Abstract
p53 is best known as a tumor suppressor that regulates cell-cycle, differentiation, and apoptosis pathways, but its potential role in embryonic development and organogenesis remains controversial. Here, p53−/− embryos bred on C57Bl6 background exhibited a spectrum of congenital abnormalities of the kidney and urinary tract, including ureteric bud (UB) ectopia, double ureters/collecting systems, delayed primary branching of the UB, and hypoplastic metanephroi. We observed ectopic UB outgrowth from the Wolffian duct (WD) in one third of p53−/− embryos. The prevalence of duplex was higher in embryos than in neonates, and ex vivo organ culture suggested that ectopic ureters can regress over time, leaving behind a dysplastic pole (“segmental dysgenesis”). Transgenic expression of dominant negative p53 or conditional inactivation of p53 in the UB but not in the metanephric mesenchyme lineage recapitulated the duplex phenotype. Mechanistically, p53 inactivation in the WD associated with enhanced sensitivity to glial cell line–derived neurotrophic factor (GDNF)-induced ectopic budding and potentiated phosphatidylinositol-3 kinase activation by GDNF in UB cells. Unlike several other models of UB ectopia, hypersensitivity of p53−/− WD to GDNF is not accompanied by reduced Sprouty-1 or anterior expansion of the GDNF domain. In summary, our data lend support for a restrictive role for p53 activity in UB outgrowth from the WD.
Organogenesis is dependent on a multitude of growth factors, signaling molecules, and transcription factors that temporally and spatially define a developmental program. Metanephric development is dependent on formation of the Wolffian duct (WD) and the adjacent metanephric mesenchyme (MM) from the intermediate mesoderm. In mice, the ureteric bud (UB) develops at embryonic day 10.5 (E10.5) from the caudal end of the WD. Inductive interactions between the UB and the MM ensure cell survival and subsequent onset of metanephrogenesis. Emergence of the UB from a specific site in the WD adjacent to the MM is controlled by the glial cell line–derived neurotrophic factor (GDNF)-cRet signaling pathway.1–6 GDNF is a growth factor that is secreted by the MM and binds to its receptor c-Ret and co-receptor GFRα1 that are expressed along the WD and UB tips.
Stimulation of the GDNF-cRet pathway results in cell proliferation and chemotaxis.7,8 After the initial broad distribution of the GDNF field along the posterior half of the WD, the GDNF expression domain is progressively restricted to the caudal end of the WD in the immediate vicinity of the site of UB outgrowth9–11; however, the potential for bud emergence remains along the length of the WD, which continues to express the c-Ret/GFRα1 receptor/co-receptor. Evidence from mutant mouse models and metanephric organ culture implicates impaired restriction of the GDNF field and the GDNF/c-Ret signaling pathway in ureter duplication. Whereas loss of any component of this pathway results in renal agenesis, ectopic GDNF expression induces appearance of supernumerary buds, resulting in multilobed kidneys sometimes with additional ureters6,12; therefore, inhibitors of GDNF/c-Ret expression or activity are necessary to restrict UB emergence to a specific site on the WD. Available data support two mechanistic scenarios on how ectopic c-Ret/GFRα1 activation might occur: (1) Anterior expansion of the GDNF field (i.e., anterior to the specified metanephric anlagen) as a result of loss of a GDNF repressor, such as Foxc1 or Slit2/Robo213,14; and (2) increased responsiveness of the duct to GDNF from loss of antagonistic signals, such as Sprouty1 in the WD,15 or around the duct in the periureteral mesenchyme, such as Bmp4 or Agtr2.16,17 Both models are accompanied by ectopic GDNF-Ret activity. Thus, there is clear evidence of control at multiple levels to ensure normal metanephric development.
The tumor suppressor functions of p53 have been well characterized. Under stress stimulation, p53 transcriptionally regulates genes that control cell-cycle arrest, apoptosis, and DNA repair. All of these processes ensure elimination of genetically damaged cells from being propagated. In addition, p53 plays a role in promoting cell differentiation.18 Less well studied are its activation and role in embryonic development and nonstressed cells. p53-null mice are viable and show developmental abnormalities with incomplete penetrance on certain genetic backgrounds.19–25 This is in contrast to embryonic lethality observed in p53-depleted Xenopus embryos.26–28 Compensation by p53 family members p63 and p73 that are present in early mouse embryo has been suggested as a possible reason for the nonlethal phenotype.29
Previous work from our laboratory has described the role of p53 in regulation of renal function genes in terminally differentiating renal epithelia.30 In this study, we found that loss of p53 also affects early events of kidney development. Metanephroi from conventional p53−/− mice exhibit ectopic UBs and duplex kidneys. Conditional deletion of p53, designed to eliminate p53 from either the WD or MM lineage, revealed the duplex ureter/kidney is phenocopied when p53 is inactivated/absent in the WD.
Results
Developmental Pattern of p53 Gene Expression during Kidney Organogenesis
We analyzed p53 mRNA expression during metanephric development by in situ hybridization. p53 expression is ubiquitous in E10.5 mouse embryo, including the WD and surrounding mesenchyme (data not shown). At E11.5, p53 is abundantly expressed in the mesonephric duct and tubules (Figure 1, A and B). p53 mRNA expression is higher in the main UB, at the first branch (T) stage, and subsequently in the trunks and tips of UB branches than the surrounding condensed MM and stroma (Figure 1, C through E). After E13.5, p53 was expressed in nephron progenitors (Figure 1, E and G). By middle to late nephric development, at E16.5, p53 is highly expressed in the differentiating collecting ducts and nephrons (Figure 1, G and H). At postnatal day 1 (PN1), renal vesicles and comma- and S-shaped structures also express p53 mRNA, albeit at lower levels than collecting ducts.
