Abstract
Until recently, the idea that estradiol could affect cellular processes independent of nuclear estrogen receptors was often dismissed as artifact. This in spite of a large number of carefully controlled studies performed both within and outside the nervous system demonstrating estrogens regulate various intracellular signaling pathways by acting at the membrane surface of cells and/or at biological rates incompatible with the time course of genomic-initiated events. The concept that estradiol can act on surface membrane receptors to regulate nervous system function is now far less controversial. However, there is evidence that there may be multiple types of estrogen receptors on the membrane surface of cells. Determining the physiological relevance of each of these receptors is currently underway. Two important membrane estrogen receptors are in fact the classical estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ) proteins, which is somewhat surprising based upon their well-established role in nuclear gene transcription. This review will focus on the mechanism by which surface-localized ERα and ERβ stimulate intracellular signaling events in cells of the nervous system through activation of metabotropic glutamate receptors (mGluRs). This mechanism of estrogen receptor function also requires caveolin proteins, which provide the subcellular compartmentalization of the particular signaling components required for appropriate cell stimulation. The review will conclude with several examples of physiological processes under the apparent regulation of ER/mGluR signaling.
Keywords: Estradiol, MGluR, Lordosis, Nociception, Membrane, Rapid actions
1. Classical estrogen receptor signaling
Researchers have studied the effects of gonadal hormones on brain function for quite some time [1]. Across the lifespan of an organism, hormones such as estrogens affect nervous system function through alterations in anatomy and/or physiology. Principle roles of estrogens include its regulation of sexual development, maturation and reproductive behaviors. Following the cloning of the first estrogen receptor [2,3], ERα was determined to be a ligand-regulated transcription factor [4,5]. This was consistent with previous work demonstrating the actions of estradiol were dependent on the translation of new protein [6,7]. Furthermore, the distribution of ERα [8,9] was tightly correlated with steroid autoradiography studies [10,11], which found the highest levels of estrogen binding in brain regions critical for reproductive success. A simple, single model for estrogen action continued to be developed as ERα was found to be located primarily in the nucleus [12,13], where it would bind DNA at estrogen response elements (EREs) as a dimer once bound to steroid [14].
These ERα-mediated changes in gene expression and protein synthesis are referred to as the classical mechanism of estrogen action. The complexity of ER-mediated gene expression has expanded with the finding of a second estrogen receptor, ERβ [15] and diverse ER interactions with various co-activators and other transcription factors [16–18]. This interplay between ERs and various other nuclear machinery involved with gene transcription account for the diversity of estrogen-regulated genes, including those which lack EREs. In addition, ERs can be activated in the absence of estrogens [19–24], making the classical model of estrogen action in brain far from simple. Yet, even with the many adaptations required to expand the original model of estrogen action to fit these additional findings, major support was lacking within the field of neuroendocrinology for estradiol affecting cell function outside of a transcriptionally-initiated event.
2. Classical versus novel mechanisms of estrogen action
Along with the evidence that the classical effects of estradiol in brain went far beyond the simple model of ERα-induced gene expression, another paradigm shift (i.e. estrogens act at the surface membrane to regulate neuronal function) attempted to gain traction. This was in response to three novel, but related, themes in the literature. The first being multiple discoveries of estrogen action within areas of the nervous system not associated with reproduction and a corresponding revelation that various non-reproductive behaviors are affected by estrogens. The second focus was that many of the effects of estradiol affecting neuronal function occur on a time scale too rapid to be accounted for by the classical mechanism of action. Third, many of these rapid effects appear to be initiated by estradiol acting at the surface of the neuronal membrane.
In neurons, Kelly et al. were the first main proponents of estradiol having rapid effects. They showed that within seconds, the hormone altered the electrical activity of preoptic and septal neurons [25]. Of note, rapid actions of estrogens were observed not just within the nervous system, but also in various other tissues. For example, one of the first reported non-classical effects of estrogens was on the accumulation of cAMP in uterine tissue. Szego and Davis reported that concentrations of cAMP increased within 15 s of estrogen application [26]. Regardless of the preparation, the work these investigators and others studying rapid actions of steroid hormones was initially met with tremendous skepticism. However, with time and continued experimental resilience, initial skepticism was gradually replaced with a general agreement that estrogens could indeed act at the neuronal membrane to affect cellular function.
