and protein expression were assessed in WT and Npt2b−/− animals 2 wk after tamoxifen dosing. Npt2b mRNA isolated from the ileum was detected by quantitative reverse-transcription PCR in WT mice but not in Npt2b−/− animals (data not shown). Similarly, Npt2b protein was observed via immunoblotting in ileum mucosal scrapings from WT mice but not those from Npt2b−/− animals (Figure 1E). Immunohistochemical analyses revealed intense fluorescence staining of Npt2b in the entire villi of the ileum brush border membrane of WT mice but not that of Npt2b−/− animals (Figure 1F). These results demonstrate that the tamoxifen dosing regimen induced complete and extended deletion of Npt2b expression from intestinal tissue in both fully differentiated epithelial and intestinal crypt precursor cells. Npt2b is expressed abundantly in lung type II alveolar cells,20 and as expected protein expression was readily detectable in WT but not in Npt2b−/− animals (data not shown).
Phenotypic Characterization of Npt2b−/− Mice
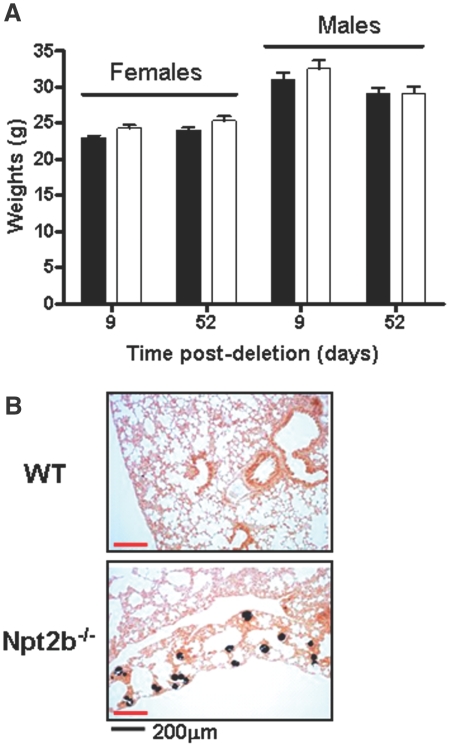
Gross phenotypic changes were not observed in Npt2b+/− or Npt2b−/− mice relative to WT animals. For simplicity, the reported data are shown for WT and Npt2b−/− animals only. Npt2b deletion did not result in weight changes (Figure 2A).
Figure 2.
Phenotypic characterization of the Npt2b−/− mouse. (A) Animal weights for both females and males were measured for 52 d after Npt2b deletion. No changes in body weights were observed in Npt2b−/− (□) compared with WT (■) animals. (B) Lungs of Npt2b−/− animals show discernible signs of pulmonary alveolar microlithiasis as indicated by von Kossa stain.
Loss of function Npt2b mutations are associated with PAM in humans.16,17 Similar to PAM patients, calcification nodules were evident in Npt2b−/− but not in WT animals 10 wk after Npt2b deletion (Figure 2B). In contrast, analysis of kidney and ileum revealed no pathologic abnormalities associated with Npt2b deletion.
Dynamic and static histomorphometric analysis of WT and Npt2b−/− bones 10 wk after Nptb2 deletion showed no differences between the genotypes (Table 1). This demonstrates that under normal conditions Npt2b plays a minor role in bone remodeling.
Table 1.
Bone histomorphometric data
| WTa | Npt2b−/−a | |
|---|---|---|
| BV/TV (%) | 5.57 ± 0.74 | 6.84 ± 0.93 |
| MS (%) | 2.63 ± 0.31 | 2.75 ± 0.38 |
| MS/BS (%) | 20.24 ± 0.82 | 19.82 ± 2.18 |
| MAR (μm/d) | 1.48 ± 0.13 | 1.53 ± 0.19 |
| BFR/BS (μm3/μm2/d) | 0.29 ± 0.03 | 0.31 ± 0.06 |
| ES/BS (%) | 2.40 ± 0.35 | 3.46 ± 0.71 |
| Ob.S/BS (%) | 26.67 ± 6.3 | 22.55 ± 5.55 |
| Oc.S/BS (%) | 1.61 ± 0.34 | 2.21 ± 0.39 |
| Tb.Th (μm) | 25.49 ± 1.52 | 27.49 ± 1.30 |
| Tb.N (/mm) | 2.12 ± 0.18 | 2.44 ± 0.25 |
| Tb.Sp (μm) | 432.3 ± 31.29 | 379.2 ± 34.82 |
Data expressed as mean ± SEM. BV/TV, bone volume/tissue volume; MS, mineralizing surface; MS/BS, mineralizing surface/bone surface; MAR, mineral apposition rate; BFR/BS, bone formation rate/bone surface; ES/BS, eroded surface/bone surface; Ob.S/BS, osteoblast surface/bone surface; Oc.S/BS, osteoclast surface/bone surface; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular spacing.
aNo statistical differences were observed in all parameters measured.
