Abstract
Patients with Alport syndrome progressively lose renal function as a result of defective type IV collagen in their glomerular basement membrane. In mice lacking the α3 chain of type IV collagen (Col4A3 knockout mice), a model for Alport syndrome, transplantation of wild-type bone marrow repairs the renal disease. It is unknown whether cell-based therapies that do not require transplantation have similar potential. Here, infusion of wild-type bone marrow-derived cells into unconditioned, nonirradiated Col4A3 knockout mice during the late stage of disease significantly improved renal histology and function. Furthermore, transfusion of unfractionated wild-type blood into unconditioned, nonirradiated Col4A3 knockout mice improved the renal phenotype and significantly improved survival. Injection of mouse and human embryonic stem cells into Col4A3 knockout mice produced similar results. Regardless of treatment modality, the improvement in the architecture of the glomerular basement membrane is associated with de novo expression of the α3(IV) chain. These data provide further support for testing cell-based therapies for Alport syndrome.
Alport syndrome is characterized by the progressive development of glomerulonephritis associated with the loss of α3α4α5 type IV collagen protomer in the glomerular basement membrane (GBM).1 The type IV collagen chain composition in the GBM is critical to the maintenance of the glomerular filtration.2,3 Genetic mutations in α3, α4, or α5 (IV) collagen chains result in the ablation of the obligatory posttranslational assembly of α3α4α5(IV) protomer, which leads to renal disease in patients with Alport syndrome.2,4–7 The engineered genetic mutation in the COL4A3 gene [encoding for α3(IV) chain; the Col4A3 knockout mouse] provides us with a mouse model that closely recapitulates the human disease.8,9 Col4A3 knockout mice develop progressive glomerulonephritis associated with the loss of the GBM α3α4α5(IV) protomer and die as a result of renal failure. Importantly, the progression of the disease in mice varies with respect to their genetic background. Col4A3 knockout mice, on the 129Sv genetic background, progress more rapidly and die at approximately 13 wk of age in comparison with Col4A3 knockout mice on the C57BL/6 background, which die of renal failure at approximately 22 wk of age in our laboratory. It is suggested that the difference in disease progression between these two strains of mice results from the compensatory effect of α5α6α5(IV) protomer in the GBM of Col4A3 knockout on C57BL/6 genetic background, which is negligible when this mutation is on the 129Sv background.10 This modifying effect suggests that type IV collagen protomer composition is critical for GBM function and suggests that modulating GBM type IV composition, by providing the missing chain collagen to the GBM of Col4A3 knockout mice, could theoretically slow down or halt the progression of the renal disease.
Previous preclinical studies demonstrated that de novo production of α3(IV) collagen in Col4A3 knockout mice that received a transplant of wild-type (WT) bone marrow (BM) is associated with significant improvement in renal function.11,12 We and others have shown that BM-derived cells specifically target the diseased glomeruli and allow for the deposition of α3(IV) chain, which results in the restoration of the α3α4α5(IV) protomer in the GBM.11,12 These results suggest that BM-derived cells provide a therapeutic benefit to the Col4A3 knockout mice. Nonetheless, a disease-modulating effect of total body irradiation on the progression of the kidney disease was recently suggested in Col4A3 knockout mice on the 129Sv genetic background.13 Unlike the human disease, kidney disease progression in Col4A3 knockout mice involves significant immune infiltration; therefore, a case can be made that total body irradiation and subsequent BM transplantation in Col4A3 knockout mice could modulate disease progression by diminishing renal immune infiltration. Our previous report demonstrated that lymphocyte ablation improves renal interstitial fibrosis in Col4a3/Rag-1 double-KO mice on C57BL/6 background16 as a result of diminished interstitial infiltrates. Recently, Katayama et al.13 provided evidence for an increase in the survival of Col4A3 knockout mice on 129Sv background, associated with improvement in renal function and histologic findings after total body irradiation; however, that study does not conclusively negate the specific therapeutic potential of BM-derived cells in the recovery of the renal phenotype.
A better understanding of the cell-based therapy in Col4A3 knockout mice is critical for future clinical development of this therapeutic strategy for patients with Alport syndrome. Here we provide a critical evaluation of a cellular process in the therapy of Col4A3 knockout mice. Our experiments unequivocally demonstrate that cell-based therapy is a viable option in the treatment of Alport syndrome.
Results
BM Transplantation in Col4A3 Knockout Mice Improves the Renal Phenotype and Is Associated with the Expression of the Missing α3 Chain of Type IV Collagen
A careful analysis of the role of total body irradiation in modulating disease progression in Col4A3 knockout mice on C57BL/6 genetic background that receive a BM transplant indicates that injection of WT BM-derived cells enable significant histologic improvement when compared with Col4A3 knockout mice that receive Col4A3 knockout -derived BM cells (see supplemental text and Supplemental Figure 1). Furthermore, our results show that both syngeneic and nonsyngeneic BM transplantations improve proteinuria and provide the missing chain of type IV collagen in Col4A3 knockout mice on the 129Sv genetic background (see supplemental supporting text and Supplemental Figure 2).
Multiple Infusions of WT BM Cells Rescue the Renal Phenotype of COL4A3KO Mice
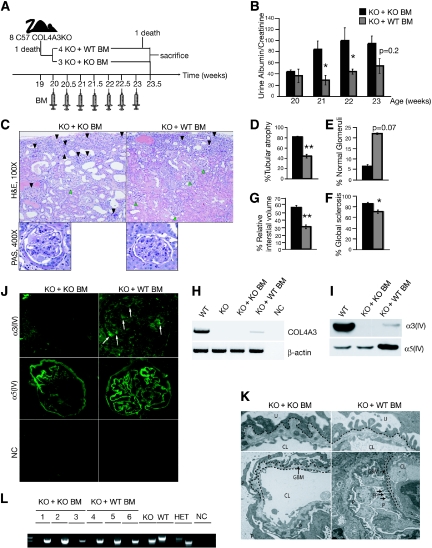
Our analysis of mice with Col4A3 knockout BM transplant reveals that BM cells with the WT allele rather than with the KO allele of the COL4A3 gene provide significant improvement in renal function and histology (Supplemental Figure 1). To provide evidence that the observed improvement in the renal disease is dependent on a BM-derived cell, we investigated whether BM cell infusions in non irradiated Col4A3 knockout mice on the C57BL/6 genetic background provide similar phenotypic improvement. We began BM infusions in a littermate cohort of eight Col4A3 knockout mice (Figure 1A). Col4A3 knockout mice on the C57BL/6 genetic background develop progressive glomerulonephritis and die from renal failure between 19 and 25 wk of age. We chose specifically to evaluate littermate Col4A3 knockout mice, which present with similar disease progression rate. When the first mouse in one of the cohort succumbed to renal failure at 19 wk of age, we began BM infusions and continued infusions during the course of the next 3 wk. After 3 wk of repeated BM infusions, all mice were killed when another Col4A3 knockout mouse, which received Col4A3 knockout BM infusion at 20 wk, died as a result of renal failure (Figure 1A).