Recent studies have demonstrated estrogen modification of cell excitability through modulation of ion channels in many other brain regions [27–29]. Various intracellular signaling proteins are also affected by membrane estrogen signaling, including activation of protein kinase A (PKA), protein kinase B (AKT), protein kinase C (PKC), phospholipase C (PLC), inositol triphosphate (IP3), and mitogen-activated protein kinase (MAPK) [30–41]. Not surprisingly then, estradiol through novel mechanisms can affect gene expression and protein synthesis through the activation of transcription factors such as cAMP response element-binding protein (CREB) [34,42–44]. These rapid actions of estrogens have been found relevant to a whole host of behaviors, such as learning and memory, motor control, mood and pain perception [45–48]. Interestingly, the regions of the nervous system critical to these behaviors were originally thought to have little or no expression of the classical ERs, ERα and ERβ. Thus, the question was raised as to the mechanism by which estradiol was able to exert these novel effects.
While novel information was escalating regarding rapid effects of estradiol in the central nervous system, the theories of possible underlying mechanisms remained very much controversial. This was by and large due to the fact that many of the reported rapid effects appeared to be initiated at the membrane surface, determined through the utilization of membrane impermeable estrogen analogs [49,50]. The persistence of a novel action of estradiol following the intracellular dialysis of a cell with the steroid also supports this mechanism of action [29]. Indeed, estradiol had been shown to bind to the membrane of endothelial cells as early as 1977 [51]. However, due to the fact that a membrane estrogen receptor had not yet been identified, these results remained controversial.
In the 1980s, researchers began testing the hypothesis that the classical ER, ERα, could localize to the membrane surface [52,53]. These reports were often improperly discredited, on the claim that these findings were due to a technical artifact; i.e. contamination of membrane fractions with transposed receptors from the nucleus during the isolation procedure. In addition, the known structure of these classical receptors provided no clue as to how they would be membrane-localized, as well as be able to activate intracellular signaling even if they were trafficked to this region of the cell. However, in 1999, overexpression of ERα and ERβ revealed that a portion of the classic ER protein was targeted to the membrane and activated intracellular signaling [54]. This simple and elegant experiment demonstrated that the same protein is capable of mediating both intracellular and membrane actions of estradiol. And while membrane-localized ERα and ERβ still maintains some controversy within the nervous system, in other cell types it is clearly well established and virtually undisputed (for numerous examples, see the other articles in this special issue).
3. Estrogen receptor interactions with mGluRs
One often studied indication of rapid estradiol action has been the phosphorylation (activation) of CREB [34,35,42,44,55]. Phosphorylation of CREB is an important node in cell signaling, critically involved in various forms of neuronal plasticity. Several laboratories have found that the activation of surface estrogen receptors leads to CREB phosphorylation via stimulation of the MAPK/ERK signaling pathway. In turn, activated CREB regulates gene expression though interaction with DNA at CREB response elements (CREs). These and other novel estradiol actions are blocked by the ER antagonist ICI 178,820, whereas ERα and ERβ agonists frequently mimic the actions of the steroid [42,56]. Such results provided pharmacological evidence that classical ERs play a role in the novel actions of estradiol. While these data still provide room to argue for a unique membrane estrogen receptor, debate whether classical estrogen receptors were at least partially responsible for mediating some of the reported rapid effects essentially ended when Herbison and colleagues, using ER knock-out mice, determined that the rapid actions of estradiol on phosphorylation of CREB and MAPK were dependent on ERα and ERβ [57]. However, with the end of one controversy, another quickly surfaced: that is, how do classical ERs initiate cell signaling when localized to the membrane? Moreover, how are ERs trafficked to the membrane in the first place?