Figure 1.
p53 gene expression is shown during metanephric development. p53 mRNA is expressed in the WD, the mesonephric tubules (Mes), and the surrounding mesenchyme (whole-mount ISH [A; section ISH [B]). (A through E, G, and H) Expression is higher in the duct and UB derivatives (black arrows) than in the mesenchyme. p53 mRNA is expressed in nephron precursors (red arrows) from the time they appear at E13.5 (E), at E16.5 (G) and PN1 (H). (F) p53 expression is developmentally regulated in the kidney. Q-PCR from metanephroi of various embryonic ages shows approximately four-fold decline in p53 mRNA expression from E15.5 to adulthood. p53 expression normalized to β-actin expression.
Quantitatively, p53 expression in the kidney is developmentally regulated through embryonic growth and declines significantly from PN20 into adulthood, after cessation of postnatal nephric development (Figure 1F). We previously showed that nuclear p53 protein levels are higher in newborn mouse and rat kidneys than in adult by Western blot analysis, electrophoretic mobility shift assay, and chromatin immunoprecipitation.30,31
p53 Null Mice Exhibit a Spectrum of Congenital Abnormalities of the Kidney and Urinary Tract
Double Ureters/Collecting System (Duplex).
In a previous study, we reported that p53−/− pups have a high prevalence of renal abnormalities, including persistent and ectopic proliferation, tubular dysgenesis, mislocalization of renal function genes, dysmorphic papillae, and hydronephrosis.30,32 Embryonic analysis of these mice revealed double ureters in one third (three of nine) of p53−/− embryos and in nearly 17% (eight of 45) of heterozygous embryos, as compared with 0% (zero of 21) in wild-type (WT; Table 1; Figure 2). Figure 2D illustrates an example of p53−/− metanephroi that exhibit an ectopic bud invading a bi-lobed MM. Invasion of the abnormal MM by the ectopic ureter occurred anterior relative to the primary UB. During the culture period of 88 h, the distinct point of emergence of each UB from the WD disappeared and the two ureters in Figure 2F seemed to have fused at the base and resemble a ureter bifurcation. Interestingly, the duplex UB observed in Figure 2G was no longer apparent at 88 h (Figure 2, G′ and G″). Although it is possible this is a culture artifact, this apparent “self-correction” may account for the higher incidence of duplexes observed in embryonic as compared with postnatal kidneys. Newborn p53−/− kidneys have an atypical extension of the nephrogenic zone deep in the tissue, demarcating the lobes of the duplex kidney (Figure 2, H through H″). Ureters in p53-knockout embryos seem shorter than those in WT littermates (compare Figure 2, A and D).
Table 1.
Spectrum of anomalies in p53-deficient mice
| Parameter | Conventional |
Hoxb7-Cre/p53loxP |
Pax3-Cre/p53loxP |
Hoxb7-p53DN |
||||
|---|---|---|---|---|---|---|---|---|
| −/− | +/+ | Cre+ | Cre− | Cre+ | Cre− | Tg+ | Tg− | |
| Hypoplasiaa | 7/7 (100%)c | 0/10 | 8/11 (73%) | 3/17 (18%) | ND | ND | 0/24 | 0/13 |
| Duplex | 3/9 (33%) | 0/21 | 4/86 (5%) | 0/52 | 0/74 | 0/62 | 15/44 (34%) | 0/23 |
| Dysmorphic papilla/medulla defectb | 24/53 (45%)d | 0/8 | ND | ND | ND | ND | 12/44 (27%) | ND |
ND, not determined.
aBased on UB counts of cytokeratin-stained whole mounts.
bPapilla/medulla defect in PN1 through 3 sections.
cFour litters, 51 to 87% of WT.
dIncludes −/− and +/− embryos, because −/− alone have duplexes and/or hydronephrosis.
Figure 2.
Duplex kidney phenotype in conventional p53-null mice and delayed primary branch formation in p53−/− metanephroi are shown. (A through F′) Growth and branching patterns of p53-null metanephroi ex vivo are examined. Metanephroi from p53+/+ and p53−/− littermates were cultured on transwell filters for 88 h, then stained with anti-cytokeratin antibody. At 0 h, the p53+/+ metanephroi (A and A′) show the T-shaped UB characteristic at E11.5. The UB in p53−/− metanephroi has not yet branched (D and D′) at E11.5, resulting in fewer UB tips at 88h (C and C′ versus F and F′). (D) White arrows point to two UBs emerging at distinct points from the WD and invading the MM (black arrow). At 40 h (E), the two points of UB emergence seem to have merged into one, thus giving the appearance of a bifurcated ureter (E and F). Larger condensates in p53−/− kidneys (black arrowheads, E and E′) than in p53+/+ littermates (B and B′). Blind ectopic UBs (E and F, yellow arrowheads) are clearly visible at 40 and 88 h. (G through G″) Regression of an ectopic ureter over time (0 versus 88 h, G and G′) results in a single ureter with an abnormal lobulated kidney. (H) Periodic acid-Schiff–stained p53−/− PN1 kidney section is shown. Red arrows point to the duplex lobes that are separated by a deep nephrogenic zone (boxed [H]; high magnification [H′]). (I) Section from a heterozygous kidney shows WT morphology.