Figure 1.
BM cell infusions in C57BL/6 Col4A3 knockout mice rescue the renal phenotype. (A) Schematic representation of experimental setup: BM infusions were administered to seven Col4A3 knockout littermates with end-stage renal failure (20 wk of age), with four Col4A3 knockout mice receiving WT BM from GFP+ donor and three Col4A3 knockout mice receiving Col4A3 knockout BM. Recipients were treated with seven consecutive BM infusions and sacrificed at 23.5 wk of age after one of the mice in the experimental group of Col4A3 knockout mice infused at 20 weeks with BM transplant from Col4A3 knockout died. (B) Urine albumin-creatinine ratio measurements in Col4A3 knockout mice that received BM transplant from Col4A3 knockout mice (n = 3) and mice that received BM transplant from WT mice (n = 4) from 20 to 23 wk of age. (C) Representative hematoxylin and eosin (H&E) staining of the kidney cortex of 23.5-wk-old mice that received BM transplant from Col4A3 knockout and mice that received BM transplant from WT mice; black arrowheads point to globally sclerosed glomeruli, and green arrowheads point to normal healthy glomeruli. Representative periodic acid-Schiff staining of glomeruli. (D through G) Morphometric analyses of percentage of tubular atrophy (D), percentage of normal glomeruli (E), percentage of glomerular global sclerosis (F), and percentage of interstitial volume of 23.5 wk-old Col4A3 knockout mice that received BM transplant from Col4A3 knockout and mice that received BM transplant from WT mice (G). (H) RT-PCR analyses for expression of Col4A3 knockout and β-actin control. (I) Western blot immunolabeling for mouse α3(IV) and mouse α5(IV) from ECM proteins from kidneys of WT, Col4A3 knockout mice that received BM transplant from Col4A3 knockout, and Col4A3 knockout mice that received BM transplant from WT mice. (J) Immunolabeling of mouse α3(IV) and α5(IV) collagen in kidney glomeruli of Col4A3 knockout mice that received BM transplant from Col4A3 knockout and mice that received BM transplant from WT mice mice; secondary FITC-conjugated antibody was used in negative control (NC). (K) Representative transmission electron microscopy images from Col4A3 knockout mice that received BM transplant from Col4A3 knockout mice and mice that received BM transplant from WT mice at 23.5 wk of age. P, podocyte; FP, foot processes. GBM is partly underlined. (L) Genotyping PCR amplification of Col4a3 WT (1000-bp product) and Col4A3 knockout (850-bp product) gene from genomic DNA extracted from the BM of Col4A3 knockout mice that received BM transplant from Col4A3 knockout mice, Col4A3 knockout mice that received BM transplant from WT mice, Col4A3 knockout, WT, and Col4A3 heterozygote (HET). NC, no template control. *P < 0.05; **P < 0.01. Magnifications: ×100 and ×400 in C; ×400 in J.
Urine albumin-creatinine ratio improved in Col4A3 knockout mice infused with WT BM at 20 wk, in contrast with mice infused with Col4A3 knockout BM, and a significant difference was noted at 21 and 22 wk of age, halfway during the course of the BM infusions (Figure 1B; Table 1). Histologic findings also revealed significant improvement in glomerular sclerosis, with an increase in percentage of normal glomeruli, tubular atrophy, and relative interstitial volume of Col4A3 knockout mice infused with WT BM in comparison with mice infused with Col4A3 knockout BM (Figure 1, C through G; Table 1). These results therefore suggest that the BM-derived cells in Col4A3 knockout mice infused with WT BM are responsible for the observed improvement in the glomerular and tubular compartments rather than a mere radiation effect.
Table 1.
Morphometric analyses and renal function tests: BM infusion
| Parameter | KO + WT BM | KO + KO BM | Statistical Significance |
|---|---|---|---|
| Urine albumin-creatinine ratio | |||
| 20 wk | 36.6 ± 12.0 | 44.0 ± 3.2 | No |
| 21 wk | 28.8 ± 4.5 | 84.0 ± 23.3 | a |
| 22 wk | 44.2 ± 9.5 | 99.6 ± 8.1 | a |
| 23 wk | 54.2 ± 11.2 | 94.3 ± 22.9 | P = 0.2 |
| Morphometric analyses (%) | |||
| normal glomeruli | 22.3 ± 0.4 | 6.5 ± 0.9 | P = 0.07 |
| global glomerular sclerosis | 70.9 ± 3.4 | 80.0 ± 2.7 | a |
| tubular atrophy | 44.4 ± 3.6 | 82.1 ± 1.6 | b |
| relative interstitial volume | 30.3 ± 2.5 | 56.4 ± 2.8 | b |
aP < 0.05.
bP < 0.01.
The phenotypic rescue observed in Col4A3 knockout mice infused with WT BM cells was associated with changes in GBM type IV collagen composition. The missing α3(IV) collagen chain was expressed in the GBM of Col4A3 knockout mice infused with WT BM mice, whereas it remained absent in mice infused with Col4A3 knockout BM (Figure 1J). The expression of α3(IV) collagen chain in GBM of Col4A3 knockout mice infused with WT BM was associated with a noticeable increase in α5(IV) collagen deposition in these mice in contrast with mice infused with Col4A3 knockout BM at 20 wk(Figure 1J). Our immunostaining data thus suggest that BM-derived cells alter the GBM type IV collagen composition in Col4A3 knockout mice, likely resulting in improved GBM stability and glomerular filtration. Reverse transcriptase–PCR (RT-PCR) for COL4A3 expression and Western blot analyses of kidney extracellular matrix (ECM) extract revealed α3(IV) collagen expression in Col4A3 knockout mice infused with WT BM at 20 wk, in contrast with mice infused with Col4A3 knockout BM (Figure 1, H and I). Furthermore, Col4A3 knockout mice infused with WT BM presented with increased levels of α5(IV) chain (Figure 1I). Taken together, these results demonstrate that WT BM-derived cells allow for significant improvement of renal histologic findings associated with changes in the GBM type IV collagen composition, de novo expression of the missing α3(IV) collagen chain, and increased α5(IV) collagen expression.