Serum, Urine, and Fecal Biochemistries
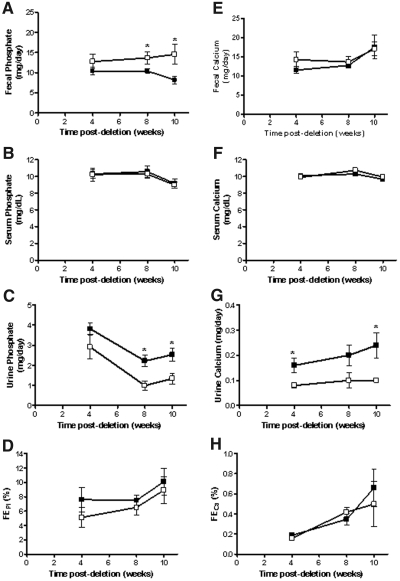
The temporal effects on phosphate homeostasis after gene ablation were analyzed over 10 wk after tamoxifen treatment. Consistent with an expected decrease in intestinal phosphate absorption, a net increase in fecal phosphate content was observed for Npt2b−/− animals, reaching approximately twofold at 10 wk (Figure 3A). Importantly, differences in serum phosphate levels were not detected between WT and Npt2b−/− animals (Figure 3B). In contrast, urinary phosphate excretion was reduced by greater than two-fold in Npt2b−/− mice relative to that in WT animals and appeared to be inversely correlated with changes in fecal phosphate excretion (Figure 3C). Fractional excretion of phosphorus was similar between WT and Npt2b−/− mice (Figure 3D), suggesting that kidney function was unaffected by Npt2b deletion. These results indicated that decreased urinary phosphate excretion compensated for reduced intestinal absorption, thereby maintaining serum balance.
Figure 3.
Phosphate excretion in Npt2b−/− animals is altered. Serum, urine, and fecal phosphate and calcium were measured at different time points. (A) Fecal phosphate excretion is elevated in Npt2b−/− mice (□) relative to that in WT (■) animals. (B) Serum phosphate was normal in Npt2b−/− mice. (C) Urine phosphate is reduced in Npt2b−/− animals relative to that in WT mice. (D) Fractional excretion of phosphate was unchanged. (E) Fecal calcium excretion was normal in Npt2b−/− mice relative to that in WT animals. (F) Serum calcium was normal in Npt2b−/− animals. (G) Urine calcium is reduced in Npt2b−/− animals relative to that in WT mice. (H) Fractional excretion of calcium was unchanged. Data expressed as mean ± SEM; *P < 0.05. Results were obtained from three independent experiments (n = 8 to 10/group).
Serum calcium levels were also maintained at normal levels in the Npt2b−/− animals (Figure 3F). Unexpectedly, urinary calcium excretion was decreased in Npt2b−/− animals when compared with that in WT animals without statistically significant changes in fecal calcium excretion or fractional urinary calcium excretion (Figure 3, E, G, and H). A trend toward increased Trpv5 mRNA expression was observed in Npt2b−/− animals (data not shown).
Effect of Npt2b Ablation on the Expression of the Renal Npt2a Cotransporter
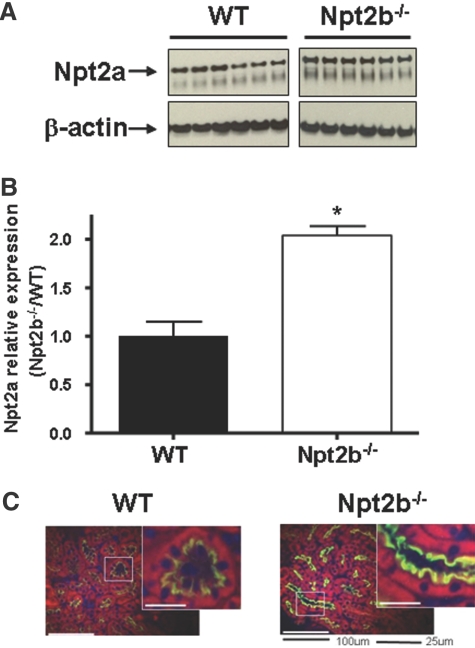
These results indicate that despite an apparent reduction in total intestinal phosphate absorption serum phosphate is maintained in the Npt2b−/− animals via reduced urinary excretion. We therefore asked whether changes in the expression of the major renal sodium phosphate cotransporter, Npt2a could be responsible for the compensatory decrease in urinary phosphate excretion. Quantitative immunoblot analysis revealed a statistically significant increase in Npt2a protein expression in kidney lysates from Npt2b−/− mice relative to that of WT littermates (Figure 4, A and B). In addition, immunohistochemical visualization revealed that Npt2a abundance was increased within the renal brush border membrane of the proximal tubules in Npt2b−/− animals compared with that of WT animals (Figure 4C). In contrast, renal expression of Npt2c mRNA was not different in Npt2b−/− mice relative to that of WT mice (data not shown).
Figure 4.
Npt2a expression in WT and Npt2b−/− animals. (A) Representative immunoblot of Npt2a expression from kidney lysates of WT and Npt2b−/− mice. (B) Quantitation of immunoblots demonstrating a significant increase in Npt2a protein expression in Npt2b−/− animals (□) when compared with that in WT animals (■) (*P < 0.05). (C) Immunohistochemistry of a cross-sectional area of kidney, demonstrating increased expression of Npt2a (green) in the proximal tubules of Npt2b−/− animals. Hoechst (Invitrogen) staining for nuclei (blue) and actin staining with phalloidin (red) are also shown.