Clues to the mechanism by which membrane-localized ERα and ERβ exert effects on cell function included numerous reports that describe estrogen action to be sensitive to G-protein manipulation [29,41]. Based upon these data, a relatively straightforward hypothesis, i.e. ERα and ERβ directly bind and activate G-proteins, was put forth [58,59]. In support of this mechanism, ERα can directly interact with G protein subunits [60]. Yet, the diverse array of signaling pathways regulated by membrane estrogen receptors suggests additional mechanisms of action. Outside of the nervous system, membrane estrogen receptors have been found to directly bind and activate surface receptors linked to various second messenger systems [61–63]. In parallel, we find membrane-localized ERα and ERβ capable of activating various metabotropic glutamate receptors (mGluRs).
Our initial experiments performed in cultured hippocampal neurons found that estradiol stimulation of ERα resulted in increased CREB phosphorylation through activation of mGluR1 [42]. mGluR1 and mGluR5 comprise the group I mGluRs, which are Gq linked and were previously shown capable of activating CREB [64,65]. Mechanistically, mGluR1 stimulation leads to MAPK-dependent CREB phosphorylation via activation of phospholipase C (PLC), protein kinase C (PKC) and inositol trisphosphate (IP3) signaling. Interestingly, the activation of ERα by estradiol was only effective in triggering CREB phosphorylation in cultures derived from female, and not male, hippocampus. The underlying cause for this sex difference is currently being investigated.
The actions of ERα on mGluR1 required only picomolar concentrations of estradiol. Furthermore, CREB phosphorylation was observed within 30 s of steroid application (with maximal responses at 2 min following estradiol administration). With the additional evidence that non-permeable estrogen analogs and ERα agonists mimicked the response of estradiol and that the pure estrogen receptor antagonist ICI 182,780 blocked the actions of estradiol, we concluded ERα at the membrane was responsible for triggering CREB phosphorylation.
As mentioned, estradiol has been observed to stimulate a variety of intracellular signaling cascades. In striatal neurons, we had previously reported that estradiol, through activation of a G-protein coupled receptor, could decrease L-type calcium channel currents [29]. This has subsequently been confirmed in various other neuronal systems [66,67]. This is of particular importance because calcium entry through L-type calcium channels can rapidly trigger CREB phosphorylation through activation of calcium calmodulin-dependent protein kinase IV (CaMKIV). Consequently, we found estradiol to also decrease L-type calcium channel-dependent CREB phosphorylation. Estrogen inhibition of L-type calcium channel-dependent CREB phosphorylation was dependent upon activation of the group II mGluRs, mGluR2 and/or mGluR3 [42]. These mGluRs are functionally linked to Gi/o second messenger signaling. The only major difference between estradiol activation of mGluR1 versus mGluR2/3 was that the latter was triggered by both ERα as well as ERβ.
It was of particular interest that we observed the bidirectional affects (i.e. activation of both mGluR1 and mGluR2/3 signaling) of estradiol upon CREB phosphorylation within the same population of cells. Isolation of one pathway versus the other was first achieved through pharmacological manipulation. However, in a follow up study, we were able to independently disrupt one signaling pathway or the other through modification of caveolin expression and/or function [68]. Caveolin proteins are membrane proteins that organize signal transduction machinery [69]. Originally described outside of the nervous system, caveolin proteins were found essential for various membrane ERα responses (reviewed in Ref. [70]). In hippocampal neurons, the caveolin-1 protein (CAV1) is essential for the functional coupling of ERα with mGluR1. Conversely, caveolin-3 (CAV3) is necessary for ERα and ERβ activation of mGluR2/3 [68]. To our knowledge, this is the first demonstration of functionally discrete microdomains within the same cell being generated by different caveolin proteins.
