Abstract
Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists not only improve metabolic abnormalities of diabetes and consequent diabetic nephropathy, but they also protect against nondiabetic chronic kidney disease in experimental models. Here, we found that the PPAR-γ agonist pioglitazone protected against renal injury in aging; it reduced proteinuria, improved GFR, decreased sclerosis, and alleviated cell senescence. Increased local expression of PPAR-γ paralleled these changes. Underlying mechanisms included increased expression of klotho, decreased systemic and renal oxidative stress, and decreased mitochondrial injury. Pioglitazone also regulated p66Shc phosphorylation, which integrates many signaling pathways that affect mitochondrial function and longevity, by reducing protein kinase C-β. These results suggest that PPAR-γ agonists may benefit aging-related renal injury by improving mitochondrial function.
Aging in most species is associated with impaired adaptive and homeostatic mechanisms, leading to susceptibility to environmental or internal stresses with increasing rates of disease and death. Kidney mass is substantially reduced in old age, by approximately 20 to 25% between the age of 30 and 80 yr1 and a 0.5-cm decrease in length per decade after middle age.2 The aging human kidney is characterized by increased glomerulosclerosis, interstitial fibrosis, tubular atrophy, and arteriosclerosis.3,4 A number of different theories of primarily disease-independent aging, which can be categorized as evolutionary, molecular, cellular, and systemic, have been put forward over the past 50 yr. From the cellular perspective, several mechanisms are considered to underlie the primary aging process and contribute to age-related changes and adaptive responses. These mechanisms include oxidative stress, mitochondrial dysfunction, telomere shortening, and various genetic modulations.5
Pioglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) agonist and member of the thiazolidinedione (TZDs) class of antidiabetic drugs, improves insulin-mediated glucose uptake into skeletal muscle without increasing endogenous insulin secretion and improves dyslipidemia. PPARγ agonist was also effective in the treatment of non–insulin-dependent diabetes mellitus.6 However, PPARγ also has nonmetabolic effects, such as regulating cell cycle, inhibiting inflammation, and modulating cytokine production. Our previous studies have shown that PPARγ agonist ameliorates nondiabetic glomerulosclerosis.7,8 Recently, a protective effect of pioglitazone against oxidative stress in liver and kidney of diabetic rabbits was reported.9 Long-term exposure to pioglitazone improved mitochondrial function. Of note, mitochondria produce more than 90% of reactive oxygen species (ROS) after related injury.10 More importantly, one study showed that the Pro/Ala PPARγ gene polymorphism, which is related to PPARγ activity and insulin sensitivity, is also associated with human longevity.11 However, there is little information about PPAR modulation during aging. Because expression of PPARγ is reduced during aging and TZDs have many beneficial effects on aging-related molecules,12 we hypothesized that pioglitazone could protect against aging-related renal injury. Our results indeed show that activation of PPARγ is protective against aging-related renal injury. Mechanisms of this effect are associated with increased klotho and reduced mitochondrial oxidation, which is signaled by increased p66Shc phosphorylation through the protein kinase C-β pathway.
Results
Pioglitazone Improves Glucose and Lipid Metabolism in Aging Rats
Pioglitazone decreased the mildly elevated serum glucose and improved triglycerides and cholesterol, especially LDL level (Table 1) in aging rats. There was no effect on insulin.
Table 1.
Effects of pioglitazone on renal function and glucose and lipid metabolism in 24-mo-old rats
| Urine Proteina(mg/24 h) | Ccr(ml/min) | Glucose(mg/dl) | Insulin(ng/ml) | Cholesterol(mg/dl) | HDL-Cholesterol(mg/dl) | LDL-Cholesterol(mg/dl) | Triglycerides(mg/dl) | |
|---|---|---|---|---|---|---|---|---|
| Control(n = 7) | 648.50 ± 79.52 | 0.38 ± 0.09 | 150.67 ± 10.44 | 0.52 ± 0.06 | 205.56 ± 23.45 | 40.22 ± 5.16 | 116.04 ± 10.71 | 246.44 ± 26.22 |
| Pioglitazone(n = 10) | 227.56 ± 71.63b | 0.83 ± 0.16b | 111.38 ± 14.81b | 0.80 ± 0.11 | 118.50 ± 5.46b | 35.38 ± 6.08 | 64.83 ± 3.16b | 91.50 ± 4.81b |
aBaseline proteinuria at 18 months before treatment was 51.59 ± 12.80 mg/24 h.
bP < 0.05 versus control.
Pioglitazone Protects against Aging-Related Renal Injury and Increases Klotho
With aging, urinary protein excretion was dramatically increased at 24 mo in untreated aging control rats (Control; 648.50 ± 79.52 versus baseline 51.59 ± 12.80 mg/24 h; P < 0.05), and creatinine clearance (Ccr) at 24 mo was decreased to 0.38 ± 0.09 ml/min (corresponding serum creatinine, approximately 4 mg/dl) compared with normal serum creatinine in our previous study in 18-mo-old rats (0.72 ± 0.06 mg/dl).13 Pioglitazone (Pio) significantly decreased proteinuria (227.56 ± 71.63 mg/24 h, P < 0.05 versus Control) and improved Ccr (0.83 ± 0.16 ml/min, P < 0.05 versus Control; Table 1).