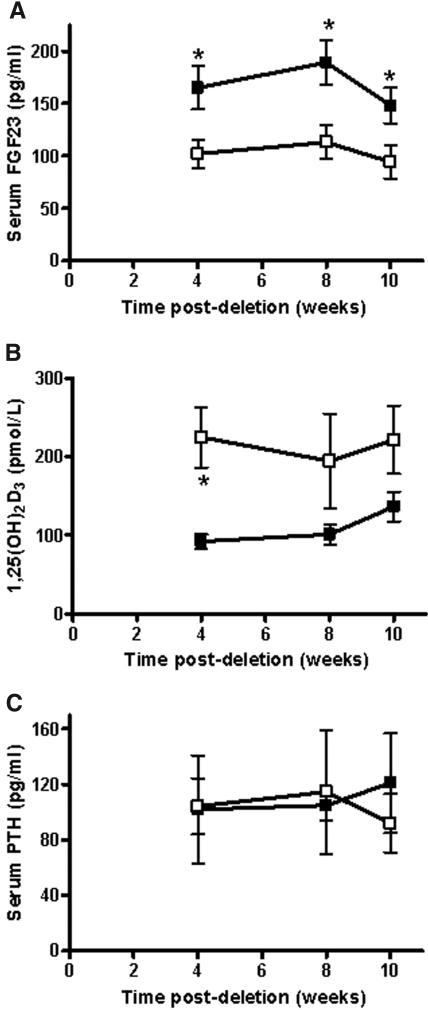
Regulation of Npt2a expression is known to occur through the actions of two phosphaturic proteins, PTH and FGF23. A significant reduction of serum FGF23 but not PTH levels was observed after tamoxifen induction (Figure 5, A and C). In addition to its control of urinary phosphorus excretion, FGF23 is a negative regulator of 1,25(OH)2D3 production. A corresponding increase in 1,25(OH)2D3 as a result of decreased FGF23 levels could account for decreased urinary calcium excretion (Figure 5B). A trend toward increased 1,25(OH)2D3 levels was demonstrated, with a statistically significant difference observed at 4 wk after tamoxifen dosing in the Npt2b−/− mice (Figure 5B). In addition, 1α-hydroxylase and 24-hydroxylase mRNA expression were increased and decreased, respectively by 2.5-fold (data not shown). These data may explain decreased calcium excretion through 1,25(OH)2D3 actions on Trpv5 renal expression.
Figure 5.
Regulators of phosphate homeostasis were altered in Npt2b−/− mice. (A) Serum fibroblast growth factor 23 (FGF23) is decreased, (B) serum 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) is increased, and (C) serum parathyroid hormone (PTH) is unchanged in Npt2b−/− mice (□) when compared with those of WT (■) control animals. Data expressed as mean ± SEM; *P < 0.05. Results were obtained from three independent experiments (n = 8 to 10/group).
Effects of Npt2b Deletion on Acute Intestinal Absorption
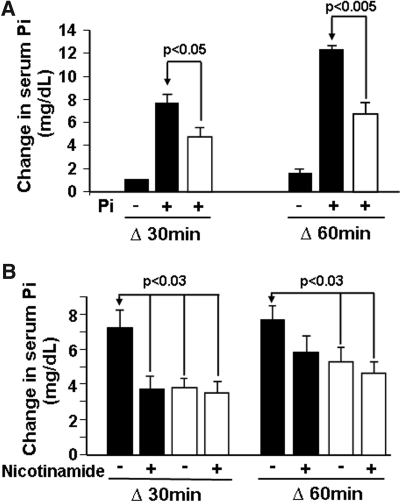
To assess the role of Npt2b in total phosphate absorption under conditions associated with high phosphorus intake, we employed an acute hyperphosphatemic mouse model. Mice were fed a low phosphate diet (0.02% phosphate) for 1 wk to induce maximal Npt2b expression in WT animals,14 followed by a 0.5 M sodium phosphate bolus. After gavage, serum phosphorus levels increased from baseline by approximately 8 mg/dl and approximately 12 mg/dl at 30 and 60 min, respectively (Figure 6A). In contrast, Npt2b−/− animals exhibited a blunted increase in serum phosphate that was approximately 40 to 45% less than the increase observed in WT animals (Figure 6A). The change from baseline was approximately 5 mg/dl and approximately 7 mg/dl at 30 and 60 min after phosphate bolus administration in Npt2b−/− animals, respectively (Figure 6A). These results suggest that when Npt2b expression is maximally induced under low phosphate conditions Npt2b is contributing slightly less than half of the total acute intestinal phosphate absorption.
Figure 6.
Acute hyperphosphatemic mouse model. The WT (■) and Npt2b−/− (□) animals were placed on a low phosphate (0.02%) diet for 1 wk and then gavaged with a hypertonic sodium phosphate solution. Serum was collected at 0, 30, and 60 min after gavage, and phosphate was measured and plotted as the difference in serum phosphate from time 0 min. (A) The WT and Npt2b−/− animals were evaluated for their ability to absorb serum phosphate after gavaging with a high phosphate solution. Npt2b deletion significantly dampened the rise in serum phosphate. (B) Nicotinamide was administered in three doses in the last 3 d before gavage with a phosphate bolus, and their serum phosphate changes were followed. Nicotinamide significantly reduced the rise in serum phosphate in WT animals but not in Npt2b−/− mice. Data expressed as mean ± SEM. Results were obtained from three independent experiments (n = 8 to 10/group).
Studies have demonstrated that nicotinamide inhibits intestinal sodium-dependent phosphate transport in rats with a corresponding decrease in Npt2b mRNA expression.21 We therefore investigated whether the acute effects of nicotinamide inhibition are mediated by Npt2b. The standard increase in serum phosphorus observed 30 min after a phosphorus bolus was blunted by approximately 50% in WT animals treated with nicotinamide compared with that in untreated mice (Figure 6B). In contrast, nicotinamide had no effect on Npt2b−/− animals. These data suggest that nicotinamide mediates acute inhibition of intestinal phosphate transport through Npt2b.