Several mouse models with supernumerary UB phenotype have been described. Loss of Foxc1/c2 and Slit2/Robo2 resulted in supernumerary phenotype attributed to an expanded GDNF domain.13,14 Sprouty1 and Bmp4 deletion also generated this phenotype but as a result of aberrant Ret activity.15,17 To determine whether ectopic UB phenotype in p53-null animals is due to anomalies in the GDNF-Ret pathway, we next examined gdnf, c-ret, and spry1 expression by in situ hybridization (ISH) in E10.5 p53-null embryos. We did not find an extended GDNF domain (Figure 3A) or altered c-Ret or Sprouty1 expression that might explain the duplex ureter/kidney phenotype (Figure 3, B and C).
Figure 3.
GDNF, c-Ret, and Spry1 mRNA levels and domains are unchanged in p53−/− embryos. E10.5 litters from p53+/− pairings were dissected and used for in situ hybridization. AP, anterior-posterior; h, hind-limb bud.
Delayed Primary Branch Formation.
To evaluate the growth of UB and MM in the absence of p53 over time, metanephroi from E11.5 p53+/+, p53+/−, and p53−/− embryos were grown in vitro up to 88 h. At 0 h, UB in p53+/+ metanephroi have branched once (T-stage), as expected in E11.5 kidneys (Figure 2, A and A′). In contrast, UB in p53−/− metanephroi lag in the first T formation (Figure 2, D and D′). Delayed entry into T-stage formation could explain, at least partially, the hypoplastic metanephroi observed in p53−/− embryos (Figure 2, C and C′, versus F and F′). In situ hybridization for Spry1 shows branch and tip formation are lagging in the p53−/− kidney, in comparison with the age-matched p53+/+ littermate metanephros (Figure 4, A and A′). In addition, p53-null kidneys exhibit decreased proliferation in both the UB and MM lineages (Figure 4B). UB tip numbers were consistently lower in p53−/− than p53+/+ metanephroi in multiple litters (Figure 4C), with the mutant metanephroi showing 30% fewer tips than in the WT (Figure 4D). The spectrum of anomalies observed in p53-deficient mice are listed in Table 1.
Figure 4.
Hypoplasia in p53−/− kidneys is shown. (A) In situ hybridization for Spry1 was done on E12.5 metanephroi. Branch and tip formation are lagging in the p53−/− metanephros in comparison with the age-appropriate branching observed in a p53+/+ littermate. There was no change in Spry1 mRNA levels. (B) Decreased proliferation in p53-null kidneys was visualized by phospho-H3 antibody staining compared with kidney section from WT littermate. (C) UB tip numbers were counted in four litters from p53+/− pairings. (D) The number of tips was consistently lower in p53−/− (yellow bar) versus p53+/+ metanephroi (purple bar), with the null metanephroi showing 30% fewer tips than the WT.
Conditional Inactivation of p53 from the Nephric Duct Lineage Induces Ectopic Bud Emergence
To distinguish whether the double collecting system is a result of loss of p53 from the WD or MM, we conditionally deleted p53 either from the WD/UB (UBΔp53) or the mesenchyme (MMΔp53) by Cre-mediated recombination. To generate UBΔp53, we crossed p53loxp/loxp mice with mice expressing Cre-recombinase under the control of a 1341-bp fragment of the Ksp-cadherin promoter or the Hoxb7 promoter. UBΔp53 mice phenocopied the duplex observed in p53−/− mice (Figure 5, A and B). Incidence of duplex ureters/kidneys was six of (5%) 116 and four (5%) of 86 for Ksp-Cre– and Hoxb7-Cre–mediated deletion, respectively. In contrast, conditional deletion of p53 from the MM using Pax3-Cre+;p53loxP mice (MMΔp53) did not result in duplex ureter formation (zero of 74), indicating that loss of p53 from the MM is not sufficient to produce the duplex renal phenotype. Pax3-driven Cre expression was localized to the nephrogenic cord (E9.5) and to the MM adjacent to the nephric duct and UB at E10.5, but no expression was detected in the nephric duct.33
Figure 5.
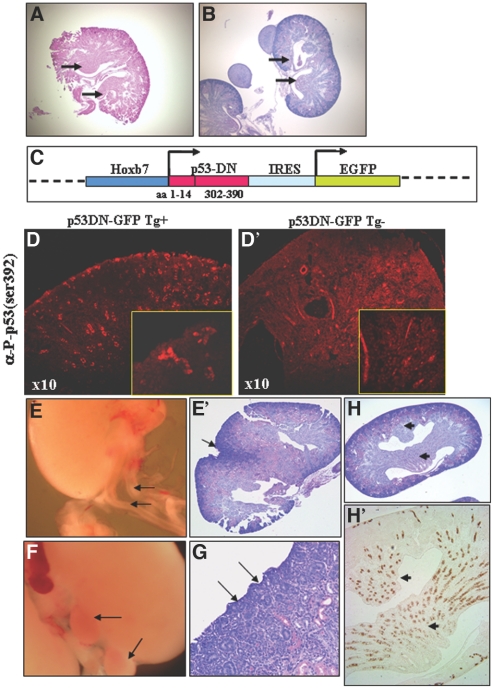
WD/UBΔp53 mice have kidneys with double papillae. (A, B, and D through H) p53 was conditionally deleted in the WD/UB by crossing Ksp-cadherin–driven (A) or Hoxb7-driven (B) Cre transgenic mice to p53flox mice or by expressing p53DN driven by Hoxb7 promoter (D through H). (C) Hoxb7-p53DN construct. p53 cDNA encoding a miniprotein (amino acids 1 through 14 and 302 through 390) was inserted into a Hoxb7-IRES-EGFP construct to drive expression of the mutant protein (p53DN) in WD lineage. (D and D′) Immunofluorescence staining with anti–p53-P-ser392 is shown. Section from a transgenic mouse kidney expressing p53DN shows intense staining in UB trunks and branches (D periphery, and inset) and deeper collecting ducts. Section from a nontransgenic mouse kidney shows much lower intensity of staining, representing detection of endogenous levels of p53-P-ser392 (D′, magnified inset). (E) Transgenic mice expressing this miniprotein exhibit duplex ureters. (E′) Periodic acid-Schiff staining of a section reveals an internalized nephrogenic zone (arrow).)H and H′) The double papilla (H, black arrows) stains positively for AQP2 (H′). (F and G) Hoxb7-p53DN gain-of-function phenotype: Extrarenal nodules (F) and pitted surface (G).