In our previous BM transplantation study,11 we demonstrated that BM derived-cells localized to the kidney in Col4A3 knockout mice infused with WT BM at 20 wk. We wondered whether the homing of the infused BM cells to the recipient's BM was a prerequisite for subsequent homing to the inflamed kidney of the Col4A3 knockout mice. We analyzed the genotype of the BM cells harvested from Col4A3 knockout recipients upon completion of the treatment. We did not detect the WT copy of the COL4A3 gene, suggesting that BM-infused cells do not home to the recipient's BM (Figure 1L). These results suggest that recruitment of BM-derived cell to the kidney does not require previous homing to the recipient's BM. Finally, electron microscopy imaging of the GBM revealed restored GBM architecture in Col4A3 knockout mice infused with WT BM at 20 wk, with decreased splitting and thickening of the GBM when compared with the mice infused with Col4A3 knockout BM (Figure 1K), consistent with renal function and histologic improvement. In addition, BM infusions increased the lifespan of Col4A3 knockout mice in comparison with mice infused with Col4A3 knockout BM, with death of four out of five mice infused with Col4A3 knockout BM at 26.5 wk of age, a time point at which five out of five mice infused with WT BM at 20 wk were still alive (Supplemental Figure 3D). Finally, we tested whether a single injection of WT unfractionated BM cells in unconditioned Col4A3 knockout recipients (at an earlier time point in the disease progression) could provide the missing chain of type IV collagen. Our results indicated a single injection of WT BM cells into 8-wk-old Col4A3 knockout mice was sufficient to provide the missing chain of type IV collagen in kidney GBM, detected by RT-PCR and Western blot analyses, 12 wk after the single infusion (see supplemental supporting text and Supplemental Figure 3).
Simple Blood Transfusion into Col4A3 Knockout Mice Improves Renal Function and Histologic Findings
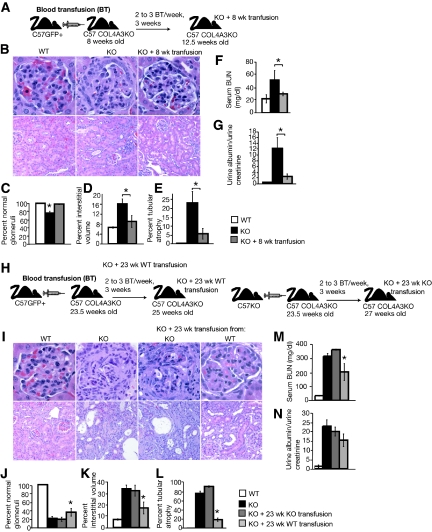
We next evaluated the feasibility of long-term blood exchange, via parabiotic pairing, in inducing therapy in the Col4A3 knockout mice (Supplemental Figure 3E). Western blot analyses of kidney ECM proteins revealed the expression of α3(IV) collagen in the recipient parabiont mouse (Supplemental Figure 3F). We then asked whether simple blood transfusions could rescue the renal phenotype in Col4A3 knockout mice. We used green fluorescence protein–positive (GFP+) transgenic mice as blood donors. Initially, we transfused 8-wk-old Col4A3 knockout mice and sacrificed the recipient mice at 12.5 wk of age (Figure 2A). Morphometric analyses of histologic findings (Figure 2B) revealed a statistically significant improvement in glomerular sclerosis, tubular atrophy, and interstitial volume (Figure 2, C through E; Table 2). Renal function tests revealed an improvement of the renal phenotype in agreement with the histologic findings. Col4A3 knockout mice transfused at 8 wk with blood from WT mice presented with significantly lower serum blood urea nitrogen (BUN) levels, similar to that of WT control mice (Figure 2F; Table 2). Similarly, urine albumin-creatinine ratio was significantly lower in transfused Col4A3 knockout mice in comparison with untreated Col4A3 knockout mice (Figure 2G; Table 2).
Figure 2.
Blood transfusions rescue Col4A3 knockout renal phenotype. (A) Schematic representation of experimental setup: WT donor mice were used for blood transfusion in 8-wk-old C57BL/6 Col4A3 knockout mice (n = 4). The mice were subsequently killed at 12.5 wk of age. (B through D) Representative H&E pictures at higher (top) and lower (bottom) magnifications of kidneys from WT (n = 3), Col4A3 knockout (n = 6), and Col4A3 knockout mice transfused at 8 wk(n = 4) (B) and morphometric analyses of percentage of normal glomeruli (C), percentage of interstitial volume (D), and percentage of tubular atrophy (E). (F and G) BUN measurements (F) and urine albumin-creatinine ratio (G) of 12.5-wk-old WT (n = 6), Col4A3 knockout (n = 3), and Col4A3 knockout mice transfused at 8 wk(n = 4). (H) Schematic representation of experimental setup: WT and Col4A3 knockout donor mice were used for blood transfusion in 23.5-wk-old Col4A3 knockout mice ([n = 3] and [n = 7], respectively). (I through L) Representative H&E pictures at higher (top) and lower (bottom) magnifications of kidneys from 21-wk-old WT (n = 3) and Col4A3 knockout (n = 3), 25-wk-old Col4A3 knockout transfused at 23 wk with Col4A3 knockout blood (n = 3), and 27-wk-old Col4A3 knockout transfused at 23 wk with WT blood (n = 7) mice (I) and morphometric analyses of percentage of normal glomeruli (J), percentage of interstitial volume (K), and percentage of tubular atrophy (L). (M and N) BUN measurements (M) and urine albumin-creatinine ratio (N) from 21 wk-old WT (n = 3) and Col4A3 knockout (n = 3), 25-wk-old Col4A3 knockout transfused at 23 wk with Col4A3 knockout blood (n = 3), and Col4A3 knockout mice transfused at 23 wk with WT blood (n = 6) mice. *P < 0.05. Magnifications: ×400 in B (top) and I (top); ×200 in B (bottom) and I (bottom).
Table 2.
Morphometric analyses and renal function tests: Blood transfusion
| Parameter | Morphometric Analyses (%) |
Renal Function Tests |
|||
|---|---|---|---|---|---|
| Normal Glomeruli | Tubular Atrophy | Relative Interstitial Volume | Serum BUN (mg/dl) | Urine Albumin-Creatinine Ratio | |
| Early transfusion | |||||
| WT | 99.6 ± 0.4 | 0.1 ± 0.1 | 6.6 ± 0.3 | 22.5 ± 6.5 | 0.5 ± 0.1 |
| KO at 12.5 wk | 75.8 ± 4.5 | 22.6 ± 6.4 | 16.1 ± 2.0 | 51.0 ± 15.1 | 12.2 ± 3.7 |
| KO + 8 wk transfusion | 97.7 ± 1.2 | 5.4 ± 3.0 | 9.0 ± 2.4 | 29.9 ± 2.9 | 2.5 ± 0.9 |
| Statistical significance | a | a | a | a | a |
| Late transfusion | |||||
| WT | 98.9 ± 0.3 | 0.1 ± 0.1 | 6.9 ± 0.6 | 33.5 ± 2.9 | 1.5 ± 0.8 |
| KO | 22.4 ± 4.1 | 77.3 ± 5.2 | 33.4 ± 3.2 | 316 ± 19.9 | 25.8 ± 3.9 |
| KO + 23 wk KO transfusion | 20.3 ± 6.8 | 92.6 ± 1.7 | 31.8 ± 4.7 | 363 ± 3.9 | 22.6 ± 2.7 |
| KO + 23 wk WT transfusion | 36.3 ± 8.7 | 19.2 ± 3.9 | 16.9 ± 5.18 | 203 ± 60.2 | 17.3 ± 3.8 |
| Statistical significance | a | a | a | a | No |
aP < 0.05.
