Abstract
Fertility rates, pregnancy, and maternal outcomes are not well described among women with a functioning kidney transplant. Using data from the Australian and New Zealand Dialysis and Transplant Registry, we analyzed 40 yr of pregnancy-related outcomes for transplant recipients. This analysis included 444 live births reported from 577 pregnancies; the absolute but not relative fertility rate fell during these four decades. Of pregnancies achieved, 97% were beyond the first year after transplantation. The mean age at the time of pregnancy was 29 ± 5 yr. Compared with previous decades, the mean age during the last decade increased significantly to 32 yr (P < 0.001). The proportion of live births doubled during the last decade, whereas surgical terminations declined (P < 0.001). The fertility rate (or live-birth rate) for this cohort of women was 0.19 (95% confidence interval 0.17 to 0.21) relative to the Australian background population. We also matched 120 parous with 120 nulliparous women by year of transplantation, duration of transplant, age at transplantation ±5 yr, and predelivery creatinine for parous women or serum creatinine for nulliparous women; a first live birth was not associated with a poorer 20-yr graft or patient survival. Maternal complications included preeclampsia in 27% and gestational diabetes in 1%. Taken together, these data confirm that a live birth in women with a functioning graft does not have an adverse impact on graft and patient survival.
One of the many perceived benefits of kidney transplantation has been restoration of pituitary-ovarian function and fertility in women of reproductive age. Prenatal advice for women with a functioning kidney transplant has been primarily based on data derived from observational research,1–13 and the reported live-birth rates achieved in such women range from 43.214 to 82%.15
Although an increased pregnancy event number has been reported for women with a functioning kidney transplant,16 little is actually known about “pregnancy rate changes” during the past 40 yr. More importantly, long-term graft and maternal survival analyses, referred to when advising women who have undergone transplantation and are considering a pregnancy, have been mostly performed without adequate matching,12 or, alternatively, matching has been used but outcomes followed up for only brief intervals14,17,18 and in small cohorts.19–22 Published graft matching studies to date suggest no adverse impact 10 yr after a live birth.14
In most instances, pregnancies in women with a kidney graft have been encouraged. Historically, renal function,8,15,17,18 baseline proteinuria,23 intercurrent hypertension,1,24 and time from transplantation1,3,5,8,14,15,18,24,25 have been used to predict adverse event risks to the mother, kidney, and offspring. To this are added the often unquantifiable inherent risks for genetically transmitted diseases or the problems associated with prematurity.26,27 More recently, epidemiologic evidence suggests low birth weight may be associated with the development of hypertension,28 cardiovascular disease,29 insulin resistance,30 and end-stage renal failure.31 Moreover, low birth weight is associated with an increased risk for hypertension, independent of genetic and shared environmental factors.32
Series published to date have not captured all pregnancy events or their outcomes. Limitations of some of the published studies include short duration of follow-up and studies with no adequate or long-term matching for decade and renal function.
We examined fertility rates, pregnancy rates, and pregnancy outcomes over 40 yr in an at-risk population, defined as women who were aged between 15 and 49 and had a functioning kidney transplant, using ANZDATA registry data. In addition, maternal and graft outcomes were analyzed, and, uniquely, a matched cohort analysis of 120 nulliparous and 120 parous women who had undergone transplantation enabled analysis of outcomes at 20 yr.
Results
There were 577 pregnancies among 381 patients (six patients with four pregnancies, 40 with three, 150 with two, and 381 with one), resulting in 588 fetuses/infants (including 11 pairs of twins). Outcomes were not known for two pregnancies. Two patients with transplant function that required temporary dialysis during pregnancy were categorized as transplant patients. Five women (resulting in two live births, two terminations, and one stillbirth) who conceived and had a functioning transplant for most of their pregnancy but returned to long-term dialysis before delivery were categorized as transplant patients.
Demographics
Women with a functioning transplant at the time of their first pregnancy had a median duration of end-stage kidney disease (ESKD) of 67 mo (interquartile range [IQR] 39 to 100 mo). The median maternal age for the cohort at the time of pregnancy was 29 yr (IQR 26 to 33). Forty-three percent (n = 246) had a “glomerulonephritis” reported as a cause of their ESKD, 36% (n = 210) reflux, 5% (n = 28) diabetes/hypertension, 4% (n = 21) analgesic nephropathy, 3% (n = 15) cystic disease, and 10% (n = 57) unknown. The median transplantation-to-pregnancy interval was 67 mo (IQR 32 to 97 mo).