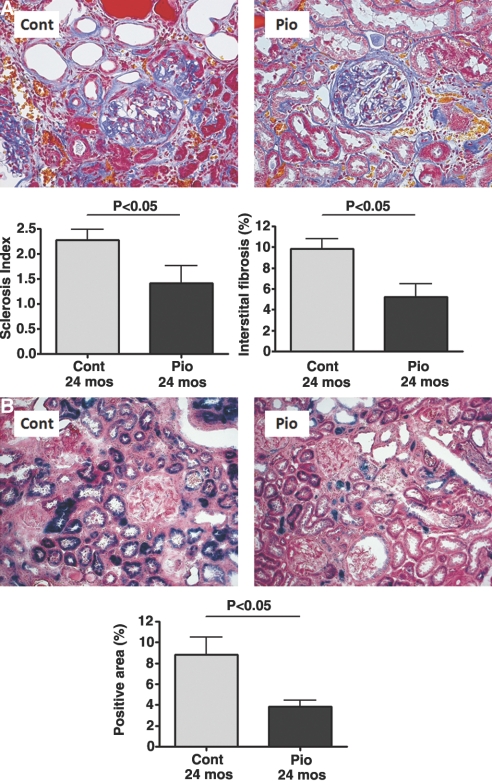
At 24 mo, glomerulosclerosis, tubular atrophy, and interstitial fibrosis were present in all rats. Rats receiving the PPARγ agonist showed significant amelioration of the development of glomerulosclerosis compared with control (sclerosis index, 0 to 4 scale; Pio 1.41 ± 0.36 versus Control 2.28 ± 0.21; P < 0.05; Figure 1A). Effects on tubulointerstitial fibrosis, assessed by point counting, paralleled glomerulosclerosis (Pio 5.21 ± 1.26 versus Control 9.83 ± 0.96%, P < 0.05; Figure 1A).
Figure 1.
Pioglitazone protects against aging-related renal injury. Compared with the control group, pioglitazone ameliorated aging-related glomerulosclerosis (scale 0 to 4) and interstitial fibrosis (point counting) (A; paraffin sections, Masson trichrome staining; magnification, ×200) and significantly decreased positive senescence-associated β-galactosidase staining, a senescence marker (B; blue, frozen sections, β-galactosidase staining; magnification, ×100).
In our previous study, plasminogen activator inhibitor-1 (PAI-1) and TGFβ were decreased by the PPARγ agonist in the puromycin-induced focal segmental glomerulosclerosis model.8 However, in this aging model, there was no significant difference in PAI-1 expression between the two groups (Pio 0.92 ± 0.24 versus Control 1.61 ± 0.22, P = not significant), whereas P-Smad2, the major downstream signaling pathway of TGFβ, was significantly decreased (Pio 0.04 ± 0.004 versus Control 0.42 ± 0.15, P < 0.05; Figure 2).
Figure 2.
Pioglitazone inhibits TGFβ activation. Smad2 phosphorylation, a major downstream signaling pathway of TGFβ, was significantly reduced by pioglitazone.
Infiltrating macrophages were reduced to about 50% by pioglitazone (Pio 17.34 ± 2.93 versus Control 34.27 ± 5.62 macrophages/HPF, P < 0.05). With aging, there were more senescent cells in the kidney, as visualized by senescence-associated β-galactosidase staining. Pioglitazone improved this aging-related phenotype (Pio 3.84 ± 0.63 versus Control 8.85 ± 1.68% senescence-associated β-galactosidase positivity, P < 0.05; Figure 1B).
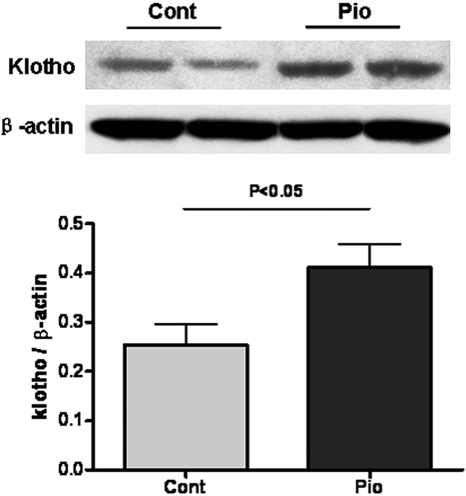
Klotho, a novel antiaging molecule with manifold effects, including on ROS, was increased in the aging kidney by pioglitazone (Pio 0.41 ± 0.05 versus Control 0.25 ± 0.04, P < 0.05; Figure 3).
Figure 3.
Pioglitazone improves klotho in aging kidney. Klotho, an anti-aging factor, protein levels in kidney homogenates were decreased in control aging compared with aging rats treated with pioglitazone.