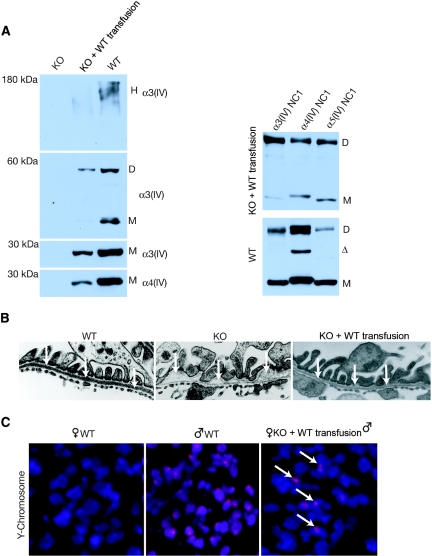
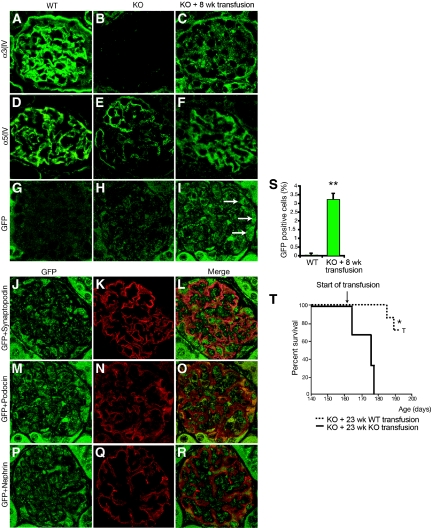
Concurrently, we also transfused 23.5-wk-old Col4A3 knockout mice with blood harvested from GFP+ or from Col4A3 knockout donor mice (Figure 2H). In such late-stage blood transfusion rescue experiment, Col4A3 knockout mice received transfusions when they developed end-stage renal failure. Importantly, the control Col4A3 knockout blood was harvested from young nonanemic Col4A3 knockout donor mice (6 wk of age). Recipients received two to three blood transfusions per week for 3 wk (the volume of each transfusion was between 200 and 300 μL). Histologic findings showed that Col4A3 knockout mice with blood harvested from GFP+ mice presented with statistically significant improvement in glomerular sclerosis, tubular atrophy, and interstitial volume in contrast with mice transfused with blood from Col4A3 knockout mice (Figure 2, I through L; Table 2). Renal function tests indicated a statistically significant improvement in proteinuria (urine albumin-creatinine ratio) and BUN in the Col4A3 knockout mice transfused with blood harvested from GFP+ micemice, in comparison with mice transfused with blood harvested from Col4A3 knockout mice (Figure 2, M and N; Table 2). Western blot analyses of kidney ECM proteins revealed expression of α3(IV) and α4(IV) type IV collagen chains in WT and Col4A3 knockout mice transfused at 8 wk with blood from WT mice, but such expression in Col4A3 knockout (KO) control mice was not observed (Figure 3A). Electron microscopy imaging of GBM from WT, Col4A3 knockout, and Col4A3 knockout mice transfused at 8 wk with blood from WT indicated a restored GBM and podocyte foot process architecture in the transfused Col4A3 knockout mice, similar to that observed in WT mice, in contrast to focal thinning and thickening of the GBM in untreated Col4A3 knockout mice (Figure 3B). Recruitment of male-derived cells in gender-mismatched blood transfusion experiments was evidenced by Y-chromosome labeling in the recipient glomeruli (Figure 3C), and immunolabeling for α3(IV) NC1 domain revealed positive GBM staining in glomeruli of WT and transfused Col4A3 knockout mice, with no staining detected in Col4A3 knockout control glomeruli (Figure 4, A through C). An increase in α5(IV) expression in transfused Col4A3 knockout mice glomeruli was detected when compared with Col4A3 knockout control mice (Figure 4, D through F). Immunohistochemical labeling of GFP+ cells revealed on average 3.3 ± 0.3% GFP+ cells per glomeruli in transfused Col4A3 knockout mice (Figure 4S), in contrast to no positive staining observed in the WT and Col4A3 knockout control kidney tissues (Figure 4, G through I). GFP labeling in the glomeruli of transfused Col4A3 knockout mice co-localizes with nephrin (Figure 4, P through R), podocin (Figure 4, M through O), and synaptopodin (Figure 4, J through L). These double-labeling experiments suggest that the GFP+ cells from the blood transfusions home to the glomeruli and express podocyte markers. Finally, we demonstrated a statistically significant increase in the survival in Col4A3 knockout mice transfused at 23 wk with blood from WT mice compared with mice transfused with blood from Col4A3 knockout mice (Figure 4T), with 100% of mice transfused with blood from WT alive at 26 wk of age but with death observed in all mice transfused with blood from Col4A3 knockout mice, suggesting that emergence of WT COL4A3-carrying cells and α3(IV) chain in the GBM is associated with increased survival (Figure 4T).
Figure 3.
Type IV collagen expression and restored GBM architecture are shown in Col4A3 knockout mice that received blood transfusions at 8 wk. (A, left) Western blot analysis reveals α3(IV) chain expression in type IV collagen hexamer (H) of WT and transfused Col4A3 knockout mice, but no expression is observed in Col4A3 knockout control mice. After denaturation of the ECM preparation, type IV collagen α3 dimers (D) and α3 and α4 monomers (M) could be detected in WT and transfused Col4A3 knockout mice but not in Col4A3 knockout mice. (Right) Immunoprecipitation of α3-, α4-, and α5-containing NC1 hexamers from collagenase-solubilized GBM by α3 antibody. Anti-α3 antibody was used to immunoprecipitate α3NC1-containing hexamers from collagenase solubilized GBM from WT and KO Blood 8 WT mice. SDS-PAGE resolves the immunoprecipitated α3NC1 hexamers into NC1 monomers and dimers. Western blot analyses reveal that α3, α4, and α5 NC1 monomers and dimers co-precipitate with α3 NC1-containing hexamers in both WT and transfused Col4A3 knockout mice, indicating the reemergence of α4 and α5 chain expression in the type IV collagen NC1 hexamers at 12.5 wk of age. Δ, Degradation product. (B) Ultrastructural analysis of the GBM by transmission electron microscopy of 12.5-wk-old WT, Col4A3 knockout, and transfused Col4A3 knockout mice. (C) Fluorescence in situ hybridization for the mouse Y-chromosome. Y-chromosome labeling shows recruitment of male blood–derived cells to glomeruli from female transfused Col4A3 knockout mice (12.5 wk of age). Magnification, ×31,000 in B; ×400 in C.
Figure 4.