Relative Role of Npt2b in Active Transport
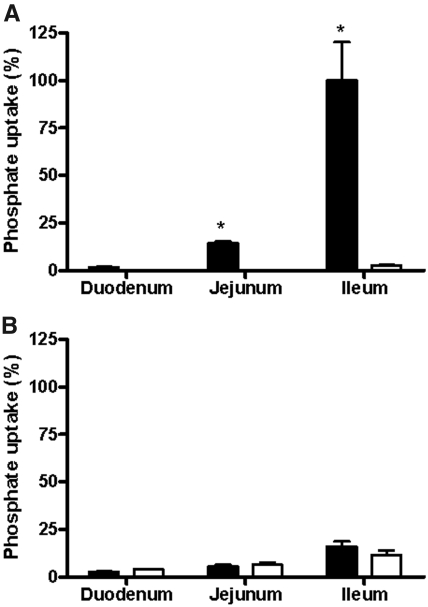
An everted sac experiment was performed to evaluate the relative role of Npt2b in sodium-dependent phosphate transport. In this experiment, intestinal pieces from each of the three major intestinal segments were excised, everted, filled, and placed in a sodium-free or sodium-containing buffer plus 1.2 mM potassium phosphate to eliminate potential electrochemical gradients driving paracellular transport. Transfer of trace radiolabeled 33P or 45Ca from bath (mucosal) to luminal side (serosal) was measured. There were no differences in calcium transport between WT and Npt2b−/− animals (data not shown). Figure 7A shows that virtually all of the sodium-dependent transport under these conditions can be attributed to Npt2b. Substantial phosphate transport was observed in the ileum and jejunum, with minimal transport in the duodenum of WT animals when compared with that of Npt2b−/− mice. These results are consistent with the findings that in mice Npt2b is expressed at low levels in jejunum and predominantly expressed in the ileum.14,22 There was no difference in the sodium-independent transport between WT and Npt2b−/− mice in each of the three segments (Figure 7B), indicating that sodium-independent pathways do not compensate for reduced uptake resulting from Npt2b deletion. In addition, sodium-dependent transport is the predominant active pathway of phosphate absorption in the mouse small intestine, ranging from 1.5-fold more than sodium-independent in the duodenum to sixfold more in the ileum.
Figure 7.
Phosphate transport in different intestinal segments. With the everted sac method, (A) sodium-dependent and (B) sodium-independent phosphate transport were measured in all three segments of the intestine. (A) Npt2b deletion significantly decreased the sodium-dependent transport. (B) No differences were observed in the sodium-independent transport between WT (■) and Npt2b−/− (□) mice. Active sodium-dependent phosphate transport is the predominant pathway in the intestine. Data expressed as mean ± SEM; *P < 0.05. Results were obtained from two independent experiments (n = 8 to 10/group).
Discussion
Maintenance of systemic phosphate balance is emerging as an important health concern in individuals with both adequate and declining kidney function. Although it is acknowledged that the kidney is the primary site of phosphate regulation, a full understanding of how other key organs contribute to overall systemic phosphate homeostasis is lacking. The intestine has not been considered a major organ for phosphate control, because more phosphate is absorbed than required by the body, with an estimated 70% of all dietary phosphate content absorbed via the intestine.23 In humans, postprandial serum phosphate increases are quickly handled by the kidney, and serum phosphate returns to basal levels within a few hours. Recent evidence suggests that the intestine may participate in acute phosphate regulation through release of phosphatonin24 and may therefore play a larger role in management of systemic phosphate homeostasis than previously recognized. A major goal of this study was to investigate the role of the sodium-dependent phosphate cotransporter Npt2b in acute and chronic maintenance of systemic phosphate balance.
To assess the importance of Npt2b, we created an Npt2b inducible conditional knockout mouse that allowed us to delete Npt2b protein across all tissues in adult animals without affecting development. Interestingly, a recent report demonstrated that developmental deletion of the Npt2b gene led to an embryonic lethal phenotype due to a defect in phosphate absorption from the maternal circulation.25
Our studies demonstrate that serum phosphorus and calcium concentrations are maintained in animals devoid of measureable Npt2b, despite clear evidence of decreased intestinal phosphate uptake (Figure 3). The simplest interpretation of this result is that increased phosphate excretion is a passive consequence of reduced phosphate absorption. However, our data suggest that compensatory changes were actively induced in animals lacking Npt2b to maintain normal systemic phosphate levels. Increased protein expression of the renal sodium phosphate cotransporter Npt2a was detected clearly in Npt2b−/− mice relative to that in WT animals, indicating that the animals had responded appropriately by increasing phosphate reabsorption (Figure 4). These changes in Npt2a expression can be explained by the decreased levels of the phosphaturic hormone FGF23 (Figure 5A).
A secondary consequence of reduced FGF23 appears to be elevation of serum 1,25(OH)2D3 (Figure 5B). Previous studies have shown that FGF23 reduces 1,25(OH)2D3 by decreasing 1α-hydroxylase and increasing 24-hydroxylase mRNA levels.26–28 Although FGF23 and 1,25(OH)2D3 changes are relatively modest, the corresponding changes in expression of the two key enzymes involved in 1,25(OH)2D3 metabolism in the Npt2b−/− animals provide evidence that chronic intestinal transport changes can influence FGF23 expression as a primary response. The known negative feedback loop controlling the interrelationship between FGF23 and 1,25(OH)2D3 further supports the notion that the primary hormone targeted by long-term changes in intestinal transport is FGF23.