Pioglitazone Induces Renal PPARγ Expression
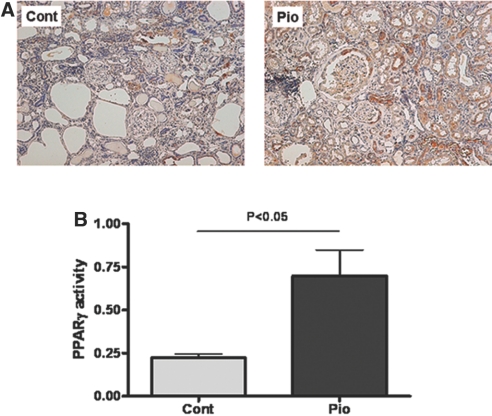
Normally, PPARγ is mainly expressed in the kidney in distal tubules, collecting ducts, and vascular smooth muscle cells. Pioglitazone stimulated PPARγ expression in proximal tubules, glomerular mesangial cells, podocytes, and parietal epithelial cells (Figure 4A). Renal PPARγ activity was significantly increased in the pioglitazone group compared with control (Pio 0.70 ± 0.15 versus Control 0.22 ± 0.02, P < 0.05; Figure 4B).
Figure 4.
PPARγ expression and activity in aging kidney. Pioglitazone increased PPARγ expression in proximal tubules, glomerular mesangial cells, podocytes, and parietal epithelial cells (A; anti-PPARγ immunostaining; magnification, ×100), as well as PPARγ activity as assessed by ELISA of kidney homogenates (B).
Pioglitazone Reduces Both Systemic and Renal Oxidative Stress
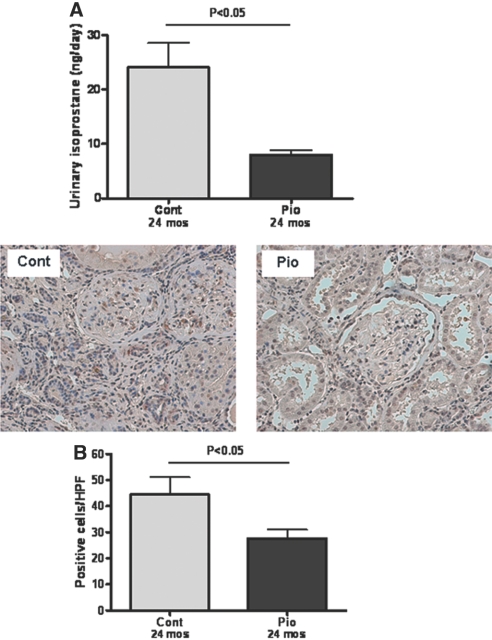
The activation of PPARγ decreased urinary isoprostane excretion, a marker for systemic lipid oxidation (Pio 7.89 ± 0.99 versus Control 24.03 ± 4.53 ng/d, P < 0.05; Figure 5A). Kidney immunostaining for 4-hydroxy-2-nonenal, another oxidative lipid marker, was also decreased in pioglitazone versus control (Pio 27.69 ± 3.26 versus Control 44.50 ± 6.56 positive cells/HPF, P < 0.05; Figure 5B). Renal superoxide dismutase protein (Cu/Zn superoxide diamutase) was increased by pioglitazone (Pio 1.64 ± 0.20 versus Control 0.91 ± 0.09, P < 0.05). The NADH oxidases, NOX2 and NOX4, were reduced 40 (Pio 0.62 ± 0.02 versus Control 1.04 ± 0.14, P < 0.05) and 21% (Pio 1.64 ± 0.20 versus Control 0.91 ± 0.09, P = not significant), respectively, by pioglitazone.
Figure 5.
Pioglitazone and oxidative stress. Activation of PPARγ significantly decreased urinary isoprostane excretion, a marker of oxidative stress (A). 4-Hydroxy-2-nonenal, another oxidative lipid marker expressed in cytoplasm (immunostaining; magnification, ×200), was decreased in the pioglitazone-treated group (B).
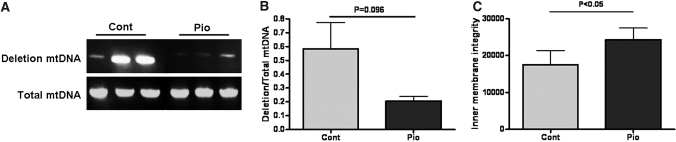
Pioglitazone Attenuates Mitochondrial Injury
More than 90% of ROS is produced by mitochondria. Previous studies have shown increased mitochondrial DNA deletion in aging kidneys.14,15 We found a trend to decrease mitochondrial DNA deletion in pioglitazone-treated rats (Pio 0.21 ± 0.03 versus Control 0.58 ± 0.19, P = 0.096; Figure 6A). However, pioglitazone significantly increased mitochondrial inner membrane integrity (Pio 24,299 ± 3131 versus Control 17,542 ± 3837 fluorescence units/mg protein, P < 0.05; Figure 6B).
Figure 6.
Mitochondrial injury in aging kidney. mtDNA deletion, a marker of mitochondrial injury, was present in control aging kidney, and pioglitazone showed a trend to reduce this marker (A). Pioglitazone significantly maintained mitochondrial inner membrane integrity (B).