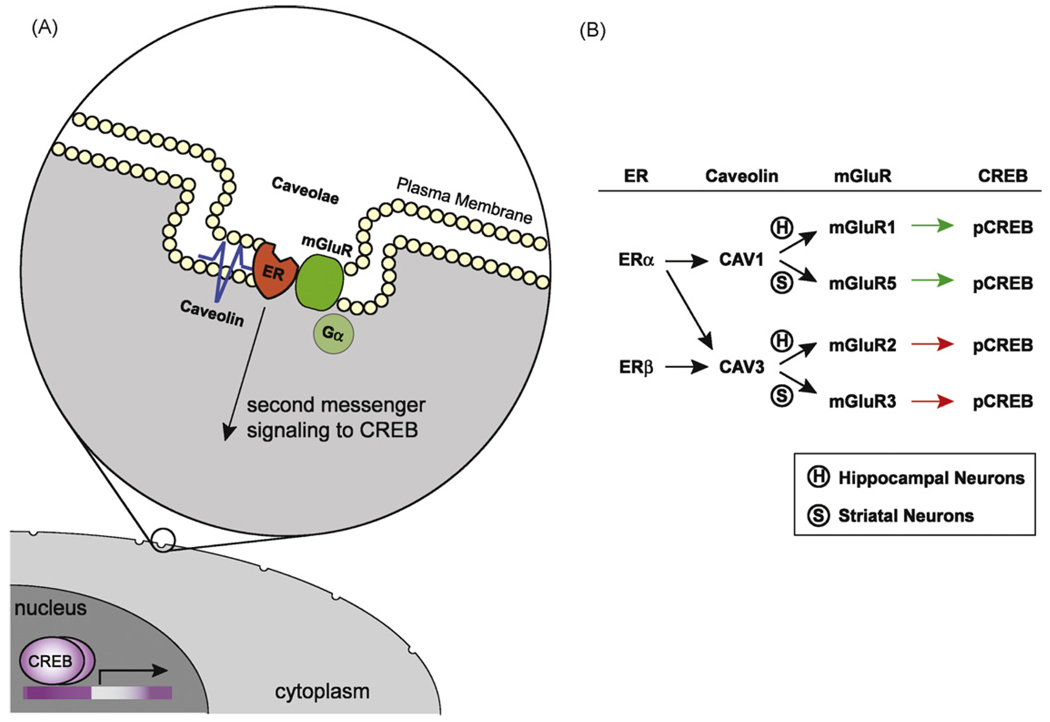
Our recent studies have examined putative ER/mGluR interactions in other brain regions. We have focused our efforts in striatum, where rapid effects of estrogens have been reported for some time [71]. Analogous to our results in hippocampal tissue, activation of ERα leads to the phosphorylation of CREB, whereas ERα and ERβ attenuate L-type calcium channel-dependent CREB phosphorylation. Both pathways were similarly dependent upon CAV1 and CAV3. To our surprise, however, the mGluRs responsible for estrogen signaling to the nucleus were different. While ERα is coupled to mGluR1 in hippocampus, it is mGluR5 in striatum. Likewise, recent findings suggest ERα/ERβ activates mGluR2 signaling in hippocampus but mGluR3 in striatum [72]. These results are particularly intriguing as all four mGluRs are expressed in both hippocampus and striatum. Regardless of the mechanism for differential pairing of ERs and mGluRs across neuronal subtypes, these data suggest ERs may be coupled to various GPCRs, and not a select population. This may account for the widespread estrogen action within the nervous system. At the very least, ERs can have diverse effects on neuronal cell excitability through pairing with different caveolin and mGluR proteins (Fig. 1).
Fig. 1.
Estrogen receptor activation of mGluR signaling through interactions with caveolin proteins. (A) Model system of estradiol-induced activation of mGluRs via caveolin-based caveolae. (B) Summary of distinct signaling pathways by which ERα and ERβ can affect CREB phosphorylation.