Pregnancy and Fertility (Live Birth) Rates
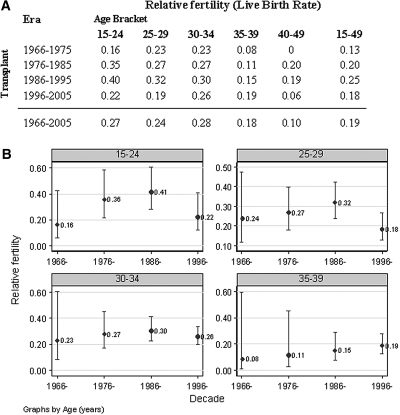
There were 28,329 patient-years of follow-up of women with a functioning kidney graft. The absolute fertility rate trend was reduced among women who were younger than 25 yr and rose for women who were older than 30 yr (Table 1). Relative to the Australian population, fertility rates in the transplant population during the four decades did not statistically differ, although, in the last decade, a trend reduction was noted. The lack of statistical significance may actually reflect the small numbers in some groups (Figure 1).
Table 1.
Pregnancy and fertility rates of women with a functioning kidney transplant in Australia and New Zealand, 1966 through 2005
| Era | Age (yr) |
||||||
|---|---|---|---|---|---|---|---|
| 15 to 19 | 20 to 24 | 25 to 29 | 30 to 34 | 35 to 39 | 40 to 49 | 15 to 49 | |
| Pregnancy rates | |||||||
| 1966 to 1975 | 27 (2) | 48 (8) | 62 (12) | 37 (7) | 13 (4) | 2 (1) | 22 (34) |
| 1976 to 1985 | 26 (6) | 51 (22) | 74 (48) | 36 (27) | 11 (8) | 2 (3) | 24 (114) |
| 1986 to 1995 | 9 (4) | 56 (46) | 76 (86) | 50 (69) | 11 (18) | 0.9 (3) | 25 (226) |
| 1996 to 2005 | 7 (3) | 20 (14) | 32 (45) | 44 (92) | 17 (42) | 0.8 (5) | 15 (201) |
| 1966 to 2005 | 13 (15) | 42 (90) | 56 (191) | 44 (195) | 14 (72) | 2 (12) | 20 (575) |
| Fertility rates | |||||||
| 1966 to 1975 | 13 (1) | 36 (6) | 41 (8) | 26 (5) | 3 (1) | 0 (0) | 13 (21) |
| 1976 to 1985 | 9 (2) | 44 (19) | 56 (36) | 24 (18) | 4 (3) | 1 (2) | 17 (80) |
| 1986 to 1995 | 5 (2) | 42 (35) | 58 (65) | 40 (55) | 6 (11) | 0.9 (3) | 19 (171) |
| 1996 to 2005 | 7 (3) | 17 (13) | 25 (36) | 39 (83) | 14 (35) | 0.3 (2) | 13 (172) |
| 1966 to 2005 | 7 (8) | 33 (73) | 43 (145) | 36 (161) | 10 (50) | 12 (7) | 16 (444) |
Data are rates per 1000 patient-years (no. of events). There were 327 live births among Australian and New Zealand patients who had a functioning transplant at the time of their pregnancy.
Figure 1.
(A) Relative fertility (live birth) rates of women with a functioning kidney transplant in Australia, 1966 through 2005, are shown. (B) Relative fertility (95% CI) rates of transplant patients in Australia, 1966 through 2005 are shown.
Maternal Survival Outcomes
In women with a graft, maternal survival rates after a live birth, regardless of subsequent changes in renal replacement modality, were 96% (95% CI 94 to 98%) at 5 yr, 92% (95% CI 89 to 95%) at 10 yr, 83% (95% CI 78 to 88%) at 15 yr, and 75% (95% CI 68 to 81%) at 20 yr. These percentages did not change across decades. Among the 69 who died after having achieved a live birth with a functioning transplanted kidney, the median time to maternal death (and therefore median age of offspring) was 12.2 yr (IQR 7.8 to 16.9 yr). No peripartum maternal deaths were reported. Five patients with a transplant returned to dialysis during or soon after a pregnancy. The maternal complication of preeclampsia was reported in 27% (n = 21; total = 81) of women. Preeclampsia was not associated with age or parity. One (1%) woman developed gestational diabetes.