Immunostaining and survival curve are shown for Col4A3 knockout mice that received a transfusion. (A through F) Type IV collagen α3 and α5 chain expression in the GBM of kidneys from WT, Col4A3 knockout, and Col4A3 knockout mice transfused at 8 wk with WT blood. No α3 chain is detected in the GBM of Col4A3 knockout control mice, and we detected faint α5 chain expression in Col4A3 knockout mice (B and E). Patchy α3 and α5 chain expression is detected in the GBM of Col4A3 knockout mice transfused at 8 wk with WT blood (C and F), similar to α3 and α5 chain expression in WT mice (A and D). (G through I) GFP labeling of kidney glomeruli: Whereas WT (G) and Col4A3 knockout (H) mice did not show any GFP labeling, Col4A3 knockout mice transfused at 8 wk with WT blood (transfused with whole blood from wild GFP+ mice) revealed GFP+ labeling in the glomeruli (I). (J through R) Co-localization of GFP labeling in glomeruli from Col4A3 knockout mice transfused at 8 wk with WT blood with the podocyte-specific markers synaptopodin (J through L), nephrin (P through R), and podocin (M through O). All tissue analyzed were from 12.5-wk-old mice. (S) Quantification of GFP+ cells revealed 3.3 ± 0.1% GFP+ cells per glomerulus at 12.5 wk of age in Col4A3 knockout mice transfused at 8 wk with WT blood (n = 3) and 0.2 ± 0.1% GFP+ cells per glomerulus at 12.5 wk of age in WT control mice (n = 3). (T) Survival curve depicts statistically significant increase in survival in Col4A3 knockout mice transfused at 23 wk with blood from WT mice (n = 7) in comparison with Col4A3 knockout mice transfused at 23 wk with blood from Col4A3 knockout mice (n = 3). T, termination, animals were killed. *P < 0.05; **P < 0.01. Magnification, ×400.
Lymphocytes, Monocytes/Macrophages and Mouse Embryonic Stem Cell Therapy in Col4A3 Knockout Mice
We tested the hypothesis that such therapeutic cells may derive from the hematopoietic compartment of the BM. A previous study using BM-derived mesenchymal stem cells in COL4A3KO mice suggested that mesenchymal stem cell therapy did not lend itself to the synthesis of the missing chain of type IV collagen14; therefore, we focused our attention on the hematopoietic compartment. We asked specifically whether lymphocytes and macrophages are among the therapeutic cells in the cell-based therapy in Col4A3 knockout mice. Using specific hematopoietic cell lineage–deficient mice in BM transplant studies in Col4A3 knockout mice, our results indicate that B/T lymphocytes and monocytes/macrophages are not required for the emergence of the missing α3(IV) in the GBM of Col4A3 knockout mice that receive BM transplant (Table 3, supplemental text and Supplemental Figure 4).
Table 3.
Morphometric analyses and renal function tests: BM transplant studies
| Parameter | Urine Albumin-Creatinine Ratio | Morphometric Analyses (%) |
|
|---|---|---|---|
| Normal Glomeruli | Tubular Atrophy | ||
| COL4A3 KO + WT BMT | 21.2 ± 4.5 | 47.5 ± 6.5 | 43.6 ± 1.9 |
| COL4A3 KO | 33.6 ± 3.0 | 32.1 ± 3.8 | 60.5 ± 1.1 |
| COL4A3 KO + Rag-1 KO BMT | 27.4 ± 8.0 | 48.4 ± 7.2 | 32.8 ± 3.3 |
| COL4A3 KO + CD11b KO BMT | 23.9 ± 9.8 | 62.2 ± 3.2 | 32.1 ± 1.9 |
| Statistical significance | No | a | a |
aP < 0.05.
In an effort to expand our understanding of the cell-based therapy in the Col4A3 knockout mouse model and establish specificity, we questioned whether pure cultures of mouse embryonic stem cells (mESCs) could offer a therapeutic benefit when transplanted into Col4A3 knockout mice by providing the missing α3 chain of type IV collagen. Our preliminary data indicated that mESCs can differentiate in vitro into renal progenitor cells expressing podocyte markers, including nephrin and podocin (Supplemental Figure 7N). These in vitro findings prompted our investigation of the therapeutic capacity of mESCs in Col4A3 knockout mice. Our results demonstrated homing of the undifferentiated mESCs to the Col4A3 knockout recipient kidney, unlike the differentiated mESCs, was associated with a statistically significant improvement in renal function and histologic findings and improvement in GBM architecture (see supplemental text and Supplemental Figures 5 through 7). Importantly, the synthesis of the missing α3 chain of type IV collagen after mESC injections resulted in the recovery in the α3α4α5 IV collagen protomer (see supplemental text and Supplemental Figure 6) (Table 4). In addition, our studies using human embryonic stem cells (hESCs) in Col4a3/Rag-1 double-KO mice demonstrate an expression of the missing chain of type IV collagen (Supplementary Text and Supplementary Figure 7) produced by the hESCs and incorporated within the mouse GBM type IV collagen network. These results support previous studies which demonstrate that human α chain of type IV collagen can form chimeric protomers in the Col4A3 knockout mice.30
Table 4.
Morphometric analyses and renal function tests: mESC studies
| Parameter | WT | KO | KO + mESCs | Statistical Significance |
|---|---|---|---|---|
| Urine albumin-creatinine ratio | ||||
| 12 wk | 0.5 ± 0.08 | 12.2 ± 3.7 | 2.2 ± 0.2 | a |
| 21 wk | 1.5 ± 0.8 | 25.8 ± 3.9 | 7.4 ± 2.1 | a |
| BUN | 49.2 ± 3.6 | 316.7 ± 19.9 | 112.7 ± 1.2 | a |
| Morphometric analyses (%) | ||||
| normal glomeruli | 98.9 ± 0.3 | 22.4 ± 4.1 | 44.7 ± 19.9 | a |
| tubular atrophy | 0.1 ± 0.1 | 77.3 ± 5.2 | 23.0 ± 4.0 | a |
| relative interstitial volume | 6.9 ± 0.6 | 33.4 ± 3.2 | 23.9 ± 3.6 | a |
aP < 0.05.
Discussion
A better understanding of the therapeutic potential of BM-derived cells and stem cells in the rescue of the renal phenotype in Col4A3 knockout mice is critical for potential testing in the clinic. A recent research report and an editorial by 22 scientists questioned the rationale of cell-based therapy for Alport syndrome and suggested that more studies are required to validate further the potential of such therapeutic option in preclinical studies in mice.13,15 Here, we oblige with more experiments and mechanistic approaches aimed to address the potential of a cell-based therapy in the Col4A3 knockout mice.