4. Physiological relevance of ER/mGluR signaling
While our work has focused on elucidating the mechanisms by which membrane ERs regulate neuronal function, and thus have used a more reductionist approach, it is essential to demonstrate these same signaling pathways are present and relevant in more intact preparations, and thus show physiological relevance. The laboratory of Micevych has examined three separate model systems previously demonstrated to be regulated by membrane estrogen receptors. In each of these systems, ER/mGluR coupling was deemed essential.
The first series of experiments examined estradiol signaling in the arcuate nucleus and its role in female sexual receptivity. In the female rat, estradiol acts on a limbic-hypothalamic circuit to allow the expression of lordosis, a stereotypic sexually receptive behavior [73,74]. It has been known for almost 30 years that estradiol-induced lordosis behavior is dependent on the transcription of new proteins [7,75]. However, priming animals with a membrane-constrained estradiol (E-6-BSA) followed with a subthreshold dose of estradiol was as efficacious as two estradiol injections [76]. Thus membrane actions of the steroid are also important for lordosis. Previous work by the Micevych lab had determined a critical, estrogen-sensitive pathway from the arcuate nucleus to the medial preoptic area [77,78]. In the arcuate nucleus, ERα was found to be co-localized with mGluR1a. In addition, estrogen action in the arcuate nucleus upon lordosis behavior was critically dependent on mGluR1 signaling [79].
This first demonstration of ER/mGluR coupling in hypothalamic neurons was followed by an equally elegant demonstration in hypothalamic glial cells. Previous studies have shown that estradiol administration results in an increase in intracellular calcium within these cells. The resulting rise in calcium is believed to be critical in the synthesis of neuroprogesterone and the LH surge [80]. Similar to the findings in neurons, the estrogen-dependent rise in intracellular calcium within glial cells was reliant upon interactions between membrane ER and mGluR1a. The estradiol-induced increase in intracellular calcium flux was blocked by both the classical ER antagonist, ICI 182,780 and the mGluR1a antagonist LY 367385 [81]. Additionally, ERα and mGluR1a were found to co-immunoprecipitate in hypothalamic glial cell membrane fractions, suggesting a direct interaction between these two receptors at the membrane surface [81].
In a third preparation, the functional coupling of ERs to mGluR2/3 was observed. The cell bodies of primary visceral spinal afferent neurons are located in the DRG. These cells transmit nociceptive information to the spinal cord. One such activator of these DRG cells is ATP, which has emerged as a putative signal for visceral pain. Noxious stimuli such as distention of the viscera or tissue damage release ATP [82], which then transduces noxious stimuli by activating purinergic, ATP-gated P2X receptors on primary afferent fibers [83]. Opening of P2X channels results in membrane depolarization sufficient to trigger action potentials and calcium influx through voltage-gated calcium channels associated with nociception [84]. The vast majority of ATP-sensitive DRG neurons respond to estradiol [66]. Estradiol was found to inhibit ATP-mediated calcium influx in small diameter DRG neurons, though inhibition of L-type calcium channels. Further, estradiol inhibition of L-type calcium channels was dependent on mGluR2/3 [85].
5. Conclusions
It has now become widely accepted that the actions of estrogens within the brain far exceed its classical effects upon sexual behavior and maturation. Indeed, estradiol has been shown to induce a multitude of rapid, membrane-initiated events within various regions of the brain. These novel effects have been demonstrated to play a crucial role in diverse behaviors such as learning and memory, motor control, mood and pain perception. Although this has become a burgeoning and exciting field of research to many, there is still much dispute regarding the underlying cellular mechanism(s) by which the steroid hormone elicits these novel actions. By further examining the interactions between membrane-localized ERs and other proteins such as mGluRs and caveolins, our understanding of the wide-ranging effects of this steroid hormone on brain function may be greatly expanded.
Acknowledgements
This work was supported by NIH grant NS41302 (PGM). The authors would like to thank Jessie Luoma for her technical assistance regarding the preparation of this manuscript and Dr. Paul Micevych for his helpful discussions.