Because Cre-mediated recombination is reportedly chimeric, we used Hoxb7 promoter–driven expression of dominant negative p53 (p53DN) as an additional method of inhibiting p53 activity in the WD/UB. p53DN contains the oligomerization domain and lacks the DNA-binding domain.34 This miniprotein forms stable mixed multimers with endogenous WT p53, thereby inhibiting its functional interaction with DNA. Kidneys from newborn pups (PN1 through 3) from 10 founder lines were initially examined. Subsequent analyses were performed on lines from two founders that expressed the transgene in the collecting ducts (Figure 5). Kidneys from transgenic mice exhibit increased distribution of p53 staining in UBs and collecting duct epithelia (Figure 5, D and D inset), in contrast to basal levels exhibited in the nontransgenic kidney. Because the transgene construct also expresses green fluorescence protein (GFP) driven by the Hoxb7 promoter, serial tissue sections were stained for GFP, p53, and lectin Dolichos biflorus demonstrating coexpression in collecting ducts (Supplemental Figure 1). Transgene expression is maintained in differentiating collecting ducts, which stain positively for aquaporin 2 (AQP2; data not shown). As expected, tissue sections from nontransgenic mice were negative for GFP (Supplemental Figure 1).
Duplex ureters and multilobed kidneys occurred at a frequency of approximately 35% in Tg+ Hoxb7-p53DN mice (Figure 5E; Table 1). In addition, kidneys from these animals showed enlarged or duplex papillae (Figure 5H), ectopic extension of the nephrogenic zone, renal nodules, and pitting of the surface as a result of patterning defects (Figure 5, E′, F, and G). The double papilla stains positively for AQP2 (Figure 5H′). Collectively, our data demonstrate that the loss of p53 function in the WD/UB causes ureter duplication.
Hypersensitivity of p53-Deficient WD to GDNF
Occurrence of duplex collecting system in UBΔp53 but not in MMΔp53 suggested a WD/UB autonomous defect. We therefore hypothesized that varying p53 activity in the WD alters the sensitivity of WD to exogenous GDNF-induced budding in vitro. E11.5 WD respond to high-dosage GDNF (50 to 200 ng/ml) by growing multiple c-Ret+ buds along the duct, as demonstrated in Figure 6, B and C.12 In comparison, a low dosage of GDNF (10 ng/ml) does not elicit bud formation in WT WD (Figure 6A). p53−/− and Hoxb7–p53DN+ WD, however, grow supernumerary buds in response to low-dosage GDNF treatment (Figure 6, D′ and E′). This response was also observed upon pharmacologic inhibition of p53 transcriptional activity in WT WD with the p53 inhibitor pifithrin-α (PFTα) in the presence of low-dosage GDNF (Figure 6F′). Conversely, treatment with Nutlin-3a, which inhibits Mdm2–p53 interaction and thus p53 degradation, prevented ectopic bud formation from the WD even at high (50 to 100 ng/ml) dosages of GDNF (Figure 6, G through I).
Figure 6.
p53-deficient nephric ducts exhibit increased sensitivity to GDNF. (A through C) WT nephric ducts grow ectopic c-Ret+ UBs in response to exposure to high dosage (>50 ng/ml) GDNF (B and C). Exposure to subthreshold dosage (10 ng/ml) does not elicit bud growth (A). (D through E′) Subthreshold dosage exposure with GDNF of p53-deficient ducts induces multiple ectopic bud formation in mutant (D′ and E′) ducts but not in ducts from WT littermates (D and E, respectively). (F and F′) WT ducts treated with p53-inhibitor Pifithrin also respond to 10-ng/ml dose of GDNF with ectopic buds (F′); the contralateral DMSO-treated duct does not (F). (G through I) p53 stabilization by treatment with 5μM (H) or 10 μM (I) Nutlin3a renders the duct refractory to 50 ng/ml GDNF, whereas DMSO-treated duct responds appropriately (G).
Because bud emergence is reportedly preceded by a spurt of cell proliferation35 and because p53 regulates cell proliferation, we examined proliferation in p53-null ducts. Because bud formation is visible by 48 h of GDNF treatment, we assayed for proliferation 24 h after treatment, before when buds are usually visible. Phospho-histone 3 (P-H3-S10) staining did not show increased proliferation in p53-null nephric ducts on comparison to WT ducts (Supplemental Figure 2), suggesting that ectopic bud formation in p53-null ducts is not a proliferation defect. We may have missed differences in proliferation as a result of the time point that was assayed, or these differences may be subtle. Nevertheless, our data demonstrate that p53 activity in the WD is required and tightly regulated to allow normal UB outgrowth.