Whereas irradiation alone may offer a small benefit in the recovery of renal function in the Col4A3 knockout mice, a significant benefit in the recovery of renal function is observed only when WT BM cells are provided along with irradiation. In addition, nonirradiated Col4A3 knockout mice that receive multiple infusions of WT BM cells demonstrate significant improvement in renal function and incorporation of the missing chain of type IV collagen in the GBM. Simple blood transfusion and injection of pure cultures of undifferentiated mouse embryonic stem cells into Col4A3 knockout mice also provide significant protection from renal disease and are also associated with incorporation of the missing α3 chain of type IV collagen into the GBM of Col4A3 knockout. These mechanistic studies provide unequivocal evidence that irradiation is not required for realizing the therapeutic benefit of cell-based therapy. Moreover, the use of undifferentiated mESCs demonstrated that stem cells possess the capacity to improve renal function and generate new collagen chains in the Col4A3 knockout mice, whereas the differentiated mESCs or cultured podocytes did not provide such benefit, further highlighting the notion that injury responses can educate undifferentiated stem cells to provide relief to the damaged kidney by repairing the GBM; however, the risk for teratoma formation in mESC-treated Col4A3 knockout mice precludes the development of therapy using these cells. We therefore attempted to better characterize the BM-derived therapeutic cell population in Col4A3 knockout mice that received a transplant. In our BM transplant studies, mature lymphocytes and monocytes seemed to play a limited role in the rescue of the renal pathology in Col4A3 knockout mice. Despite the significant protection of the tubulointerstitial compartment after ablation of mature B and T lymphocytes,16 this improvement did not result in an increase in the lifespan of Col4a3/Rag-1 DKO in comparison with Col4A3 knockout mice. Ablation of B and T cells and monocytes with the WT allele for COL4A3KO in the reconstituted BM did not preclude the expression of the missing chain of type IV collagen in Rag-1KO or CD11bKO BM-transplanted Col4A3 knockout mice. Although our findings indicate that these mature immune cells are not required for the de novo deposition of α3(IV) chain in Col4A3 knockout mouse GBM, the identity of the therapeutic cell population in the BM remains unclear. Our experiments with ESCs, however, suggest that cell plasticity is desirable property for the synthesis of type IV collagen in the GBM in the context of a cell-based therapy in Col4A3 knockout mice. The BM-derived therapeutic cell population may possess features shared by mESCs, namely cell plasticity and totipotency. Indeed, differentiated mESCs did not provide Col4A3 knockout mice with a therapeutic advantage, thus suggesting that preserving the pluripotency and cellular plasticity of mESCs is a prerequisite to successful cell-based therapy in Col4A3 knockout mice. Our experiments, however, lead us to conclude that B and T cells as well as macrophages are dispensable to restoring the renal phenotype in Col4A3 knockout mice, thereby supporting the notion that cell-based therapy is an attractive option for development in the clinic. Keeping in mind the challenges of potential clinical trial designs, we also evaluated the relative efficiency of syngeneic versus nonsyngeneic BM transplant in Col4A3 knockout mice on 129Sv genetic background. In contrast to the analysis of BM transplant therapy in Col4A3 knockout mice on 129Sv genetic background by Katayama et al.,13 our results indicate that BM transplantation significantly improves proteinuria status, in both syngeneic and nonsyngeneic transplant settings. Our results contradict the experiments published by Katayama et al.,13 and such differences could partly be explained by the timing of the therapy and method of BM transplantation. Interestingly, our results demonstrate a relative difference in the renal function recovery between syngeneic and nonsyngeneic BM transplantations, possibly highlighting an inherent modifying effect provided by BM cells from the C57BL/6 donor mice.
Our results demonstrate that a cell carrying the WT allele for the missing α3(IV) chain in Col4A3 knockout mouse recipient can synthesize the missing chain, which subsequently assembles into protomers found in the GBM type IV collagen network. The recovered α3(IV) chain combined with its α4 and α5(IV) binding partners, and the newly assembled α3α4α5(IV) protomer dramatically improves the GBM architecture and reverses the renal phenotype in these mice. Our results suggest that a relatively small amount of the newly synthesized α3(IV) chain in the Col4A3 knockout mouse leads to a significant increase in α5(IV) chain expression in the GBM, and together this change in the chain composition in the GBM type IV collagen network brings about the dramatic improvement of the kidney disease in the Col4A3 knockout mice treated with WT cells, from BM or blood source. A small amount of recovered α3α4α5(IV) protomer in the GBM could dramatically strengthen the structural integrity of the GBM type IV collagen network via the network interactions between α3α4α5(IV), α1α2α1(IV) and α5α6α5(IV) protomers, thus partly accounting for the concurrent increase in α5(IV) expression upon α3(IV) chain-mediated rescue.
Collectively, our studies demonstrated that multiple approaches of providing cells and stem cells to the Col4A3 knockout mice rescue the renal disease in these mice without the need for total body irradiation. The underlying mechanism for this cell-based therapy—cell transdifferentiation or cell fusion—remains unclear, and future studies are required to understand this phenomenon further. In addition, we demonstrated that simple blood transfusion is sufficient to provide therapeutic benefit in Col4A3 knockout mice and enable the production and incorporation of the missing type IV collagen chain. Moreover, it has not escaped our attention that such benefit via simple blood transfusion, if real, can be easily converted into a protocol for therapeutic testing in the clinic. Such efforts are ongoing in the laboratory, and future studies will also address whether simple blood transfusion can also benefit mice with diverse glomerular and tubular kidney diseases.
In conclusion, our work supports the development of a cell-based therapy for patients with Alport syndrome. Currently, therapies that can dramatically change the disease progression in patients with Alport syndrome are lacking. The experiments highlighted in this report offer hope for future potential cell-based therapy for our patients.
Concise Methods
Mice
Generation and renal disease progression in Col4A3 knockout mice were previously described.2,8 The mice were backcrossed (more than 10 generations) into the C57BL/6 background. Col4A3 knockout mice were also maintained in the 129Sv background. Rag-1 KO, CD11bKO, and GFP+ transgenic C57BL/6 mice were purchased from Jackson Laboratory. All animals were housed under standard conditions in the Beth Israel Deaconess Medical Center animal facility. All animal studies were reviewed and approved by the Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. For genotyping and BM genomic DNA PCR, see Supplemental Material and Methods.
BM Transplantation and BM Infusions
BM transplantation of 8-wk-old C57BL/6 Col4A3 knockout mice was performed as described previously.11 BM transplantation of 129Sv Col4A3 knockout mice was performed as follows: Five-week-old mice were sublethally irradiated (total body irradiation, 8 Gy) and received a transplant of 106 unfractionated BM cells harvested from 129Sv or C57BL/6 WT and Col4A3 knockout donors. The 129Sv mice that received a transplant were killed at 10 wk of age, 5 wk after BM transplantation. BM infusions were given to nonirradiated, unconditioned 20-wk-old C57BL/6 Col4A3 knockout mice. Seven consecutive injections, twice weekly, of 107 BM cells from unconditioned Col4A3 knockout and GFP+ mice were given retro-orbitally. BM was harvested from donors as described previously.11 We evaluated a cohort of 20-wk-old Col4A3 knockout littermates, at which point the mice presented with severe renal insufficiency, with one mouse dying from renal failure before treatment began at 19 wk of age. Treatment was completed at 23 wk of age. In the Col4A3 knockout mice treated with Col4A3 knockout BM, another mouse died from renal failure at 23.5 wk of age. All remaining mice were killed at 25 wk of age. For single BM infusion, 8-wk-old C57BL/6 Col4A3 knockout mice were systematically administered an injection of 107 unfractionated BM cells harvested from C57BL/6 WT and Col4A3 knockout donor. The mice were then killed at 20 wk of age, 12 wk after treatment.