Graft Survival and Function after Live Birth
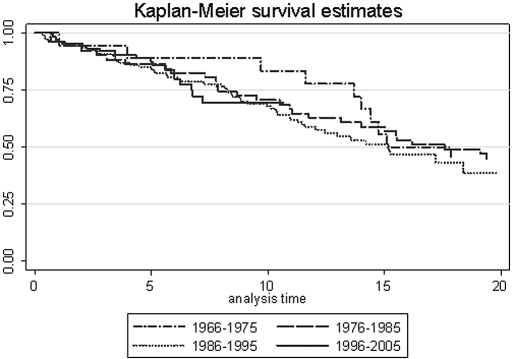
The risk for graft loss did not vary across decades after a live birth (Figure 2). Compared with 1996 through 2005, the hazard ratio (HR) for graft loss at 0 to 10 yr after a live birth was 0.46 (95% confidence interval [CI] 0.14 to 1.59; P = 0.22) for 1966 through 1975, 0.89 (95% CI 0.45 to 1.78; P = 0.74) for 1976 through 1986, and 0.98 (95% CI 0.55 to 1.75; P = 0.95) for 1986 through 1995. Compared with 1986 through 1995, the HR for graft loss at 10 to 15 yr after a live birth was 1.17 (95% CI 0.42 to 3.27; P = 0.76) for 1966 through 1975 and 0.60 (95% CI 0.23 to 1.58; P = 0.30) for 1976 through 1985. In addition, there was no significant change across decades after adjustment for maternal age and time from ESKD to transplantation. When live births during 1976 through 1995 were adjusted for the last predelivery creatinine, they were not significantly affected (data not shown).
Figure 2.
Kaplan-Meier kidney graft survival estimates after a live birth by decade are shown.
A total of 64 paired preconception and 3-mo postdelivery serum creatinine concentrations were available for analysis from 110 live births reported after October 1, 2001. Median preconception creatinine (n = 76) was 1.13 mg/dl (IQR 0.98 to 1.47), and median 3-mo postdelivery creatinine (n = 72) was 1.24 mg/dl (IQR 1.05 to 1.47); there was no significant difference (P = 0.08).
Matched Cohort
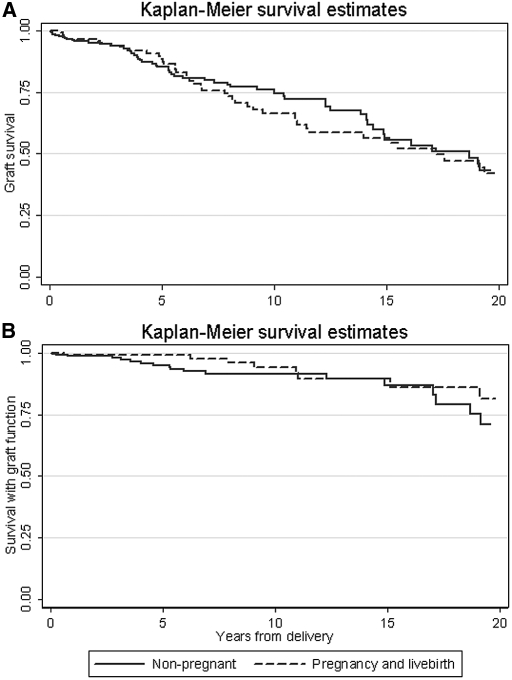
Of 298 women with a first live birth (achieved while their graft functioned), 120 patients could be matched to nonpregnant/nulliparous control subjects who had undergone transplantation. Two patients who returned to long-term dialysis before delivering a live birth were excluded from this analysis. Delivering a live birth was not associated with a higher 20-yr risk for graft loss (HR 1.11; 95% CI 0.70 to 1.76; P = 0.7), even after adjustment for potential confounders (adjusted HR 1.04; 95% CI 0.63 to 1.70; P = 0.5). Five-year unadjusted graft survival rates for patients and control subjects were 89% (95% CI 81 to 93%) and 85.3% (95% CI 79.0 to 89.9%), respectively; 10-yr rates were 66.4% (95% CI 55.3 to 75.4%) and 74.8% (95% CI 66.4 to 81.3%), respectively; and 15-yr rates were 56.5% (95% CI 44.4 to 66.9%) and 55.7 (95% CI 43.6 to 66.2%), respectively. Median follow-up time was 5.6 yr. Unadjusted patient survival rates censored for graft failure for patients and control subjects at 5 yr were 99.2% (94.2 to 99.9%) and 95.2% (95% CI 90.5 to 97.6%), respectively; 10-yr patient survival rates were 94.0% (95% CI 84.3 to 97.8%) and 91.7% (95% CI 85.5 to 95.3%), respectively; and 15-yr survival rates were 89.6% (95% CI 77.6 to 95.4%) and 86.8% (95% CI 76.1 to 92.9%), respectively. Statistical comparisons were not performed because of the small number of deaths. Kaplan-Meier survival estimates from the matching study for patients and nonpregnant/nulliparous control women are depicted for grafts (Figure 3A) and patients with a functioning graft (Figure 3B).
Figure 3.
Kaplan-Meier graft survival estimates in nulliparous and parous women with a kidney graft are shown. (A) Graft survival. (B) Survival with graft function.