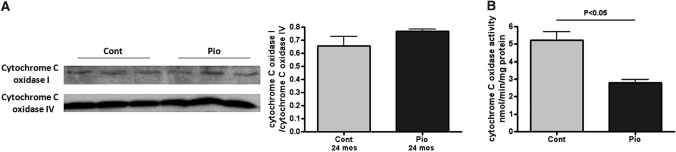
Cytochrome C oxidase (also known as complex IV) is the terminal complex of the mitochondrial respiratory chain and is comprised of 13 different subunits encoded by 3 mitochondrial genes (subunits I, II, and III) and 10 nuclear genes (subunits IV–VIII). Cytochrome C oxidase IV remains relatively stable and therefore can be used as an internal control. Although there was no significant change in cytochrome C oxidase I/IV, pioglitazone did decrease total cytochrome C oxidase activity (Pio 2.81 ± 0.18 versus Control 5.23 ± 0.46 nmol/min/mg protein, P < 0.05; Figure 7).
Figure 7.
Pioglitazone and cytochrome C oxidase. Pioglitazone did not change cytochrome C oxidase I expression, a marker of mitochondrial oxidative stress in aging kidney. However, pioglitazone significantly reduced cytochrome C oxidase activity in mitochondria.
Pioglitazone Decreases p66Shc Phosphorylation through the Protein Kinase C-β2 Pathway
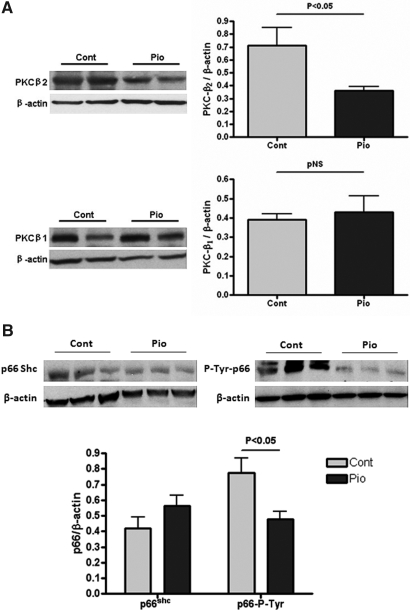
As an anti-diabetic drug, pioglitazone is known to decrease protein kinase C-β expression.16 In our model, pioglitazone decreased protein kinase C-β2 in kidney (Pio 0.36 ± 0.03 versus Control 0.71 ± 0.14, P < 0.05; Figure 8A) but did not change protein kinase C-β1 expression.
Figure 8.
Pioglitazone modulates mitochondrial function through the protein kinase C–p66ShC pathway in aging kidney. Pioglitazone reduced protein kinase C-β2 expression in the kidney but did not change protein kinase C-β1 expression (A). Pioglitazone reduced p66ShC phosphorylation on its tyrosine site (B) (PKC, protein kinase C).
Phosphorylated p66Shc serves as an integration point for many signaling pathways that affect mitochondrial function and longevity. Once activated, p66Shc oxidizes cytochrome C and induces opening of the mitochondrial permeability transition pore, generates more reactive oxygen species, and releases more pro-apoptotic factors into the cytosol. More importantly, p66Shc can be regulated by the protein kinase C-β pathway.17 In our study, pioglitazone did not significantly change the total expression level of p66Shc but reduced the phosphorylation on its tyrosine site (Pio 0.48 ± 0.05 versus Control 0.77 ± 0.10 nmol/min/mg protein, P < 0.05; Figure 8B).
Discussion
Renal aging is associated with renal disease and nonrenal clinical complications in humans.18 Using a suitable animal model, we report that pioglitazone protects renal injury in aging by reducing proteinuria, improving GFR, decreasing sclerosis, and alleviating cell senescence. We found decreased mitochondrial injury through reduced protein kinase C-β and p66Shc phosphorylation. These results suggest novel effects of PPARγ agonist on mitochondrial injury that may be of particular relevance in aging-related renal injury.
To our knowledge, this is the first study of long-term TZD treatment in aging. Interestingly, in this nondiabetic aging model, pioglitazone dramatically improved aging-related glomerulosclerosis, interstitial fibrosis, cell senescence, and renal function. Diminished PPARγ in adipose tissue and skeletal muscle and insulin resistance have been found in aging.19–21 Previous studies in the kidney showed that PPARγ activity was decreased in aging rats.12 Our data showed that pioglitazone increased PPARγ expression and activity in the aging kidney, which paralleled decreased glomerulosclerosis and interstitial fibrosis. In addition, systemic oxidative stress and intima thickness in renal arteries were decreased in the treatment group (data not shown). Pioglitazone also improved the mildly elevated blood glucose and increased lipid in aging rats, which may also have contributed to its beneficial effects on renal injury. These data suggest that not only systemic but also local actions of pioglitazone are involved in protection of injury in the aging kidney.