For the survival study, 10 unconditioned, nonirradiated 22.5-wk-old C57BL/6 Col4A3 knockout mice were administered an infusion of unfractionated 106 BM cells harvested from C57BL/6 Col4A3 knockout mice or WT mice that were ≤6 wk of age. Additional infusions were given at 23.5 and 24.5 wk.
Parabiotic Pairing and Blood Transfusions
Parabiotic pairing of 5-wk-old C57BL/6 WT and Col4A3 knockout mice was performed as described previously.17 The parabiotic pair was sacrificed 6 wk after pairing. Blood transfusions were performed as follows: Eight-week-old C57BL/6 Col4A3 knockout mice received unfractionated blood from GFP+ donor mice. A total of 200 μl of whole blood was injected retro-orbitally two to three times per week during the course of 3 wk. The recipients were then sacrificed at 12.5 wk of age. Next, 23.5-wk-old C57BL/6 Col4A3 knockout were administered retro-orbitally 200 μl of whole blood harvested from healthy 6-wk-old Col4A3 knockout or GFP+ mice. After the treatment, two to three injections per week during the course of 3 wk, the mice were sacrificed at 25 and 27 wk of age or kept alive to monitor survival.
Human Kidney Tissue
Normal human kidney tissue was obtained from rejected kidney grafts and was provided by Dr. C. Shield, III, under appropriate patient consent and institutional approval.
ESC and Podocyte Cell Culture and Systemic Injection
mESCs cells expressing GFP were a gift from Dr. George Daley (Children's Hospital, Boston, MA). Undifferentiated mESCs were cultured on primary mouse embryonic fibroblast feeder layer (Chemicon International, Temecula, CA) in DMEM (Life Technologies), supplemented with 15% FBS (Life Technologies), 1 M HEPES buffer (Sigma, St. Louis, MO), 100 mM sodium pyruvate (Sigma), 0.12% monothioglycerol (Sigma), and 1000 U/ml recombinant leukemia inhibitory factor (Chemicon). On the day of mESC injection, feeder cells were removed by incubation of the cell suspension twice with PBS for 30 min at 37°C, and single-cell mESCs, free from feeder fibroblasts, were resuspended in PBS for injection into KO mice. For differentiation, feeder cells were removed by incubation of the cell suspension twice with DMEM for 30 min at 37°C. mESCs were resuspended in culture medium lacking leukemia inhibitory factor. To induce embryoid body formation, mESCs were transferred to plastic Petri dishes to allow their aggregation and prevent adherence to the plate. The embryoid bodies were cultured for 5 d and then dissociated with trypsin, and single cells were resuspended in PBS for injection into KO mice. The trypsinized differentiated mESCs were also plated onto a type IV collagen–coated surface (0.1 mg/ml) for immunolabeling.
Human ESCs (hESCs; approved H1 cell line) were purchased from WiCell International Stem Cell Bank (Madison, Wisconsin). Undifferentiated hESCs were propagated on irradiated mouse embryonic fibroblast feeder cell layer in DMEM F12 cell culture medium supplemented with 15% knockout FBS, 2 mM nonessential amino acids, 2 mM l-glutamine, 0.1 mM β-mercaptoethanol, and 10 ng/ml basic fibroblast growth factor. On the day of hESC injection, hESC colonies were dissociated from mouse embryonic fibroblast MEF feeder layer, and single-cell hESCs were resuspended in PBS for injection into COL4A3/Rag-1 DKO mice so as to give 1 to 2 million cells systematically per recipient.
Conditionally immortalized podocytes were a gift from Dr. Peter Mundel (Mt. Sinai School of Medicine, New York, NY) and were cultured as described previously.18 In brief, cells were maintained in RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humid atmosphere with 5% CO2. Under permissive conditions at 33°C and supplemented with 10 U/ml, IFN-γ cells proliferate. Under nonpermissive conditions at 37°C and without IFN-γ, cells differentiate, expressing podocyte-specific markers. For differentiation, cells were passaged and then plated onto type I collagen–coated dishes (0.1 mg/ml) and allowed to differentiate for 12 to 14 d. The cells were trypsinized and resuspended in PBS for injection into KO mice or used for immunolabeling.
Eight- and 13-wk-old Col4A3 knockout mice were anesthetized by isoflurane inhalation and either 106 undifferentiated or differentiated mESCs or podocytes (permissive culture conditions at 33°C and supplemented with γ-IFN) were injected retro-orbitally. Mice were killed at 12.5 and 21.0 wk of age, and tissues were collected for analyses.
RT-PCR
Kidneys were homogenized in TRIzol (Invitrogen) and extracted according to the manufacturer's directions. The purified RNA was digested with DNAseI (Invitrogen; according to the manufacturer's directions), and cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems; according to the manufacturer's directions). The cDNA product was then digested with RNAseH (Invitrogen; according to the manufacturer's directions and using 10× Promega PCR reaction buffer). The following primers (and product size) were used for the RT-PCR: β-Actin 5′-CGTGGGCCGCCCTAGGCACCA-3′ and 5′-TTGGCCTTAGGGTTCAGGGGGG-3′ (200 bp), and COL4A3 5′-AAACGTGCACATGGACAAGA-3′ and 5′-CTCAGAGCCTGCACTTGTGA-3′ (200 bp).
PCR reaction was 40 cycles at 95°C for 30s, 60°C for 30 s, and 72°C for 30 s. The PCR products were migrated by gel electrophoresis using 2% agarose gel in 1× TAE.
Light Microscopy Staining and Morphometric Analyses
Kidneys were harvested and fixed in formalin. Paraffin sections were used for hematoxylin and eosin and Periodic acid-Schiff staining under standard conditions (Histology Core Facility, Beth Israel Deaconess Medical Center, Boston, MA). Morphometric analyses for the histologic assessment of renal injury, here glomerular sclerosis, tubular atrophy, and interstitial volume were performed as described previously.11,16
Immunocytochemistry
Thin frozen sections (4 μm) from kidneys embedded in OCT compound were denatured with 6 M urea/0.1M glycine (pH 3.5) and immunostained against rabbit anti-mouse α3 and rabbit anti-mouse α5 type IV collagen chains (a gift from Dr. Cosgrove, Boys Town National Research Center, Omaha, NE) as described previously.11 FITC-conjugated secondary antibodies (Jackson Immunoresearch) were used at a 1:300 dilution. For labeling for SV40 T-Ag, nephrin, podocin, synaptopodin, CD3, and CD19, thin frozen sections (0.6 μm) were fixed in ice-cold acetone (20 min) and immunostained against podocin, nephrin, and synaptopodin, staining the podocyte (all at 1:200 dilution, gifts from Dr. Peter Mundel, Mount Sinai School of Medicine, New York, NY), CD3 (1:200 dilution; eBioscience), and CD19 (1:250 dilution; Serotec). GFP immunolabeling was performed using the mouse anti-GFP antibody (Abcam; 1:100). TRITC- and FITC-conjugated secondary antibodies (Jackson Immunoresearch) were used at a 1:200 dilution. The slides were mounted with Vectashield Mounting Medium with DAPI (H1200; Vectashield) and glass coverslip and analyzed using the Axioskop 2 fluorescence microscope, AxioCam HRC camera, and Axiovision 4.3 software.