Pregnancy Outcomes
Medical surgical terminations became lawful in most states of Australia in 1969. In women with a functioning graft, the proportion of terminations decreased six-fold in the last decade (Table 2; P < 0.001). Once terminations were excluded, the proportion of other outcomes was similar across decades (P = 0.09).
Table 2.
Pregnancy outcomes in women with a functioning kidney transplant in Australia and New Zealand, 1966 through 2005
| Era | Terminations | Transplant Pregnancy Outcomes |
|||
|---|---|---|---|---|---|
| Spontaneous Abortions | Stillbirths | Live Births | Total | ||
| 1966 to 1975 | 10 (30%) | 3 (9%) | 0 (0%) | 21 (62%) | 34 |
| 1976 to 1985 | 21 (18%) | 10 (9%) | 5 (4%) | 80 (69%) | 116 |
| 1986 to 1995 | 35 (15%) | 15 (7%) | 8 (3%) | 171 (75%) | 229 |
| 1996 to 2005 | 10 (5%) | 24 (12%) | 1 (0.5%) | 172 (83%) | 207 |
| All eras | 76 (13%) | 52 (9%) | 14 (2%) | 444 (76%) | 586 |
Spontaneous abortion was defined as delivery of a nonviable fetus ≤20 wk of gestation. Stillbirth was defined as delivery of a nonviable fetus >20 wk of gestation.
Table 3 shows the adjusted and unadjusted odds ratios (ORs) for factors associated with a live birth for women with a functioning graft at the time of their pregnancy. Because the reporting of predelivery serum creatinine was relatively imprecise and not available for 13% of patients, we examined multivariable models with and without creatinine. Factors associated with a live birth were reflux nephropathy, “other” diseases causing ESKD, and lower serum creatinines. Maternal age showed an inverted U-shaped relationship, with the 20- to 24-yr-old group achieving a greater number of live births. Importantly, this relationship was statistically significant only in the 35- to 49-yr-old group.
Table 3.
Transplant patient factors associated with a live birth
| Factor | Unadjusted OR (95% CI)(n = 510) | Adjusted OR (95% CI) |
|
|---|---|---|---|
| Includes Creatinine(n = 442) | Excludes Creatinine(n = 509) | ||
| Era | |||
| 1966 to 1975 | 1.0 (0.3 to 3.7) | 0.6 (0.1 to 3.2) | 0.9 (0.2 to 3.5) |
| 1976 to 1985 | 0.8 (0.4 to 1.6) | 0.5 (0.2 to 1.2) | 0.6 (0.3 to 1.4) |
| 1986 to 1995 | 1.1 (0.6 to 2.0) | 0.8 (0.4 to 1.6) | 0.9 (0.5 to 1.8) |
| 1996 to 2005 | 1.0 | 1.0 | 1.0 |
| Maternal age (yr) | |||
| 15 to 19 | 0.5 (0.1 to 5.3) | a | 0.6 (0.1 to 5.6) |
| 20 to 24 | 1.0 | 1.0 | 1.0 |
| 25 to 29 | 0.4 (0.2 to 1.2) | 0.4 (0.1 to 1.2) | 0.4 (0.1 to 1.1) |
| 30 to 34 | 0.5 (0.2 to 1.4) | 0.4 (0.1 to 1.2) | 0.5 (0.2 to 1.3) |
| 35 to 49 | 0.2 (0.1 to 0.7)b | 0.2 (0.1 to 0.9)b | 0.2 (0.1 to 0.8)b |
| Cause of ESKD (%) | |||
| glomerulonephritis | 1.0 | 1.0 | 1.0 |
| cystic | 0.5 (0.1 to 1.9) | 1.3 (0.2 to 1.6) | 0.9 (0.2 to 4.3) |
| diabetes/hypertension | 0.9 (0.3 to 2.9) | 1.0 (0.3 to 3.7) | 0.9 (0.3 to 2.8) |
| reflux | 2.2 (1.2 to 4.1)b | 1.7 (0.9 to 3.4) | 2.1 (1.1 to 4.1)b |
| analgesic | 1.2 (0.3 to 4.2) | 3.9 (0.5 to 34.0) | 1.5 (0.4 to 5.7) |
| other/unknown | 4.8 (1.1 to 20.7)b | 3.4 (0.8 to 15.3) | 5.2 (1.2 to 22.9) |
| Duration of ESKD (each additional year) | 1.0 (1.0 to 1.1) | 1.0 (0.9 to 1.2) | 1.0 (1.0 to 1.1) |
| Duration of transplant (each additional year) | 1.0 (1.0 to 1.1) | 1.0 (0.9 to 1.2) | 1.0 (0.9 to 1.1) |
| Serum creatinine (each additional 10 μmol/L) | 0.9 (0.8 to 1.0) | 0.9 (0.8 to 1.0)b | – |
Terminations were excluded from the analysis. Creatinine was defined as the last available predelivery serum creatinine.
aThis age group was not included in the model because all pregnancies resulted in live births.
bP < 0.05.