References
- 1.Beach F. Hormones and behavior. New York: Hoeber; 1948. [Google Scholar]
- 2.Koike S, Sakai M, Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic Acids Res. 1987;15:2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spreafico E, Bettini E, Pollio G, Maggi A. Nucleotide sequence of estrogen receptor cDNA from Sprague–Dawley rat. Eur J Pharmacol. 1992;227:353–356. doi: 10.1016/0922-4106(92)90016-o. [DOI] [PubMed] [Google Scholar]
- 4.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 5.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 7.Rainbow TC, Davis PG, McEwen BS. Anisomycin inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1980;194:548–555. doi: 10.1016/0006-8993(80)91240-8. [DOI] [PubMed] [Google Scholar]
- 8.Shughrue PJ, Komm B, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in the rat hypothalamus. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- 9.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 10.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 11.Stumpf WE, Sar M. Steroid hormone target sites in the brain: the differential distribution of estrogin, progestin, androgen and glucocorticosteroid. J Steroid Biochem. 1976;7:1163–1170. doi: 10.1016/0022-4731(76)90050-9. [DOI] [PubMed] [Google Scholar]
- 12.King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- 13.Welshons WV, Lieberman ME, Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984;307:747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- 14.O’Malley BW, Tsai MJ. Molecular pathways of steroid receptor action. Biol Reprod. 1992;46:163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonard DM, Tsai SY, O’Malley BW. Selective estrogen receptor modulators 4-hydroxytamoxifen and raloxifene impact the stability and function of SRC-1 and SRC-3 coactivator proteins. Mol Cell Biol. 2004;24:14–24. doi: 10.1128/MCB.24.1.14-24.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uht RM, Anderson CM, Webb P, Kushner PJ. Transcriptional activities of estrogen and glucocorticoid receptors are functionally integrated at the AP-1 response element. Endocrinology. 1997;138:2900–2908. doi: 10.1210/endo.138.7.5244. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O’Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA. 2000;97:6379–6384. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-alpha: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- 20.Etique N, Flament S, Lecomte J, Grillier-Vuissoz I. Ethanol-induced ligand-independent activation of ERalpha mediated by cyclic AMP/PKA signaling pathway: an in vitro study on MCF-7 breast cancer cells. Int J Oncol. 2007;31:1509–1518. [PubMed] [Google Scholar]
- 21.Han F, Miksicek R, Clarke R, Conrad SE. Expression of an estrogen receptor variant lacking exon 3 in derivatives of MCF-7 cells with acquired estrogen independence or tamoxifen resistance. J Mol Endocrinol. 2004;32:935–945. doi: 10.1677/jme.0.0320935. [DOI] [PubMed] [Google Scholar]
- 22.Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, et al. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- 23.Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zava DT, Chamness GC, Horwitz KB, McGuire WL. Human breast cancer: biologically active estrogen receptor in the absence of estrogen? Science. 1977;196:663–664. doi: 10.1126/science.193182. [DOI] [PubMed] [Google Scholar]
- 25.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 26.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;45:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 28.Joels M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- 29.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986;233:226–228. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- 31.Minami T, Oomura Y, Nabekura J, Fukuda A. 17 Beta-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- 32.Mobbs CV, Kaplitt M, Kow LM, Pfaff DW. PLC-alpha: a commonmediator of the action of estrogen and other hormones? Mol Cell Endocrinol. 1991;80:C187–C191. doi: 10.1016/0303-7207(91)90136-g. [DOI] [PubMed] [Google Scholar]
- 33.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Q, Moss RL. 17 Beta-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 36.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen onmitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 37.Wiebe JP. Nongenomic actions of steroids on gonadotropin release. Recent Prog Horm Res. 1997;52:71–99. discussion 101. [PubMed] [Google Scholar]
- 38.Marino M, Pallottini V, Trentalance A. Estrogens cause rapid activation of IP3-PKC-alpha signal transduction pathway in HEPG2 cells. Biochem Biophys Res Commun. 1998;245:254–258. doi: 10.1006/bbrc.1998.8413. [DOI] [PubMed] [Google Scholar]