The mechanism by which intestinal transport alters FGF23 regulation is unknown. The actions of the recently described intestinal phosphatonin appear to be independent from serum phosphate, PTH, FGF23, and other known phosphaturic factors.24 The FGF23 changes observed in the Npt2b−/− mouse could be due to the chronic effects of decreased phosphate absorption rather then an acute effect. Alternatively, Npt2b may serve as a sensing molecule regulating the release of the unknown enterocyte-specific factors. Although this factor does not appear to regulate FGF23 release directly, it is conceivable that it induces secretion of a renal specific regulator of FGF23.
Expression of the type II phosphate cotransporters Npt2a and Npt2b has been identified in osteoclasts in situ and in osteoblast-like cell lines.29,30 Bone histomorphometric analysis did not identify differences between WT and Npt2b−/− mice (Table 1). These observations mirror those observed in the Npt2c−/− mouse where differences in bone changes relative to WT were not found.11 Npt2a−/− mice have bone abnormalities that are reversed with age.10 Taken together, these data suggest that the type II transporters play a minor role in bone remodeling or may compensate for each other when one is absent. It has been proposed that the type III phosphate cotransporter Pit1, which also is expressed in osteoclasts and osteoblasts,29 may be the critical phosphate transporter in bone.
Relative Importance of Npt2b in Intestinal Phosphate Transport
Limitations of each of the various models used to assess intestinal transport have made it difficult to determine the relative importance of Npt2b. The contribution of each intestinal absorption pathway is dependent on the protein expression of the transport machinery, the actual luminal phosphate concentration in a given intestinal segment, and the movement of phosphate through the intestinal tract. Paracellular transport is associated with passive ion movement through tight junctions between adjacent epithelial cells in response to electrochemical gradients.31 It is expected that paracellular transport induced by large phosphate concentrations after a meal might be the predominant postprandial pathway and largely responsible for overall phosphate absorption. However, early studies suggest that although this pathway can be favored under specific experimental conditions the intestinal epithelial wall is not readily permeable to phosphate.32 Additional studies utilizing a variety of in vivo and ex vivo techniques suggest that sodium-dependent mechanisms may contribute as much as 33 to 75% of total intestinal phosphate absorption.12,13,33 These methods generally depend on sodium depletion to separate the different types of active transport. Because it is impossible to completely deplete sodium under physiologic conditions, these methods may underestimate the contribution of Npt2b-dependent transport. It is also important to recognize that results from studies measuring transport in isolated intestinal segments may be skewed by the static residency time where natural movement of substances down the intestinal tract does not occur. Several studies have demonstrated that, although absolute transport rates in rats appear greatest in the duodenum, in whole animals the ileum as the longest segment absorbs the greatest net amount of phosphate.23 Cumulatively, these studies result in an incomplete understanding of intestinal phosphate regulation.
To assess Npt2b's contribution to total phosphate transport under postprandial conditions, we utilized an acute model that would maximize the potential contribution for each of the transport mechanisms. Under these conditions, the postprandial phosphate surge in Npt2b−/− animals was reproducibly reduced by approximately 45 to 50% relative to that observed in WT animals (Figure 6A). Additionally, nicotinamide, an agent known to reduce serum phosphorus with a corresponding decrease in Npt2b expression,21 reduced the serum surge in WT animals to the same degree as that observed by deleting Npt2b (Figure 6B). Taken together, these results demonstrate that under these defined in vivo conditions Npt2b-dependent phosphate transport contributes as much as 45% of total phosphate transport within the first hour postmeal.
In addition to a chronic low phosphate diet, several agents have been shown to regulate Npt2b expression, including 1,25(OH)2D3, estrogen, and EGF.34–36 Studies in both the vitamin D receptor knockout mouse and the 1α-hydroxylase knockout mouse have shown that Npt2b regulation by modulating dietary phosphate occurs through a vitamin-D-independent pathway.37,38 It is important to point out that our estimate of Npt2b-dependent phosphate transport may represent the maximum potential contribution, which may vary depending on physiologic conditions and dietary intake.
In addition to Npt2b, two additional sodium-dependent phosphate cotransporters are localized to the intestine, Pit1 and Pit2. Our studies using isolated everted intestinal sacs suggest that under the conditions examined transport through Npt2b is the major mechanism of active intestinal transport, accounting for more than 90% of sodium-dependent phosphate transport (Figure 7).
Altogether, these data suggest that Npt2b is important in phosphate homeostasis and further demonstrate that organ cross-talk involving the intestine, bone, and kidney and the FGF23/1,25(OH)2D3 hormonal axis has a physiologic role in regulation of renal phosphate excretion.
Concise Methods
Mice
All studies were approved by the institutional animal care committee. Mice were maintained in a virus- and parasite-free barrier facility and exposed to a 12-h light/dark cycle. Unless otherwise specified, animals were maintained on standard rodent chow diet (PicoLab Rodent Diet 20, #5053; LabDiet) containing 0.63% phosphate, 0.81% calcium, and 2.2 IU/g vitamin D3.