Discussion
This is the first longitudinal study to confirm an equivalent 20-yr graft and maternal survival outcome in women who achieved a live birth while transplanted with a functioning graft as compared with nulliparous women with a graft. This historical population described, unlike more recent cohorts, of note those in the United States, was composed of only 4% of patients with diabetes or hypertension. The cause of ESKD in this cohort was dominated by glomerular (43%) and reflux disease (36%). It is imperative to consider that patients who have diabetes and receive a transplant have poorer survival than transplant recipients who do not have diabetes,33 and this historical outcome analysis of pregnancies may not be generalizable to patients with type 1 or type 2 diabetes or cohorts that are composed of patients with a different spectrum of diseases.
Women who had a graft and achieved a live birth during 1966 through 1975 seem to have had enjoyed better graft survival 10 yr after a live birth. This difference diminished thereafter and may reflect the small number of pregnancies during that decade (n = 22), rather than a true difference in outcomes. In addition, it was common practice for clinicians to advise against pregnancy during this period, and it is probable that women with poorer graft function at the time were more likely to proceed to a surgical termination, thereby biasing these results. Excluding this “first decade ”effect for the 1966 through 1975 cohort, despite significant immunosuppressive regimen advances during four decades, graft survival among cohorts achieving a live birth remained unchanged at 15 and 20 yr.
In this cohort, the median age at time of pregnancy significantly increased across decades to a peak of 32 in the last decade (P < 0.001). Women with a functioning kidney transplant beyond 35 yr were significantly less likely to achieve a live birth compared with women who were aged 20 to 24 yr, and this is consistent with the known age effects in women without ESKD.
Despite the reported acceptable and favorable outcomes for the graft, mother, and neonate, fertility rates declined in the last decade, even though an increase in the number of live births in transplant populations has been reported.16 This reduction can be attributed solely to a nearly 50% reduction in the pregnancy and fertility (live birth) rates in 20- to 29-yr-olds. Pregnancy and fertility rates have decreased in most Westernized countries and the rate change observed in the transplant population is in keeping with broader trends and may reflect a genuine desire to avoid pregnancy in the setting of improved contraception options.
Pregnancy and fertility rates for this cohort over 40 yr were 20 and 16 per 1000 patient-years, respectively, in women with a functioning kidney transplant. It is imperative not to overlook the “relative infertility” of women with a functioning transplant. The relative fertility rate after direct comparison with the Australian background population was 0.18 for women with a transplant. In effect, only 20% of the expected number of births was reported. Many factors drive this low rate, beyond the fear of graft loss, including infertility,34 inadequate renal function rendering women anovulatory,35 advanced age at the time of transplantation, and finally an unpartnered status.
An increased in the number of live births as the decades advanced was noted, whereas the number of stillbirths and spontaneous abortions remained unchanged, suggesting this live birth increment may relate to a decreased number of surgical terminations opted for across decades (P < 0.001). A reduction in the number of terminations might be caused by many factors, including changing physician attitudes to pregnancies in the transplant population, better contraception, and societal attitude change to single parenting. Importantly, one confounder may have been a reporting bias if terminations performed away from primary centers remained undisclosed.
In the last decade, 83% of women who had a functioning transplant and became pregnant achieved a live birth, and this is similar to the percentage reported by other investigators.1 Women with reflux as their primary disease causing their ESKD were more likely to achieve a live birth (OR 2.2).
There was a small but statistically insignificant postdelivery serum creatinine rise 3 mo after a live birth. Given the small sample size (n = 64) and the large proportion of missing data on creatinine, this result should be interpreted with caution. Other series have also reported favorable outcomes in patients with preconception creatinines of <1.5 mg/dl. Predictors of preterm delivery published to date include maternal hypertension (≥140/90; OR 6.336),1 proteinuria (≥0.3 g/d; OR 11.736),5 and serum creatinine (≥1.5 mg/dl),1,36 before pregnancy. Traditionally, these predictors of risk have been used to advise women about the likely outcome of their pregnancy.