In Situ Hybridization for the Mouse Y-Chromosome (Fluorescence In Situ Hybridization Analysis)
Fluorescence in situ hybridization analysis for the mouse Y-chromosome in kidneys from gender-mismatched blood transfusion and male-derived mESCs injected into female COL4A3 KO recipients, as well as in cultured mESCs, was performed as described previously.11
Urine Albumin, Urine Creatinine and Blood Urea Nitrogen (BUN) Measurements
Urine samples were collected at the indicated times. Creatinine concentration was measured using the colorimetric assay Quantichrome (DICT-500) from BioAssays (Hayward, CA) according the manufacturer's directions, as well as using the colorimetric assay from Oxford Biomedical Research (Oxford, MI), also according to the manufacturer's directions. Albumin concentrations were measured using the Mouse Albuminuria ELISA (Bethyl Laboratory, Montgomery, TX) according to the manufacturer's directions. BUN levels were measured as described previously.11,16
Electron Microscopy
Electron microscopy analyses were performed as described previously.11,16
Immunoprecipitation
Immunoprecipitation was performed as described previously.2 For each immunoprecipitation sample, 100 μg of extracted native NC1 hexamers containing solution in 500 μl of extraction buffer (50 mM TRIS [pH7.5], 150 mM NaCl, and 1% Triton X100) with “Complete” Protease Inhibitor Cocktail (Roche) was incubated with 10 μl of type IV collagen α3 chain antibody or anti-FLAG M2 antibody as a control (Sigma) overnight at 4°C under constant gentle rotation. Protein A/G Plus-Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) were then added and incubated for 2 h at 4°C under constant gentle rotation. The beads were washed five times in 1 ml of extraction buffer, and bound proteins were eluted by boiling in 100 μl of Laemmli buffer. Eluates were analyzed by SDS-PAGE and immunoblotting.
ECM Protein Extraction and Western Blot Analysis of Type IV Collagen Chain Expression
Kidney ECM proteins were prepared as described previously.11 Briefly, kidneys were homogenized in PBS with proteinase inhibitors before DNAse I digestion in 1 M NaCl. The proteins were then incubated in 2% sodium deoxycholate and collagenase digested (Chromatographically purified collagenase, Worthington, NJ). Type IV collagen hexamers were found in the supernatant of the collagenase digest and were precipitated with 95% ethanol. Before Western blotting, the proteins were reduced and denatured in SDS-Laemmli buffer supplemented with 10% β-mercaptoethanol. Such treatment allows for the immunoblotting of NC1 type IV collagen monomers. Western blot analysis was performed as described previously11 using 1:10,000 rabbit anti-mouse α3 or anti-mouse α4 antibodies in 5% milk in TBS-T.
Statistical Analysis
SEs were calculated, and t test and ANOVA were used to determine statistical differences. Bonferroni post hoc test was used after ANOVA. Kaplan-Meier curves were used for survival study, and the log-rank (Mantel-Cox) test was used to determine statistical significance. P < 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
We thank Dr. Keizo Kanasaki for help with statistical analyses. We thank Dr. Dominic Cosgrove (Boys Town National Research Center, Omaha, NE) for help in performing the electron microscopy experiments and providing the electron microscopy analysis for the blood transfusion experiments and mESC-related experiments.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, ”Cell Therapy for Alport Syndrome,“ on pages 2279–2281.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Kashtan CE: Alport syndrome: An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore) 78: 338–360, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R, Cosgrove D: Assembly of type IV collagen: Insights from alpha3(IV) collagen-deficient mice. J Biol Chem 275: 12719–12724, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Miner JH, Sanes JR: Molecular and functional defects in kidneys of mice lacking collagen alpha 3(IV): Implications for Alport syndrome. J Cell Biol 135: 1403–1413, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: Structure, gene organization, and role in human diseases—Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 268: 26033–26036, 1993 [PubMed] [Google Scholar]
- 5.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R: Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3: 422–433, 2003 [DOI] [PubMed] [Google Scholar]
- 7.LeBleu VS, Macdonald B, Kalluri R: Structure and function of basement membranes. Exp Biol Med (Maywood) 232: 1121–1129, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC: Collagen COL4A3 knockout: A mouse model for autosomal Alport syndrome. Genes Dev 10: 2981–2992, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove D, Kalluri R, Miner JH, Segal Y, Borza DB: Choosing a mouse model to study the molecular pathobiology of Alport glomerulonephritis. Kidney Int 71: 615–618, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Kang JS, Wang XP, Miner JH, Morello R, Sado Y, Abrahamson DR, Borza DB: Loss of alpha3/alpha4(IV) collagen from the glomerular basement membrane induces a strain-dependent isoform switch to alpha5alpha6(IV) collagen associated with longer renal survival in Col4a3−/− Alport mice. J Am Soc Nephrol 17: 1962–1969, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT: Bone marrow derived-cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24: 2448–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Katayama K, Kawano M, Naito I, Ishikawa H, Sado Y, Asakawa N, Murata T, Oosugi K, Kiyohara M, Ishikawa E, Ito M, Nomura S: Irradiation prolongs survival of Alport mice. J Am Soc Nephrol 19: 1692–1700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A, Huss R, Akis N, Schlöndorff D, Anders HJ: Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int 70: 121–129, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gross O, Borza DB, Anders HJ, Licht C, Weber M, Segerer S, Torra R, Gubler MC, Heidet L, Harvey S, Cosgrove D, Lees G, Kashtan C, Gregory M, Savige J, Ding J, Thorner P, Abrahamson DR, Antignac C, Tryggvason K, Hudson B, Miner JH: Stem cell therapy for Alport syndrome: The hope beyond the hype. Nephrol Dial Transplant 24: 731–734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBleu V, Sugimoto H, Miller CA, Gattone VH, 2nd, Kalluri R: Lymphocytes are dispensable for glomerulonephritis but required for renal interstitial fibrosis in matrix defect induced Alport renal disease. Lab Invest 88: 284–292, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Soutter AD, Ellenbogen J, Folkman J: Splenosis is regulated by a circulating factor. J Pediatr Surg 29: 1076–1079, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]