p53 Loss Potentiates Phosphatidylinositol-3 Kinase Activation by GDNF in UB Cells
To explore the mechanism for increased sensitivity of p53-null ducts to GDNF, we used a cell-culture system. UB cells were transfected with p53 small hairpin RNA (shRNA) plasmid or small interfering RNA (siRNA) and treated with GDNF/GFRa1 before harvest. Loss of p53 resulted in dramatic upregulation of phospho-Akt, measured as readout of phosphatidylinositol-3 kinase (PI3K) activity (Figure 7A), without any change in total Akt levels. Upregulation in p-Akt levels was dependent on the extent of p53 knockdown. We observed a variable two- to 10-fold decrease in p53 protein levels and corresponding two- to six-fold increase in p-Akt in comparison with negative control samples that were transfected with a scrambled siRNA. Next we measured phosphatase and tensin homolog (PTEN) levels, because PTEN is positively regulated by p5336 and inhibits P-Akt production via phosphatidylinositol 3, 4, 5-triphosphate. Surprisingly, PTEN levels did not decrease with p53 knockdown. Phospho-extracellular signal–regulated kinase 1/2 levels were not measured in this study. Protein levels were normalized to β-actin or total Akt. These data provide biochemical evidence that p53 antagonizes the GDNF→c-Ret→PI3K pathway.
Figure 7.
(A) p53 knockdown by shRNA potentiates PI3K activation by GDNF in UB cells. UB cells were transfected with GFP, scrambled, or p53 shRNA plasmids for 72 h. Transfected cells were treated with GDNF+GFRα1 20 h before harvesting for Western blot analyses. Decrease in p53 levels is accompanied by increase in P-Akt, without an increase in total Akt. PTEN levels did not decrease with p53 knockdown. Fold differences in levels are normalized for total Akt. (B) Model of p53 function in WD is shown. p53 opposes pathways activated by growth stimuli, such as GDNF→cRet→PI3K. Antagonism may occur at more that one site along the pathway; p53 regulates expression of genes such as PTEN that antagonize PI3K function,36 or p21/Cip1 and FAK, inhibitors of proliferation and cell migration, respectively.62,63 p53 negatively regulates expression of PI3KCA, the catalytic subunit of PI3K.64
Discussion
In this study, we provide evidence that p53 function is required for early events of kidney development. p53 activation in response to genotoxic stressors is well documented.37,38 Initial reports on p53-null mice did not accredit p53 with a developmental role22; however, subsequent analyses of the mouse models showed genetic background–dependent anomalies,19 including female exencephaly,25 craniofacial and ocular abnormalities,19,23 embryonic lethality, and defects in spermatogenesis.39 We found that loss of embryonic p53 caused maldevelopment of the kidney and urinary tract in mice on a C57BL6 background.
We previously described a role for p53 in regulating expression of renal function genes in terminally differentiating renal epithelium.30 Observation of duplex renal papillae in PN1 kidneys from p53-null neonates prompted us to analyze the effects of p53 deficiency on early kidney development. Up to one third of p53−/− embryos on C57Bl6 background exhibit a unilateral duplex phenotype. Whereas conditional UBΔp53 mice exhibit the duplex ureter/kidney phenotype, the incidence of duplex ureters is four- to six-fold lower than that observed in conventional p53−/− mice. Lower penetrance of the duplex in conditional versus conventional p53-null mice may be an effect of different genetic backgrounds. Alternatively, the level of p53 knockdown achieved with Cre-mediated recombination may not be robust or is compensated by family members p73/p63. To address this possibility, we overexpressed a p53DN miniprotein34 in the WD under the control of the Hoxb7 promoter (UBp53DN). One third of UBp53DN mice exhibit duplex ureters/kidneys, as observed in conventional p53-null embryos. p53DN forms stable mixed multimers with endogenous WT p53, thereby inhibiting its functional interaction with DNA and effectively blocking WTp53 function to levels that may not have been achieved in the Cre-recombination models. Alternatively, p53DN may have a gain-of-function effect that allows duplex formation by a p53-independent mechanism (e.g., by antagonizing p73/p63 activity). The latter effect may explain the extrarenal nodules and pitting of the kidney surface as a result of patterning defects observed in UBp53DN mice.
Several current models of supernumerary budding cite aberrant expression or signaling along the GDNF-cRet-Spry1 pathway as the primary defect leading to ectopic budding13,14,17,40; however, there are other genetic models, such as conditional β-cateninWD and Lim1 mice,41,42 that do not. In our model of ectopic budding, we did not observe an expanded GDNF domain or changes in c-Ret or Sprouty1 expression. Unlike the β-catenin and lim-1 models, we found p53 deficiency in the WD/UB lineage does confer enhanced sensitivity to GDNF-induced budding but not via increased proliferation. Differential cell adhesion has been postulated as a cause of ectopic bud formation.41,42 p53 is a known regulator of adhesion molecule expression such as E-cadherin,43 as well as of adhesion-associated molecules such as Perp.44,45 Comparing adhesion and migration differences in cells from p53-mutant ducts, although challenging, will offer much insight into mechanisms of UB outgrowth.
How might increased sensitivity of the WD to low-dosage GDNF occur in the absence of functional p53? Our data are consistent with a model in which p53 deficiency or inactivation releases inhibitory input on cell adherence/migratory/proliferative signaling mediated by the Ret tyrosine kinase receptor in the WD (Figure 7); this in turn results in emergence of accessory UBs and abnormal branching patterns. Even though we did not find decreased PTEN levels with p53 knockdown that could explain increased P-Akt levels, it should be noted that PTEN activity is regulated by posttranslational modifications and may be the case in this system. Another possible mechanism involves variability in heparin sulfate proteoglycans levels that would alter activity of signaling proteins such as GDNF, fibroblast growth factors, bone morphogenic proteins, and WNTs.46–49 p53 modulates expression of protein regulators of extracellular heparin sulfate, such as heparanase and extracellular heparin sulfate 6-O-endosulfatases.50
Transgenic mice overexpressing WT p53 in the kidney exhibit differentiation defects in the UB.51 A consequence of abnormal signaling to the MM by the UB is increased mesenchymal apoptosis and decreased mesenchyme to epithelial transition, resulting in hypoplastic kidneys. This is probably the case in p53-null kidneys as well, which are also hypoplastic. This phenotype was surprising, considering the p53-null nephric duct has ectopic UB outgrowth; however, as stated already, ectopic UB formation is not a consequence of increased proliferation. p53-null metanephroi at E11.5 show delayed T formation that may contribute to a decreased number of UB tips. In addition, p53-null kidneys exhibit decreased proliferation that would also impair UB morphogenesis.