A safe interval between transplantation and pregnancy seems to be 1 to 4 yr, with women achieving a live birth in 72.7% of cases. Standard clinical practice has been to advise women against pregnancy in their first posttransplantation year.37 Seventy-three percent of women from this longitudinal series who became pregnant beyond their first transplant year achieved a live birth. The mean transplantation-to-pregnancy interval for women who achieved a live birth was 94.45 ± 54.89 mo. In contrast, the mean interval for women who had a stillbirth or spontaneous abortion was 86.38 ± 48.26 and 82.36 ± 48.41 mo, respectively, and these interval differences were NS; however, women who opted for surgical termination had a reduced interval (70.97 ± 48.73 mo), and this was significantly different from the interval for women who achieved a live birth (P < 0.01). Ninety-seven percent of this cohort became pregnant beyond their first transplantation year, suggesting adherence to the current recommendations.
This unique data set has allowed us to establish that maternal survival censored for graft failure and graft survival after a live birth, at 20 yr, are identical to rates in nulliparous women with a functioning graft. A superior graft survival advantage at 10 yr was noted for the 1966 through 1975 cohort. No other decade, at any time point, was associated with a more favorable outcome for either variable, despite significant alterations in immunosuppressive regimens and medical practices. This analysis also highlights the reduced and decreasing pregnancy and fertility rates in women who receive a transplant.
Before conception, most women who receive a transplant anticipate that their renal disease may shorten their lifespan. A 75% survival rate at 20 yr may assist some women in opting for a pregnancy, knowing they will probably raise their child to an age of independence.
Concise Methods
The Australian and New Zealand Dialysis and Transplant (ANZDATA) Registry has been collecting data on all consenting patients who receive long-term renal replacement therapy (RRT)—dialysis or kidney transplantation—in Australia and New Zealand since 1963. One hundred percent of all units in Australia and New Zealand participate. Since 1963, approximately 50,000 patients have required RRT. Fewer than 10 patients have deferred data accruement. Audits of some data items collected (but not the pregnancy data) have been performed but not published. To date, data integrity audits have deemed collections adequate. Specific information about pregnancy outcomes has been collected since 1963. All women who had a functioning kidney transplant and delivered an infant or had a spontaneous abortion, stillbirth, or termination of pregnancy from January 1, 1966, through December 31, 2005, were included in this analysis. Before October 2001, only delivery dates were collected. Since 2001, the estimated date of conception, complications of preeclampsia and diabetes, and preconception and 3-mo postdelivery serum creatinine were also collected. Information about kidney transplant function routinely collected and available for analysis includes serum creatinine concentrations at 1, 2, 3, 5, 7, 10, 15, and 20 yr after transplantation.
Pregnancy and Fertility (Live Birth) Rates
Age- and decade-stratified pregnancy and fertility rates were calculated for transplant patients. Pregnancy rate was defined as the number of pregnancies (regardless of outcome) per 1000 person-years. Fertility rate was defined as the number of live births per 1000 person-years. Fertile age was defined as age 15 to 49 yr, in accordance with the general population definitions used in Australia and New Zealand. Live twin births were counted as two live births but one pregnancy. Transplant patients were defined as patients who conceived and delivered while having a functioning kidney transplant, including patients who required temporary dialysis during pregnancy or returned to long-term dialysis shortly (<1 wk) before delivery. Age at delivery and year of transplantation were analyzed as categories. Relative fertility rates of patients (defined as fertility rate in RRT patients/fertility rates in the general population in Australia) were calculated using the population age- and decade-stratified fertility rates from the Australian Bureau of Statistics for comparative purposes.
Maternal and Graft Outcomes
We examined trends in maternal characteristics (age, race, RRT modality, and cause of ESKD) during the 40-yr period. Race was self-reported and categorized as indigenous (Australian Aborigines, Torres Strait Islanders, Maori, or Pacific Islanders) and nonindigenous.
Graft survival after the first live birth (for that graft) was calculated using Kaplan-Meier methods. Patients were followed until graft loss or December 31, 2006. Comparison across decades was by multivariable Cox regression, adjusted for maternal age, time from ESKD to transplantation, and last predelivery creatinine.
Graft and maternal survival rates (censored at graft failure) were directly compared with the rates observed in nulliparous patients (with a functioning kidney transplant) using a matched survival analysis. Each patient was matched to female control subjects by year of transplantation, duration of transplant, age at transplantation (±5 yr), and predelivery creatinine (±25 μmol/L) (or the corresponding time point from transplantation in control subjects). Survival analysis was by Cox regression stratified by patient/control groups and adjusted for age at transplantation, predelivery creatinine, donor source (live or deceased), graft number (primary or repeat), and time from ESKD to transplantation. Indigenous patients were excluded in this analysis because of insufficient numbers.
Maternal survival rates after a live birth in women with a graft, regardless of subsequent changes in RRT modality, were calculated using Kaplan-Meier analysis. The median maternal time to death was used to estimate the age of offspring at maternal death. Survival was not censored at subsequent live birth, because patients with more than one live birth per graft were likely to have better graft function and survival, leading to informative censoring.