In our study, pioglitazone reduced a key TGFβ signaling pathway and oxidative stress and improved mitochondrial function. Most of these actions are mediated through the PPARγ acting on PPARγ response elements in gene promoter segments. Pioglitazone suppression of TGFβ1-induced fibronectin mRNA expression is dependent on PPARγ activation.22 A functional PPARγ response element has recently been identified in the rat catalase promoter of brain microvascular endothelial cells.23 Klotho is a newly identified anti-aging factor with effects on insulin/IGF-1 signaling, ROS, and phosphate/calcium homeostasis. The klotho gene has two upstream noncanonical PPAR-γ binding sites, and in normal young mice, PPARγ increases renal tubular klotho mRNA and protein expression in vitro and in vivo.24 We found that pioglitazone treatment in aging also increased renal klotho expression by more than 60%, another possible contributor to its effects on senescence and ROS.
The free radical theory, one of the controversial theories of aging, proposes that free radical reactivity is inherent in biology and results in cumulative damage and senescence. Elevated levels of both oxidant-damaged DNA and protein are found in aged organisms.25 We found increased urinary and renal lipid oxidation in aging rats, and pioglitazone attenuated those aging-related changes. Mitochondria are the major source of ROS in cells.26 Because the mitochondrial chromosome codes for some electron carriers, mtDNA damage may indirectly inhibit respiration and stimulate ROS formation. In our study, pioglitazone had a trend to decrease mitochondrial DNA deletion and significantly increased mitochondrial inner membrane integrity. Previous data showed that PPARγ activation induced mitochondrial dysfunction and increased ROS production (oxidative ATP formation), resulting in a compensatory increase in glucose used and ATP formed by glycolysis.27 However, those studies were done on liver, muscle, or astrocytes and observed the rapid and direct effects on mitochondria by PPARγ.28 The conflicting results may point to a tissue or time specificity with regard to the relative importance of different target sites.
Mechanisms of TZD's effect on mitochondria are complicated.27 First, pioglitazone increases mtDNA.10 Second, PPARγ and nuclear respiratory factor 1 transcription factors share a common coactivator, PPAR coactivator 1α,29 and nuclear respiratory factor 1 can promote mitochondrial biogenesis. Third, TZDs may affect coupling-uncoupling dynamics, which could in turn increase glucose utilization and influence free radical production/oxidative stress.30,31 Last, several findings suggest that activation of PPARγ may exert an antioxidant activity by favorably altering the expression of specific enzymes participating in the production and/or elimination of ROS in mitochondria, including e.g. NAD(P)H and catalase.23,32–34 We showed that pioglitazone also decreased total cytochrome C oxidase activity, although not its expression. More importantly, we found that phosphorylation of p66Shc, a key modulator of mitochondrial function, was decreased in pioglitazone-treated rats. p66Shc is a newly recognized gene associated with longevity in mice, and also serves as an integration point for many signaling pathways that affect mitochondrial function.17,35 The extended life span of mice lacking p66Shc has been correlated with a decrease in mitochondrial metabolism and reactive oxygen species production.36,37 Once activated, p66Shc oxidizes cytochrome C and induces opening of the mitochondrial permeability transition pore, generates more ROS and releases more pro-apoptotic factors into the cytosol. Inhibiting or silencing protein kinase C-β protects cells against H2O2 challenge.38 Furthermore, over-expression of protein kinase C-β reproduces the mitochondrial fragmentation and Ca2+ signaling defect in cells expressing p66Shc, but not in cells lacking p66Shc. Cells expressing a mutant form of p66Shc (p66ShcS36A) that cannot be phosphorylated also lack the early mitochondrial response to protein kinase C-β activity, indicating a requirement for p66Shc phosphorylation. Interestingly, TZDs corrected the impaired protein kinase C pathway in our aging model, similar to observations in diabetic rats or high glucose-treated endothelial cells.15,39 This finding may be related to 5′-AMP-activated protein kinase (AMPK) or diacylglycerol kinase activation, two upstream inhibitors, which can be directly induced by TZDs.16,40
In our study, we did not assess PPARγ-independent pathways that also may contribute to the beneficial effects of TZDs. Data suggest that TZDs modulate nitric oxide reduction, TNF-α production, and endothelial cell proliferation by PPARγ-independent mechanisms.41,42 In vitro studies in renal tubular epithelium showed that lowering pioglitazone concentration activated both PPARγ-driven gene expression and the PPARγ-independent AMPK pathway, whereas a high dose of troglitazone activated the P-extracellular signal–regulated kinase pathway.28 These findings indicate the effects of TZDs depend both on the specific agent and the dose, resulting in activation of different signaling pathways. Both PPARγ activation and decreased protein kinase C, which is downstream of AMPK, were found in our study in response to pioglitazone. Thus, we cannot exclude that a PPARγ-independent pathway could also contribute to the observed effects.
In summary, our results support the hypothesis that increased oxidative stress associated with mitochondrial injury contributes to renal dysfunction, senescence, glomerulosclerosis, and proteinuria in the aging kidney. Our results further suggest that age-dependent renal injury can be protected by activation of PPARγ with associated increased klotho and reduced protein kinase C-β and p66Shc phosphorylation.