Generation of the Npt2b Targeting Vector and Conditional Allele in Mice
Sequences bearing a single loxP site were inserted in intron 6 at a unique HindIII site. A neomyocin drug selection marker gene and a herpes simplex virus thymidine kinase (TK) minigene were flanked by loxP sites and inserted into an XbaI site located in intron 5 (Figure 1A). The vector was linearized and electroporated into AMK06 (129SV) embryonic stem (ES) cells. Clones surviving G418 were arrayed in a 96-well format and screened by Southern blot analysis using a 3′ external probe generated by PCR for sequences in exon 12 and 13. An EcoRV digest of genomic DNA resulted in the appearance of a 12.4 kb WT fragment and an 8.2 kb targeted fragment. Three positive clones were electroporated with a Cre expression vector to excise the floxed drug selection cassettes. After selection in 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil for loss of the TK gene, the surviving clones were arrayed and screened for the conditional allele containing the loxP sites flanking exon 6 of the Npt2b gene. Southern blot analysis was performed on EcoRV digests, and 3′ external (exons 12 and 13) or internal (exon 5) probe confirmed proper targeting (Figure 1, B and C). These clones were microinjected into C57Bl/6 mouse blastocysts. Breeding of chimeric male mice to C57Bl/6 wild-type female mice resulted in the generation of numerous agouti F1 mice. Genotype analysis of genomic DNA isolated from tail biopsies of the agouti mice confirmed the presence of the Npt2b conditional allele (Npt2bfl/fl) in these mice (Figure 1D). Animals were generated by Rodent Experimental Models, Inc. (Worcester, MA).
Breeding Strategy and Induction of Npt2b Knockdown
Npt2bfl/fl mice were mated with CreERT2 mice (Artemis).19 CreERT2 is a fusion protein consisting of Cre recombinase and a mutated ligand-binding domain of the estrogen receptor (CreERT2). The Cre recombinase is activated upon tamoxifen administration. Mice were mated through several rounds to obtain litters generating only WT (Npt2bfl/fl:Cre−/−) and Npt2b−/− (Npt2bfl/fl:Cre+/−) mice. Tamoxifen (Sigma) was administered to Npt2b−/− and WT animals at a dose of 3 mg by oral gavage for five continuous days. Animals were allowed to recover for at least 7 d before any analysis. Loss of Npt2b protein in each knockout animal was confirmed by immunoblot analysis.
Tissue Harvest
Whole kidney, lung, and ileum were removed at indicated time intervals after tamoxifen administration. Isolated whole kidney and lung tissue were placed in Trizol and frozen at −80°C for RNA analysis or fixed in 10% neutral buffered formalin and embedded in paraffin for histologic analysis. The last 10 cm of the small intestine (ileum) was removed and flushed with 0.9% saline. The ileum was fixed in 10% neutral buffered formalin for histologic analysis. Of the remaining 5 cm, mucosal villi were gently scraped off. Of this, half were saved for RNA preparation, and the remainder were saved for immunoblot analysis.
Histology
Kidney, ileum, and lung tissue were harvested fixed in 10% formalin for 24 h and then placed in 70% ethanol. The tissues were then processed, embedded in paraffin, and sectioned. Sections were dewaxed in xylene, rehydrated by ethanol gradient, and stained in hematoxylin and eosin. Lung sections were stained by the von Kossa method to visualize calcium phosphate deposition. For bone analysis, mice were given two intraperitoneal injections of calcein (20 mg/kg) 5 d apart. Femurs were collected 3 d after the second injection. Bones were fixed in 40% alcohol and processed in methyl methacrylate. Each femur was sectioned in the ventral/dorsal plane. Two levels per block separated by 100 μm were performed. At each level, five serial sections were taken at 5 μm. Goldner's Trichrome and von Kossa sections were analyzed for static measurements. Unstained serial sections were used for dynamic measurements using the Osteo II Bioquant system.
Immunofluorescence
Fixed ileum and kidney were embedded in paraffin, sectioned, and stained by the following procedure. Slides were dewaxed and rehydrated, followed by antigen retrieval using sodium citrate solution, pH 9.9 for ileum sections or pH 6.0 for kidney sections (Dako), and placed in pressure cooker for 15 min. After being cooled, slides were blocked in 5% goat serum/0.1% Triton X-100/PBS for 1 h. Ileum sections were incubated with rabbit anti-mouse Npt2b antibody (1 μg/ml), raised against a carboxy terminal peptide (CQVEVLSMKALSNTTVF). Kidney sections were incubated with rabbit anti-mouse Npt2a antibody (1 μg/ml), raised against a carboxy terminal peptide (CLALPAHHNATRL). Blocking and primary antibody incubations were in 5% goat serum/PBS. Goat anti-rabbit IgG conjugated to Alexa Fluor 488 (Invitrogen) was used to detect rabbit anti-mouse primary antibody, and phalloidin conjugated to Alexa Fluor 594 (Invitrogen) was used to detect actin filaments. Coverslips were mounted with VectaShield hard set mounting media (VectorLabs). Images were captured on a Zeiss Axiovert 200.
Immunoblot Analysis
Tissue isolated as described above was homogenized in TPER (Pierce) in the presence of complete protease inhibitors (Roche) and PMSF (Sigma). Total lysate from ileum scrapings (20 μg), lung (5 μg), or whole kidney (5 μg) tissue were subjected to electrophoresis on a NuPAGE gel (Invitrogen) and detected using rabbit anti-mouse Npt2b (0.1 μg/ml) or rabbit anti-mouse Npt2a (0.1 μg/ml). Anti-rabbit IgG, horseradish peroxidase (HRP)-linked antibody (Cell Signal Technologies) was used as a secondary antibody. Enhanced chemiluminescence (Pierce Supersignal West Dura) was used as a detection method. Luminescent signal was read on a Typhoon 2000 scanner, and bands were quantified using ImageQuant software (Molecular Dynamics). For loading control, blots were normalized to β-actin primary antibody directly conjugated to HRP (Cell Signal Technologies).