Future studies will define additional mechanisms of p53 function in metanephric development, which in its absence results in WD hypersensitivity to growth stimulation but growth inhibition in the mesenchyme.
Concise Methods
Mice
Conventional p53−/− mice (trp53tm1Tyj) on C57Bl6 background were purchased from the Jackson Laboratory, and heterozygotes were used as breeder pairs. A neomycin cassette replaces exons 2 through 6 and eliminates p53 mRNA synthesis.52 Conditional lineage-specific p53 deletion was achieved by crossing p53LoxP/LoxP mice (gift from NCI/generated by Anton Berns),53 with nephric lineage-specific Cre expressers Ksp-cadherin-Cre (gift from Peter Igarashi),54,55 Hoxb7-Cre (gift from Carlton Bates),56 and Pax3-Cre (gift from Feng Chen and Jonathan Epstein).57,58 Transgenic Hoxb7-p53DN-IRES-GFP mice with targeted expression of p53DN in the WD/UB-derived structures (Hoxb7-p53DN) were generated at the Tulane Transgenic Mouse Facility. p53DN encodes a miniprotein containing amino acids 1 through 14 fused to the last 89 residues of murine WT p53. The p53 miniprotein cDNA construct was a gift from M. Oren.34 The 309-bp cDNA was amplified with forward and reverse primers containing KpnI restriction sites at the 5′ overhang. The PCR product was digested with KpnI and subcloned into KpnI-digested Hoxb7-IRES-EGFP plasmid (gift from Carlton Bates).12 Analysis was done on progeny from 10 founders. All animal experiments were approved by the Tulane University Institutional Animal Care Committee.
Organ Culture
E11.5 WDs or E12.5 metanephroi were cultured on Transwell filters in DMEM/F12 medium with 10% FCS at 37°C/5% CO2. For branching morphogenesis studies, metanephroi were cultured for 48 to 72 h and stained, and tips/branch points were counted. For GDNF sensitivity assay, WDs from p53+/+ and p53−/− mice were treated with 10 ng/ml GDNF for 72 to 96 h. In addition, WDs from WT CD1 mice were treated with low-dosage GDNF (10 ng/ml; R&D Systems) in the presence of p53 inhibitor PFTα (Sigma) or DMSO. To stabilize p53 levels, Nutlin-3, a small-molecule inhibitor of MDM2 (10 μM; Cayman Chemicals), was added to the medium with high-dosage GDNF (50 to 100 ng/ml). Media containing GDNF and PFTα/Nutlin-3/DMSO were replaced every 24 h.
Immunofluorescence Staining
Whole Mount.
Metanephric explants were fixed in ice-cold methanol for 10 min, washed twice in PBS/0.1% Tween for 30 min each, and incubated with primary antibody overnight at 4°C. Monoclonal anti-pancytokeratin antibody (Sigma; 1:200), and Lotus Tetragonolobus agglutinin (Vector Laboratories; 1:100) were used to stain collecting ducts and proximal tubules, respectively. A polyclonal anti-Pax2 antibody (1:200; Zymed) was used to stain the MM and UB in urogenital blocks. After multiple washes in PBS/Tween, explants were incubated with Alexa-fluor–tagged secondary antibody overnight at 4°C, washed, and mounted on a slide with fluorescence mounting medium (Dako). Images of stained tissue were captured using an Olympus BX51 fluorescence microscope.
Sections.
Metanephroi or E11.5 embryos were fixed in 10% formalin and processed for paraffin embedding and sectioning. Immunostaining was done as described previously.31 Antibodies for the following proteins were used: p53-P-Ser392 (Cell Signaling; 1:50), GFP (Invitrogen), Dolichos biflorus agglutinin (Sigma; 1:40), and AQP2 (Santa Cruz Biotechnology; 1:400).
In Situ Hybridization
Section and whole-mount ISH were performed as described at http://www.hhmi.ucla.edu/derobertis. E10.5 to E11.5 mouse embryos or E12.5 kidneys were fixed overnight in 4% formaldehyde in PBS, then dehydrated in methanol and stored at −20°C until use. Digoxigenin-dUTP–labeled RNA probes were used at 0.5 μg/ml. Alkaline phosphatase–conjugated anti-digoxigenin Fab fragments were used at 1:5000. Color reactions were carried out from overnight (c-Ret, p53, and Spry1) to several days (GDNF). Embryos were photographed in glycerol. The following probes were used for whole-mount ISH: p53 (clone ID 1349046, antisenseT3/senseT7; Invitrogen Clone Collections), c-Ret,59 GDNF,60 and Sprouty1.15
Reverse Transcriptase–PCR
Quantitative reverse transcriptase–PCR was performed on RNA from embryonic kidneys. Real-time primers for p53 were ordered from Applied Biosystems (Taqman Gene Expression Assay, ID Mm01731287_m1). β-Actin primers and probe sequences are as follows: Forward 5′-ACGGCCAGGTCATCACTATTG; reverse 5′-CAAGAAGGAAGGCTGGAAAAG; and probe [5HEX]-CAACGAGCGGTTCCGATGCCC.