Pregnancy Outcomes
Pregnancy outcomes were categorized as termination of pregnancy, spontaneous abortion (≤20 wk of gestation), stillbirth (>20 wk of gestation), and live birth. Transplant patient outcomes were compared with maternal age, decade, cause of ESKD, duration of ESKD, duration of transplant, and last serum creatinine using uni- and multivariable logistic regression analysis. Medically induced terminations were excluded for these comparisons (because decisions for termination were temporally influenced by medical practices), and spontaneous abortions and stillbirths were combined.
Statistic Analysis
Comparisons of continuous variables were by paired or unpaired t test, Wilcoxon rank sum and signed ranks test, ANOVA, and Kruskal-Wallis test as appropriate. Comparisons of categorical variables were by Pearson's χ2 test, Fisher exact test, and logistic regression as appropriate. Statistical analyses were performed using Stata 10 (StataCorp, College Station, TX). Medians were presented with IQRs. Point estimates were presented with 95% CIs and medians with IQRs. P < 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
This work was supported by research grants to V.L. from the National Health and Medical Research Council of Australia.
V.L. was primarily involved in manuscript preparation and literature review; S.C. and S.M. were involved in data analysis and manuscript preparation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ: Pregnancy after organ transplantation: A report from the UK Transplant Pregnancy Registry. Transplantation 83: 1301–1307, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Rahamimov R, Ben-Haroush A, Wittenberg C, Mor E, Lustig S, Gafter U, Hod M, Bar J: Pregnancy in renal transplant recipients: Long-term effect on patient and graft survival—A single-center experience. Transplantation 81: 660–664, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Naqvi R, Noor H, Ambareen S, Khan H, Haider A, Jafri N, Alam A, Aziz R, Manzoor K, Aziz T, Ahmed E, Akhtar F, Naqvi A, Rizvi A: Outcome of pregnancy in renal allograft recipients: SIUT experience. Transplant Proc 38: 2001–2002, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kallen B, Westgren M, Aberg A, Olausson PO: Pregnancy outcome after maternal organ transplantation in Sweden. BJOG 112: 904–909, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ghanem ME, El-Baghdadi LA, Badawy AM, Bakr MA, Sobhe MA, Ghoneim MA: Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol 121: 178–181, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chou CY, Ting IW, Lin TH, Lee CN: Pregnancy in patients on chronic dialysis: A single center experience and combined analysis of reported results. Eur J Obstet Gynecol Reprod Biol 136: 165–170, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Armenti VT, Radomski JS, Moritz MJ, Philips LZ, McGrory CH, Coscia LA: Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 123–134, 2000 [PubMed] [Google Scholar]
- 8.Armenti VT, Radomski JS, Moritz MJ, Gaughan WJ, Philips LZ, McGrory CH, Coscia LA: Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 121–130, 2002 [PubMed] [Google Scholar]
- 9.Armenti VT, Radomski JS, Moritz MJ, Gaughan WJ, Philips LZ, McGrory CH, Coscia LA: Report from the national transplantation pregnancy registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 97–105, 2001 [PubMed] [Google Scholar]
- 10.Armenti VT, Radomski JS, Moritz MJ, Gaughan WJ, McGrory CH, Coscia LA: Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 131–141, 2003 [PubMed] [Google Scholar]
- 11.Armenti VT, Radomski JS, Moritz MJ, Gaughan WJ, Hecker WP, Lavelanet A, McGrory CH, Coscia LA: Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 103–114, 2004 [PubMed] [Google Scholar]
- 12.Armenti VT, Radomski JS, Moritz MJ, Gaughan WJ, Gulati R, McGrory CH, Coscia LA: Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 69–83, 2005 [PubMed] [Google Scholar]
- 13.Armenti VT, Moritz MJ, Davison JM: Pregnancy in female pediatric solid organ transplant recipients. Pediatr Clin North Am 50: 1543–1560, xi, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Pour-Reza-Gholi F, Nafar M, Farrokhi F, Entezari A, Taha N, Firouzan A, Einollahi B: Pregnancy in kidney transplant recipients. Transplant Proc 37: 3090–3092, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Toma H, Tanabe K, Tokumoto T, Kobayashi C, Yagisawa T: Pregnancy in women receiving renal dialysis or transplantation in Japan: A nationwide survey. Nephrol Dial Transplant 14: 1511–1516, 1999 [DOI] [PubMed] [Google Scholar]