Concise Methods
Experimental Design and Animals
Aging male Sprague-Dawley rats from Zivic Miller Laboratories (Zellenople, PA) were studied. Aging rats were housed under controlled conditions with a 12-h light/dark cycle, at 70°F with 40% humidity and 12 air exchanges per hour. Rats received normal rat chow and water ad libitum (“5001” diet; rodent diet, 23.4% protein, 4.5% fat, 6.0% fiber, 0.40% sodium; Purina Laboratory, St. Louis, MO). At 18 mo of age, treatment with the PPARγ agonist pioglitazone added to chow (10 mg/kg per d, Pio, n = 10) was begun, and rats were killed at the age of 24 mo and compared with age-matched untreated controls (Cont, n = 7) housed under identical conditions.
Blood and Urinary Measurements
Animals were placed in metabolic cages for 24 h for urine collection at 18 and 24 mo. Urine protein was measured by Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). Serum and urine creatinine was measured by Vitros CREA slides (Johnson & Johnson Clinical Diagnostics, Rochester, NY). Urinary excretion of isoprostane was measured by the EIA kit (Oxford Biomedical Research, Rochester Hills, MI).
Evaluation of Renal Tissue
Kidney tissue from rats at death was immersion fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) solution and routinely processed, and 4-μm sections were stained with periodic acid-Schiff and Masson's trichrome. A semiquantitative score (sclerosis index [SI]) was used to evaluate the degree of glomerulosclerosis. The severity of sclerosis for each glomerulus was graded from 0 to 4+ as follows: 0 represents no lesion, and 1+ represents sclerosis of <25% of the glomerulus, whereas 2+, 3+, and 4+ represent sclerosis of 25 to 50, 50 to 75, and >75% of the glomerulus, respectively. A whole kidney average SI was obtained by averaging scores from all glomeruli on one section.
Cortical interstitial volume fraction (V/v interstitium/cortex) was assessed by point counting at ×200 on Masson trichrome–stained cortical sections. Tubulointerstitial fibrosis was also evaluated qualitatively on Masson's trichrome–stained section. All sections were examined without knowledge of the treatment protocol.
Immunohistochemistry
Four-micrometer sections were microwaved in 0.01 M sodium citrate (pH 6.0) three times for 5 min each. The primary antibodies used were rabbit anti-rat PPARγ (1:200; Cayman, Ann Arbor, MI), ED1 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit anti-mouse 4-hydroxy-2-nonenal (1:800; Cell Signaling). Sections were incubated overnight at 4°C. Immunoperoxidase staining was performed with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA), with diaminobenzidine as a chromogen. Hematoxylin was used as a counterstain.
Senescence-Associated β-Galactosidase Activity
Senescence-associated galactosidase activity was examined as described previously.43 Briefly, frozen sections were washed and incubated at 37°C with freshly prepared senescence-associated β-galactosidase stain solution: 1 mg of 5-bromo-4-chloro-3-indolyl β-d-galactoside/ml (stock solution, 20 mg/ml of dimethylformamide), 40 mM citric acid, sodium phosphate, pH 6.0, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2. Staining was evident in 16 h. The percentage of β-galactosidase–positive area was measured by point counting on 20 consecutive fields from kidneys from each rat evaluated at a magnification of ×100.
Western Blot
Frozen kidney tissue samples were transferred in radioimmunoprecipitation assay plus buffer containing 1:100 phosphatase inhibitor cocktail I, 1:100 phosphatase inhibitor cocktail II (Sigma, St. Louis, MO), 1:100 proteinase inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany), samples were homogenized and centrifuged, and the protein concentration was measured using the Dc Protein Assay kit (Bio-Rad Laboratories, Hercules, CA). p66Shc (1:2000; Abcam, Cambridge, MA), P-Tyr (1:400; Santa Cruz Biotechnology), cytochrome C oxidase I (1:400; Santa Cruz Biotechnology) and IV (1:2500; Santa Cruz Biotechnology), protein kinase C-β1 (1:400; Santa Cruz Biotechnology), protein kinase C-β2 (1:1000; Sigma), PAI-1 (1:400; R&D Systems, Minneapolis, MN), NAPDH oxidase (NOX)4 (1:100; Abcam), NOX2 (1:250; Millipore), Cu/Zn SOD (1:1000; Millipore), klotho (1:500; R&D Systems), P-Smad2 (1:500; Cell Signaling, Boston, MA), and total-Smad2 (1:250; Zymed) were detected by using the corresponding antibody overnight at 4°C. Horseradish peroxidase–labeled donkey anti-rabbit or anti-mouse IgG secondary antibody (1:2500 in 5% milk TBS-T) was added and incubated at room temperature for 1 h. Protein bands on Western blots were visualized by ECL Plus (Amersham, Arlington Heights, IL) according to the manufacturer's instructions and were developed on film. β-actin was detected using mouse anti-β-actin polyclonal antibody (Sigma). The ratio of specific message to β-actin or P-Smad2 to Total-Smad2 was used to quantify expression for each tissue sample.