Western Blotting
UB cells were transfected with p53-shRNA plasmid (SuperArray Biosciences) or siRNA (ID s75472 and s75474; Applied Biosystems) for 72 h. Cy3-labeled negative control 1 siRNA (AM4621; Applied Biosystems) or scrambled negative control plasmid (SuperArray) was used as control. Twenty hours before harvesting proteins, cells were treated with recombinant GDNF 50 ng/ml and GFRα1 100 ng/ml (R&D Systems) as described previously.61 Proteins were harvested in RIPA buffer supplemented with protease and phosphates inhibitors, resolved on 4 to 20% SDS-PAGE, and transferred to nitrocellulose membrane for immunoblotting with the following antibodies: p53 (SC6243; Santa Cruz Biotechnology), P-473-Akt and Akt (Cell Signaling), and PTEN (Cell Signaling).
Disclosures
None.
Acknowledgments
This work was supported by a Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology and Centers of Biomedical Research Excellence 1P20 RR-017659 awarded to Z.S. S.S.E.-D. is supported by National Institutes of Health grants DK56264 and DK62250.
We are grateful Jake Fontenot for excellent technical assistance. We thank Dr. Oliver Wessely and the Tulane Developmental Nephrology Group for insightful discussions and Uyen Tran-Wessely for help with p53 section in situ. We also thank Dr. Peter Igarashi for the Ksp-cadherin-Cre mice, Dr. Carlton Bates for the Hoxb7-Cre mice and the Hoxb7-IRES-GFP construct, Dr. Feng Chen for the Pax3-Cre mice, and Dr. Moshe Oren for the p53DN miniprotein cDNA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Costantini F, Shakya R: GDNF/Ret signaling and the development of the kidney. Bioessays 28: 117–127, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Davies JA: Morphogenesis of the metanephric kidney. ScientificWorldJournal 2: 1937–1950, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Towers PR, Woolf AS, Hardman P: Glial cell line-derived neurotrophic factor stimulates ureteric bud outgrowth and enhances survival of ureteric bud cells in vitro. Exp Nephrol 6: 337–351, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Herzlinger D: Inductive interactions during kidney development. Semin Nephrol 15: 255–262, 1995 [PubMed] [Google Scholar]
- 6.Shakya R, Jho EH, Kotka P, Wu Z, Kholodilov N, Burke R, D'Agati V, Costantini F: The role of GDNF in patterning the excretory system. Dev Biol 283: 70–84, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Tang MJ, Worley D, Sanicola M, Dressler GR: The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration and chemoattraction of epithelial cells. J Cell Biol 142: 1337–1345, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D, Dressler GR: PTEN modulates GDNF/RET mediated chemotaxis and branching morphogenesis in the developing kidney. Dev Biol 307: 290–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellmich HL, Kos L, Cho ES, Mahon KA, Zimmer A: Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech Dev 54: 95–105, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A: Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76–79, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Pepicelli CV, Kispert A, Rowitch DH, McMahon AP: GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol 192: 193–198, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Maeshima A, Vaughn DA, Choi Y, Nigam SK: Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct. Dev Biol 295: 473–485, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR: SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 6: 709–917, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kume T, Deng K, Hogan BL: Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127: 1387–1395, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD: Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 8: 229–239, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hohenfellner K, Hunley TE, Schloemer C, Brenner W, Yerkes E, Zepp F, Brock JW, 3rd, Kon V: Angiotensin type 2 receptor is important in the normal development of the ureter. Pediatr Nephrol 13: 187–191, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I: Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao R, Gish K, Murphy M, Yin Y, Notterman D, Hoffman WH, Tom E, Mack DH, Levine AJ: Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev 14: 981–993, 2000 [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR: High-frequency developmental abnormalities in p53-deficient mice. Curr Biol 5: 931–936, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Baatout S, Jacquet P, Michaux A, Buset J, Vankerkom J, Derradji H, Yan J, von Suchodoletz H, de Saint-Georges L, Desaintes C, Mergeay M: Developmental abnormalities induced by X-irradiation in p53 deficient mice. In Vivo 16: 215–221, 2002 [PubMed] [Google Scholar]
- 21.Fulci G, Van Meir EG: p53 and the CNS: Tumors and developmental abnormalities. Mol Neurobiol 19: 61–77, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Harvey M, McArthur MJ, Montgomery CA, Jr, Bradley A, Donehower LA: Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J 7: 938–943, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Ikeda S, Hawes NL, Chang B, Avery CS, Smith RS, Nishina PM: Severe ocular abnormalities in C57BL/6 but not in 129/Sv p53-deficient mice. Invest Ophthalmol Vis Sci 40: 1874–1878, 1999 [PubMed] [Google Scholar]
- 24.Ohyama K, Chung CH, Chen E, Gibson CW, Misof K, Fratzl P, Shapiro IM: p53 influences mice skeletal development. J Craniofac Gen Dev Biol 17: 161–171, 1997 [PubMed] [Google Scholar]
- 25.Livni N, Eid A, Ilan Y, Rivkind A, Rosenmann E, Blendis LM, Shouval D, Galun E: p53 expression in patients with cirrhosis with and without hepatocellular carcinoma. Cancer 75: 2420–2426, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Amariglio F, Tchang F, Prioleau MN, Soussi T, Cibert C, Mechali M: A functional analysis of p53 during early development of Xenopus laevis. Oncogene 15: 2191–2199, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Wallingford JB, Seufert DW, Virta VC, Vize PD: p53 activity is essential for normal development in Xenopus. Curr Biol 7: 747–757, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Hoever M, Clement JH, Wedlich D, Montenarh M, Knochel W: Overexpression of wild-type p53 interferes with normal development in Xenopus laevis embryos. Oncogene 9: 109–120, 1994 [PubMed] [Google Scholar]