- 16.McKay DB, Josephson MA: Pregnancy in recipients of solid organs: Effects on mother and child. N Engl J Med 354: 1281–1293, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kashanizadeh N, Nemati E, Sharifi-Bonab M, Moghani-Lankarani M, Ghazizadeh S, Einollahi B, Lessan-Pezeshki M, Khedmat H: Impact of pregnancy on the outcome of kidney transplantation. Transplant Proc 39: 1136–1138, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez MJ, Acebedo-Ribo M, Garcia-Donaire JA, Manzanera MJ, Molina A, Gonzalez E, Nungaray N, Andres A, Morales JM: Pregnancy in renal transplant recipients. Transplant Proc 37: 3721–3722, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Salmela KT, Kyllonen LE, Holmberg C, Gronhagen-Riska C: Impaired renal function after pregnancy in renal transplant recipients. Transplantation 56: 1372–1375, 1993 [DOI] [PubMed] [Google Scholar]
- 20.First MR, Combs CA, Weiskittel P, Miodovnik M: Lack of effect of pregnancy on renal allograft survival or function. Transplantation 59: 472–476, 1995 [PubMed] [Google Scholar]
- 21.Sturgiss SN, Davison JM: Effect of pregnancy on the long-term function of renal allografts: An update. Am J Kidney Dis 26: 54–56, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Rizzoni G, Ehrich JH, Broyer M, Brunner FP, Brynger H, Fassbinder W, Geerlings W, Selwood NH, Tufveson G, Wing AJ: Successful pregnancies in women on renal replacement therapy: Report from the EDTA Registry. Nephrol Dial Transplant 7: 279–287, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Kozlowska-Boszko B, Durlik M, Kuczynska-Sicinska J, Lao M: Predictor of transplanted kidney deterioration following pregnancy: Daily urine protein loss or serum creatinine concentration? Ann Transplant 1: 30–31, 1996 [PubMed] [Google Scholar]
- 24.Thompson BC, Kingdon EJ, Tuck SM, Fernando ON, Sweny P: Pregnancy in renal transplant recipients: The Royal Free Hospital experience. QJM 96: 837–844, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Miniero R, Tardivo I, Curtoni ES, Bresadola F, Calconi G, Cavallari A, Centofanti P, Filipponi F, Franchello A, Goggi C, La Rocca E, Mammana C, Nino A, Parisi F, Regalia E, Rosati A, Segoloni GP, Setti G, Todeschini P, Tregnaghi C, Zanelli P, Dall'Omo AM: Outcome of pregnancy after organ transplantation: A retrospective survey in Italy. Transpl Int 17: 724–729, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ: Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA 288: 728–737, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, Jaakkola MS: Preterm delivery and asthma: A systematic review and meta-analysis. J Allergy Clin Immunol 118: 823–830, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hardy R, Kuh D, Langenberg C, Wadsworth ME: Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. Lancet 362: 1178–1183, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Catov JM, Newman AB, Roberts JM, Sutton-Tyrrell KC, Kelsey SF, Harris T, Jackson R, Colbert LH, Satterfield S, Ayonayon HN, Ness RB: Association between infant birth weight and maternal cardiovascular risk factors in the health, aging, and body composition study. Ann Epidemiol 17: 36–43, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Hovi P, Andersson S, Eriksson JG, Jarvenpaa AL, Strang-Karlsson S, Makitie O, Kajantie E: Glucose regulation in young adults with very low birth weight. N Engl J Med 356: 2053–2063, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM: Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 19: 151–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergvall N, Iliadou A, Johansson S, de Faire U, Kramer MS, Pawitan Y, Pedersen NL, Lichtenstein P, Cnattingius S: Genetic and shared environmental factors do not confound the association between birth weight and hypertension: A study among Swedish twins. Circulation 115: 2931–2938, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y: Patient survival and cardiovascular risk after kidney transplantation: The challenge of diabetes. Am J Transplant 8: 593–599, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Palmer BF: Sexual dysfunction in uremia. J Am Soc Nephrol 10: 1381–1388, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Lim VS, Henriquez C, Sievertsen G, Frohman LA: Ovarian function in chronic renal failure: Evidence suggesting hypothalamic anovulation. Ann Intern Med 93: 21–27, 1980 [DOI] [PubMed] [Google Scholar]
- 36.Kurata A, Matsuda Y, Tanabe K, Toma H, Ohta H: Risk factors of preterm delivery at less than 35 weeks in patients with renal transplant. Eur J Obstet Gynecol Reprod Biol 128: 64–68, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Armenti VT, Daller JA, Constantinescu S, Silva P, Radomski JS, Moritz MJ, Gaughan WJ, McGrory CH, Coscia LA: Report from the National Transplantation Pregnancy Registry: Outcomes of pregnancy after transplantation. Clin Transpl 57–70, 2006 [PubMed] [Google Scholar]