Serum and Urine Analysis
Whole blood was collected under isoflurane via eye bleed and stored at 4°C after settling for 10 min. Serum was divided into aliquots and frozen at −80°C for subsequent analysis. Serum phosphate, calcium, and blood urea nitrogen (BUN) were quantitated according to the manufacturer's instructions (Stanbio Laboratories). Intact PTH (Immutopics), 1,25(OH)2D3 (IDS), and FGF23 (Kainos) ELISAs were performed according to the manufacturers' instructions. Animals were placed in metabolic cages, and urine was collected over a 24-h period. Urine was centrifuged to remove particulates, and the resultant supernatant volume was recorded and analyzed on Synchron CX5 (Beckman) for phosphate and calcium. Serum and urine creatinine were measured on an Integra 400 bioanalyzer (Roche Diagnostics) using the enzymatic method. Fractional excretion of phosphate or calcium was calculated using the following formula:
Fecal Phosphate and Calcium Analysis
Stool samples were collected over a 24-h period; contaminating food was removed. Fecal samples were weighed, after being dried under nitrogen for 3 h. Approximately half of the dry weight of stool sample was reconstituted in 0.6 M HCl (Sigma) to a final concentration of 50 mg/ml for 3 d under gentle rotation. On the second day of extraction, samples were homogenized and left to mix for 24 h. Samples were centrifuged at 2000 × g for 5 min to remove particulate matter. A portion of the supernatant was diluted 1:80 with HPLC-grade water (Baker). Fecal phosphate and calcium were quantified according to the manufacturer's protocol (Stanbio Laboratories).
Gene Expression Analysis
Tissues were harvested and placed in Trizol (Sigma). RNA was extracted using the chloroform and isopropanol precipitation method. The extracted RNA was purified on a Qiagen column and eluted in RNAse free water. A reverse transcriptase reaction was performed. The generated cDNA was used in Taqman low-density arrays (Applied Biosystems) containing genes of interest according to the manufacturer's protocol.
Acute Hyperphosphatemic Mouse Model
Animals were placed on a low phosphorus diet (0.02% Pi, TD06216) for 1 wk. Baseline eye bleeds were taken at t = 0 min. Animals were then gavaged with a 0.5 M NaH2PO4 solution in an approximately 200 μl volume, and serum was collected 30 and 60 min postgavage by eye bleed. Serum phosphorus was measured as described above. Where indicated, nicotinamide was given intraperitoneally 3 d before phosphate bolus administration.
Intestinal Phosphate and Calcium Transport
Intestinal calcium and phosphate transport was determined by the everted gut sac assay.39,40 A 5-cm segment of duodenum was removed from the mice proximally to the pyloric junction for calcium and phosphate transport, or 5 cm of jejunum and 5 cm of ileum were removed from the mice distally to the cecum for phosphate transport. A blunt-ended 20-gauge needle was passed into the lumen of the intestinal segment, and the distal end of the segment was tied to the end of the needle. The intestinal segment was everted by rolling the proximal end slowly along the needle. The intestinal segment was then filled with 400 μl of transport buffer [128 mM NaCl/choline Cl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 19 mM NaHCO3/NH4Cl3 (pH 7.4), at 37°C] for phosphate transport or [125 mM NaCl, 10 mM fructose, 1.3 mM Hepes, and 0.25 mM CaCl2] for calcium transport. The 20-gauge needle was pulled out, the knot of the second ligation was tied, and the sacs were incubated in 10 ml-Falcon tubes containing transport buffer containing 33P orthophosphate or 45CaCl (20,000 cpm/ml) (NEN Life Science Products) and kept in a water bath at 37°C for 1 h aerated continuously with 95% O2/5% CO2. At the end of the incubation, sacs were removed from the tubes, gently blotted dry, and cut open, and the internal fluid from each sac as well as samples from the external incubation medium were assayed in for 33P or 45Ca using a scintillation counter. The accumulation of 33P or 45Ca in the inside of the sac was expressed as a ratio of the final concentration of 33P inside/outside. The everted gut sac assay selectively measures active intestinal phosphate and calcium transport.
Statistical Analysis
A t test was used to analyze significance between two groups. P < 0.05 was considered significant.
Disclosures
All authors are employees of Genzyme Corporation and own stock in the company.
Acknowledgments
We are thankful to Drs. Sylvia Christakos, Dare Ajibade, and Puneet Dhawan from the University of Medicine and Dentistry of New Jersey for providing their expertise in the everted sac method. We also are thankful to Drs. Ronaldo Ferraris and Veronique Douard from the University of Medicine and Dentistry of New Jersey for providing their protocol for phosphate transport in everted sacs.