- 29.Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S: Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell 113: 301–314, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Saifudeen Z, Dipp S, El-Dahr SS: A role for p53 in terminal epithelial cell differentiation. J Clin Invest 109: 1021–1030, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saifudeen Z, Dipp S, Fan H, El-Dahr SS: Combinatorial control of the bradykinin B2 receptor promoter by p53, CREB, KLF-4, and CBP: Implications for terminal nephron differentiation. Am J Physiol 288: F899–F909, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Saifudeen Z, Marks J, Du H, El-Dahr SS: Spatial repression of PCNA by p53 during kidney development. Am J Physiol 283: F727–F733, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM: Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291: 325–339, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Shaulian E, Zauberman A, Ginsberg D, Oren M: Identification of a minimal transforming domain of p53: Negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol 12: 5581–5592, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michael L, Davies JA: Pattern and regulation of cell proliferation during murine ureteric bud development. J Anat 204: 241–255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW: Regulation of PTEN transcription by p53. Mol Cell 8: 317–325, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Diggle CP, Pitt E, Harnden P, Trejdosiewicz LK, Southgate J: Role of p53 in the responses of human urothelial cells to genotoxic damage. Int J Cancer 93: 199–203, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Vousden KH: Outcomes of p53 activation: Spoilt for choice. J Cell Science 119: 5015–5020, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Embree-Ku M, Boekelheide K: FasL deficiency enhances the development of tumors in p53+/− mice. Toxicol Pathol 30: 705–713, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD: Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol 299: 466–477, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Marose TD, Merkel CE, McMahon AP, Carroll TJ: Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol 314: 112–126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen A, Skjong C, Shawlot W: Lim 1 is required for nephric duct extension and ureteric bud morphogenesis. Dev Biol 288: 571–581, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Fan H, Harrell JR, Dipp S, Saifudeen Z, El-Dahr SS: A novel pathological role of p53 in kidney development revealed by gene-environment interactions. Am J Physiol 288: F98–F107, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Reczek EE, Flores ER, Tsay AS, Attardi LD, Jacks T: Multiple response elements and differential p53 binding control Perp expression during apoptosis. Mol Cancer Res 1: 1048–1057, 2003 [PubMed] [Google Scholar]
- 45.Marques MR, Horner JS, Ihrie RA, Bronson RT, Attardi LD: Mice Lacking the p53/p63 target gene Perp are resistant to papilloma development. Cancer Res 65: 6551–6556, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Rickard SM, Mummery RS, Mulloy B, Rider CC: The binding of human glial cell line-derived neurotrophic factor to heparin and heparan sulfate: Importance of 2-O-sulfate groups and effect on its interaction with its receptor, GFRalpha1. Glycobiology 13: 419–426, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Rider CC: Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem Soc Trans 34: 458–460, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Forsten-Williams K, Chu CL, Fannon M, Buczek-Thomas JA, Nugent MA: Control of growth factor networks by heparan sulfate proteoglycans. Ann Biomed Eng 36: 2134–2148, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colombres M, Henríquez JP, Reig GF, Scheu J, Calderón R, Alvarez A, Brandan E, Inestrosa NC: Heparin activates Wnt signaling for neuronal morphogenesis. J Cell Physiol 216: 805–815, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Baraz L, Haupt Y, Elkin M, Peretz T, Vlodavsky I: Tumor suppressor p53 regulates heparanase gene expression. Oncogene 25: 3939–3947, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Godley LA, Kopp JB, Eckhaus M, Paglino JJ, Owens J, Varmus HE: Wild-type p53 transgenic mice exhibit altered differentiation of the ureteric bud and possess small kidneys. Genes Dev 10: 836–850, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA: Tumor spectrum analysis in p53-mutant mice. Curr Biol 4: 1–7, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A: Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 29: 418–425, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Shao X, Somlo S, Igarashi P: Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Igarashi P, Shashikant CS, Thomson RB, Whyte DA, Liu-Chen S, Ruddle FH, Aronson PS: Ksp-cadherin gene promoter: II. Kidney-specific activity in transgenic mice. Am J Physiol 277: F599–F610, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM: Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276: 403–415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA: Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol 280: 396–406, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F: Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest 113: 1051–1058, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pachnis V, Mankoo B, Costantini F: Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119: 1005–1017, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Srinivas S, Wu Z, Chen CM, D'Agati V, Costantini F: Dominant effects of RET receptor misexpression and ligand-independent RET signaling on ureteric bud development. Development 126: 1375–1386, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressler GR: Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol 243: 128–136, 2002 [DOI] [PubMed] [Google Scholar]
- 62.el-Deiry WS, Harper JW, O'Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y, et al. : WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 54: 1169–1174, 1994 [PubMed] [Google Scholar]
- 63.Golubovskaya V, Kaur A, Cance W: Cloning and characterization of the promoter region of human focal adhesion kinase gene: Nuclear factor kappa B and p53 binding sites. Biochim Biophys Acta 1678: 111–125, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Singh B, Reddy PG, Goberdhan A, Walsh C, Dao S, Ngai I, Chou TC, O-Charoenrat P, Levine AJ, Rao PH, Stoffel A: p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev 16: 984–993, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]