PPARγ Activity
The activity of PPARγ was assessed by ELISA using a TransAM PPARγ Transcription Factor Assay Kit (Active Motif, Carlsbad, CA). This method is based on reaction of nuclear extracts (30 μg protein) with specific oligonucleotide sequences coated on a microtiter plate. According to the manufacturer's instructions, the binding of activated PPARγ is shown by the addition of a primary polyclonal anti-PPARγ antibody, followed by a secondary antibody conjugated with horseradish peroxidase and the 3,3′,5,5′-tetramethylbenzidine substrate. Absorbance was read at 450 nm and, after the reaction was stopped with sulfuric acid, at 655 nm using a microtiter plate reader.
Mitochondrial Cytochrome C Activity
Isolation of rat renal mitochondria was performed with a mitochondrial isolation kit (Sigma). The mitochondrial inner membrane integrity was measured by the uptake of the fluorescence carbocyanine dye JC-1 into the mitochondria. The protein content of mitochondria and the mitochondrial supernatants was determined by the Biuret method with bovine serum albumin as standard. The mitochondrial-specific cytochrome C oxidase activity in the soluble and membrane-bound mitochondria sample was assayed for mitochondria activity using a commercial kit (Biochain Institute, Hayward, CA).
Mitochondrial DNA Deletion
Cortical tissue (100 mg) was homogenized, and mitochondrial DNA was extracted using an mtDNA extractor CT kit (Wako Pure Chemical Industries). The primers L4395 (5′-AGGACTTAACCAGACCCAAACACG-3′) and H5164 (5′-CCTCTTTTCTGATAGGCGGG-3′) were used to amplify a 770-bp DNA fragment, a highly conserved region that serves as a reference for total mtDNA, and primers L7825 (5′-TTTCTTCCCAAACCTTTCCT-3′) and H13117 (5′-AAGCCTGCTAGGATGCTTC-3′) were used for the amplification of a 459-bp PCR product from 4834 bp–deleted mtDNA. The PCR reaction was performed in a 50-μl reaction mixture containing 100 ng of total mtDNA, 0.2 mM of each dNTP, 1.0 μl (5 units) of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA), 0.5 μM of each primer, 15 mM Tris–HCl (pH 8.0), 50 mM KCl, 3 mM MgCl2, and 6 μg/ml of albumin. After a hot start at 95°C for 12 min, amplification was performed for 30 cycles with 10 s of denaturation at 94°C, 30 s of annealing at 60°C, and 2 min of extension at 72°C, and a final extension was performed for 5 min at 72°C. The relative density of the 459-bp PCR product against the 770-bp product was calculated.44
Statistical Analysis
Results are expressed as mean ± SEM. Statistical difference was assessed by a single-factor variance (ANOVA) followed by unpaired t test as appropriate. Nonparametric data were compared by Mann-Whitney U-test. P < 0.05 was considered significant.
Disclosures
None.
Acknowledgments
This work was supported in part by NIH NIDDK P50 DK79341 and by Takeda Pharmaceutical Company Ltd. The color illustrations and publication costs were supported by Takeda Pharmaceuticals North America, Inc.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Beck LH: Changes in renal function with aging. Clin Geriatr Med 14: 199–209, 1998 [PubMed] [Google Scholar]
- 2.McLachlan M, Wasserman P: Changes in sizes and distensibility of the aging kidney. Br J Radiol 54: 488–491, 1981 [DOI] [PubMed] [Google Scholar]
- 3.Fuiano G, Sund S, Mazza G, Rosa M, Caglioti A, Gallo G, Natale G, Andreucci M, Memoli B, De Nicola L, Conte G: Renal hemodynamic response to maximal vasodilating stimulus in healthy older subjects. Kidney Int 59: 1052–1058, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Melk A, Halloran PF: Cell senescence and its implications for nephrology. J Am Soc Nephrol 12: 385–393, 2001 [DOI] [PubMed] [Google Scholar]
- 5.McLean AJ, Le Couteur DG: Aging biology and geriatric clinical pharmacology. Pharmacol Rev 56: 163–184, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Sugiyama Y, Taketomi S, Shimura Y, Ikeda H, Fujita T: Effects of pioglitazone on glucose and lipid metabolism in Wistar fatty rats. Arzneimittelforschung 40: 263–267, 1990 [PubMed] [Google Scholar]
- 7.Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB: Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int 59: 1899–1910, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Yang HC, Ma LJ, Ma J, Fogo AB: Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int 69: 1756–1764, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Gumieniczek A: Effect of the new thiazolidinedione-pioglitazone on the development of oxidative stress in liver and kidney of diabetic rabbits. Life Sci 74: 553–562, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S, Patel N, Rahn D, McAllister J, Sadeghi S, Horwitz G, Berry D, Wang KX, Swerdlow RH: The thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Mol Pharmacol 71: 1695–1702, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Barbieri M, Bonafe M, Rizzo MR, Ragno E, Olivieri F, Marchegiani F, Franceschi C, Paolisso G: Gender specific association of genetic variation in peroxisome proliferator-activated receptor (PPAR)gamma-2 with longevity. Exp Gerontol 39: 1095–1100, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Sung B, Park S, Yu BP, Chung HY: Modulation of PPAR in aging, inflammation, and calorie restriction. J Gerontol A Biol Sci Med Sci 59: 997–1006, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ma LJ, Nakamura S, Whitsitt JS, Marcantoni C, Davidson JM, Fogo AB: Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in PAI-1. Kidney Int 58: 2425–2436, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kujoth GC, Bradshaw PC, Haroon S, Prolla TA: The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet 3: e24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA: Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Verrier E, Wang L, Wadham C, Albanese N, Hahn C, Gamble JR, Chatterjee VK, Vadas MA, Xia P: PPARgamma agonists ameliorate endothelial cell activation via inhibition of diacylglycerol-protein kinase C signaling pathway: Role of diacylglycerol kinase. Circ Res 94: 1515–1522, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hajnoczky G, Hoek JB: Cell signaling. Mitochondrial longevity pathways. Science 315: 607–609, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Hotta K, Bodkin NL, Gustafson TA, Yoshioka S, Ortmeyer HK, Hansen BC: Age-related adipose tissue mRNA expression of ADD1/SREBP1, PPARgamma, lipoprotein lipase, and GLUT4 glucose transporter in rhesus monkeys. J Gerontol A Biol Sci Med Sci 54: B183–B188, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Ye P, Zhang XJ, Wang ZJ, Zhang C: Effect of aging on the expression of peroxisome proliferator-activated receptor gamma and the possible relation to insulin resistance. Gerontology 52: 69–75, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Knight JA: The biochemistry of aging. Adv Clin Chem 35: 1–62, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M: Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes 53: 200–208, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Girnun GD, Domann FE, Moore SA, Robbins ME: Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol Endocrinol 16: 2793–2801, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N: Klotho is a target gene of PPAR-gamma. Kidney Int 74: 732–739, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Ames BN, Shigenaga MK, Hagen TM: Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A 90: 7915–7922, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cadet J, Douki T, Ravanat JL: Artifacts associated with the measurement of oxidized DNA bases. Environ Health Perspect 105: 1034–1039, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C: Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol 70: 177–188, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Turturro F, Oliver R, 3rd, Friday E, Nissim I, Welbourne T: Troglitazone and pioglitazone interactions via PPAR-gamma-independent and -dependent pathways in regulating physiological responses in renal tubule-derived cell lines. Am J Physiol Cell Physiol 292: C1137–C1146, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Scarpulla RC: Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 286: 81–89, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Digby JE, Montague CT, Sewter CP, Sanders L, Wilkison WO, O'Rahilly S, Prins JB: Thiazolidinedione exposure increases the expression of uncoupling protein 1 in cultured human preadipocytes. Diabetes 47: 138–141, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW, Cascieri MA, Moller DE: Peroxisome proliferator-activated receptors gamma and alpha mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression Endocrinology 139: 4920–4927, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Hwang J, Kleinhenz DJ, Lassegue B, Griendling KK, Dikalov S, Hart CM: Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol 288: C899–905, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mahgoub MA, Abd-Elfattah AS: Diabetes mellitus and cardiac function. Mol Cell Biochem 180: 59–64, 1998 [PubMed] [Google Scholar]
- 34.Inoue I, Goto S, Matsunaga T, Nakajima T, Awata T, Hokari S, Komoda T, Katayama S: The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism 50: 3–11, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Camougrand N, Rigoulet M: Aging and oxidative stress: Studies of some genes involved both in aging and in response to oxidative stress. Respir Physiol 128: 393–401, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Giorgio MME, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG: Electron transfer between cytochrome C and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122: 221–233, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, Finkel T: The mammalian longevity-associated gene product p66Shc regulates mitochondrial metabolism. J Biol Chem 281: 10555–10560, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R: Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315: 659–663, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Yamagishi S, Ogasawara S, Mizukami H, Yajima N, Wada R, Sugawara A, Yagihashi S: Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated-gamma-ligand, in insulin-deficient diabetic rats. J Neurochem 104: 491–499, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A: Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol 27: 2627–2633, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sasaki M, Jordan P, Welbourne T, Minagar A, Joh T, Itoh M, Elrod JW, Alexander JS: Troglitazone, a PPAR-gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-alpha. BMC Physiol 5: 3, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu HB, Hu YS, Medcalf RL, Simpson RW, Dear AE: Thiazolidinediones inhibit TNFalpha induction of PAI-1 independent of PPARgamma activation. Biochem Biophys Res Commun 334: 30–37, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Takaki A, Jimi S, Segawa M, Iwasaki H: Cadmium-induced nephropathy in rats is mediated by expression of senescence-associated beta-galactosidase and accumulation of mitochondrial DNA deletion. Ann N Y Acad Sci 1011: 332–338, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Filser N, Margue C, Richter C: Quantification of wild-type mitochondrial DNA and its 4.8-kb deletion in rat organs. Biochem Biophys Res Commun 233: 102–107, 1997 [DOI] [PubMed] [Google Scholar]