Part of this work was presented at the 2008 Annual Meeting of the American Society of Nephrology, Philadelphia, PA, November 4 through 9.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Berndt T, Kumar R: Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda) 24: 17–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hruska KA, Slatopolsky E: Disorders of phosphorus, calcium, and magnesium metabolism. In: Diseases of the Kidney, edited by Schrier R, Gottschalk C.London, Little, Brown and Company, 1996, pp 2477–2526 [Google Scholar]
- 3.Sabbagh Y, Tenenhouse HS, Econs MJ: Mendelian hypophosphatemias. In: The Online Metabolic and Molecular Basis of Inherited Disease, edited by Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A.New York, McGraw-Hill, 2008, pp 1–171 [Google Scholar]
- 4.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM: Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695–701, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Portale AA, Halloran BP, Morris RC, Jr: Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest 83: 1494–1499, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS: Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95: 5372–5377, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K: Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol 20: 104–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danisi G, Straub RW: Unidirectional influx of phosphate across the mucosal membrane of rabbit small intestine. Pflugers Arch 385: 117–122, 1980 [DOI] [PubMed] [Google Scholar]
- 13.Walton J, Gray TK: Absorption of inorganic phosphate in the human small intestine. Clin Sci (Lond) 56: 407–412, 1979 [DOI] [PubMed] [Google Scholar]
- 14.Radanovic T, Wagner CA, Murer H, Biber J: Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-Pi diet of the type IIb Na+-Pi cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 288: G496–G500, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J: Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci USA 95: 14564–14569, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, Yildirim Z, Gocmen A, Tolun A: Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet 79: 650–656, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huqun, Izumi S, Miyazawa H, Ishii K, Uchiyama B, Ishida T, Tanaka S, Tazawa R, Fukuyama S, Tanaka T, Nagai Y, Yokote A, Takahashi H, Fukushima T, Kobayashi K, Chiba H, Nagata M, Sakamoto S, Nakata K, Takebayashi Y, Shimizu Y, Kaneko K, Shimizu M, Kanazawa M, Abe S, Inoue Y, Takenoshita S, Yoshimura K, Kudo K, Tachibana T, Nukiwa T, Hagiwara K: Mutations in the SLC34A2 gene are associated with pulmonary alveolar microlithiasis. Am J Respir Crit Care Med 175: 263–268, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Eto N, Miyata Y, Ohno H, Yamashita T: Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant 20: 1378–1384, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Seibler J, Zevnik B, Kuter-Luks B, Andreas S, Kern H, Hennek T, Rode A, Heimann C, Faust N, Kauselmann G, Schoor M, Jaenisch R, Rajewsky K, Kuhn R, Schwenk F: Rapid generation of inducible mouse mutants. Nucleic Acids Res 31: e12, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feild JA, Zhang L, Brun KA, Brooks DP, Edwards RM: Cloning and functional characterization of a sodium-dependent phosphate transporter expressed in human lung and small intestine. Biochem Biophys Res Commun 258: 578–582, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Katai K, Tanaka H, Tatsumi S, Fukunaga Y, Genjida K, Morita K, Kuboyama N, Suzuki T, Akiba T, Miyamoto K, Takeda E: Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant 14: 1195–1201, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Marks J, Srai SK, Biber J, Murer H, Unwin RJ, Debnam ES: Intestinal phosphate absorption and the effect of vitamin D: A comparison of rats with mice. Exp Physiol 91: 531–537, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Cramer CF: Progress and rate of absorption of radiophosphorus through the intestinal tract of rats. Can J Biochem Physiol 39: 499–503, 1961 [DOI] [PubMed] [Google Scholar]
- 24.Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R: Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA 104: 11085–11090, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibasaki Y, Etoh N, Hayasaka M, Takahashi MO, Kakitani M, Yamashita T, Tomizuka K, Hanaoka K: Targeted deletion of the tybe IIb Na+-dependent Pi-co-transporter, NaPi-IIb, results in early embryonic lethality. Biochem Biophys Res Commun 381: 482–486, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HA, Hsieh JC, Slater SA, Hsieh G, Kaczmarska M, Jurutka PW, Kolek OI, Ghishan FK, Haussler MR: 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol 103: 381–388, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK: 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289: G1036–G1042, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N: Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1α,25-dihydroxyvitamin D3 production. J Biol Chem 278: 2206–2211, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Khadeer MA, Tang Z, Tenenhouse HS, Eiden MV, Murer H, Hernando N, Weinman EJ, Chellaiah MA, Gupta A: Na+-dependent phosphate transporters in the murine osteoclast: Cellular distribution and protein interactions. Am J Physiol Cell Physiol 284: C1633–C1644, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Lundquist P, Murer H, Biber J: Type II Na+-Pi cotransporters in osteoblast mineral formation: regulation by inorganic phosphate. Cell Physiol Biochem 19: 43–56, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Will C, Fromm M, Muller D: Claudin tight junction proteins: Novel aspects in paracellular transport. Perit Dial Int 28: 577–584, 2008 [PubMed] [Google Scholar]
- 32.Cross HS, Debiec H, Peterlik M: Mechanism and regulation of intestinal phosphate absorption. Miner Electrolyte Metab 16: 115–124, 1990 [PubMed] [Google Scholar]
- 33.McHardy GJR, Parsons DS: The absorption of inorganic phosphate from the small intestine of the rat Q J Exp Physiol 41: 398–412, 1956 [Google Scholar]
- 34.Katai K, Miyamoto K, Kishida S, Segawa H, Nii T, Tanaka H, Tani Y, Arai H, Tatsumi S, Morita K, Taketani Y, Takeda E: Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem J 343: 705–712, 1999 [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Collins JF, Bai L, Kiela PR, Ghishan FK: Regulation of the human sodium-phosphate cotransporter NaPi-IIb gene promoter by epidermal growth factor. Am J Physiol Cell Physiol 280: C628–C636, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Uno JK, Inouye M, Xu L, Drees JB, Collins JF, Ghishan FK: Regulation of intestinal NaPi-IIb cotransporter gene expression by estrogen. Am J Physiol Gastrointest Liver Physiol 285: G1317–G1324, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, St-Arnoud R, Murer H, Biber J: Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1αOHase-deficient mice. Am J Physiol Cell Physiol 288: C429–C434, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K: Intestinal Na-Pi cotransporter adaptation to dietary Pi content in vitamin D receptor null mice. Am J Physiol Renal Physiol 287: F39–F47, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S: Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 149: 3196–3205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirchner S, Muduli A, Casirola D, Prum K, Douard V, Ferraris RP: Luminal fructose inhibits rat intestinal sodium-phosphate cotransporter gene expression and phosphate uptake. Am J Clin Nutr 87: 1028–1038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]