- 39.Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Brain Res Mol Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- 41.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, et al. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 45.Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–59. doi: 10.1016/0091-3057(87)90476-x. [DOI] [PubMed] [Google Scholar]
- 46.Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 47.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Ann Neurol. 2002;51:599–603. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- 48.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19:1606–1617. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]
- 50.Zheng J, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17beta-estradiol-[125I]bovine serum albumin conjugate. J Steroid Biochem Mol Biol. 1997;62:327–336. doi: 10.1016/s0960-0760(97)00037-x. [DOI] [PubMed] [Google Scholar]
- 51.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 52.Sadler SE, Maller JL. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. J Biol Chem. 1982;257:355–361. [PubMed] [Google Scholar]
- 53.Sadler SE, Bower MA, Maller JL. Studies of a plasma membrane steroid receptor in Xenopus oocytes using the synthetic progestin RU 486. J Steroid Biochem. 1985;22:419–426. doi: 10.1016/0022-4731(85)90448-0. [DOI] [PubMed] [Google Scholar]
- 54.Razandi M, Pedram A, Greene GL, Levin ER. Cellmembrane and nuclear estrogen receptors (ERs) originate froma single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 56.Bulayeva NN, Wozniak AL, Lash LL, Watson CS. Mechanisms of membrane estrogen receptor-alpha-mediated rapid stimulation of Ca2+ levels and prolactin release in a pituitary cell line. Am J Physiol Endocrinol Metab. 2005;288:E388–E397. doi: 10.1152/ajpendo.00349.2004. [DOI] [PubMed] [Google Scholar]
- 57.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 58.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;9:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 60.Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, et al. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i) J Biol Chem. 2001;276:27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- 61.Cardona-Gomez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:51–60. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 62.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 63.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 64.Choe ES, Wang JQ. Group I metabotropic glutamate receptor activation increases phosphorylation of cAMP response element-binding protein, Elk-1, and extra-cellular signal-regulated kinases in rat dorsal striatum. Brain Res Mol Brain Res. 2001;94:75–84. doi: 10.1016/s0169-328x(01)00217-0. [DOI] [PubMed] [Google Scholar]
- 65.Warwick HK, Nahorski SR, Challiss RA. Group I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to cyclic AMP response element binding protein (CREB) through a common Ca2+ and protein kinase C-dependent pathway. J Neurochem. 2005;93:232–245. doi: 10.1111/j.1471-4159.2005.03012.x. [DOI] [PubMed] [Google Scholar]
- 66.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 67.Lee DY, Chai YG, Lee EB, Kim KW, Nah SY, Oh TH, et al. 17Betaestradiol inhibits high-voltage-activated calcium channel currents in rat sensory neurons via a non-genomic mechanism. Life Sci. 2002;70:2047–2059. doi: 10.1016/s0024-3205(01)01534-x. [DOI] [PubMed] [Google Scholar]
- 68.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luoma JI, Boulware MI, Mermelstein PG. Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol. 2008;290:8–13. doi: 10.1016/j.mce.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grove-Strawser D, Mermelstein PG. Estrogen receptors activate different mGluRs across distinct brain regions; Abstract 195.13/XX10 in Society for Neuroscience Annual Meeting 2007; 2007. [Google Scholar]
- 73.Micevych P, Ulibarri C. Development of the limbic-hypothalamic cholecystokinin circuit: amodel of sexual differentiation. Dev Neurosci. 1992;4:11–34. doi: 10.1159/000111643. [DOI] [PubMed] [Google Scholar]
- 74.Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Mol Neurobiol. 2003;27:197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- 75.Gorski RA, Yanase M. Estrogen facilitation of lordosis behavior in the female rat. Exp Brain Res. 1981 Suppl. 3:222–237. doi: 10.1007/978-3-642-45525-4_18. [DOI] [PubMed] [Google Scholar]
- 76.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sinchak K, Romeo HE, Micevych PE. Estrogen and progestin regulation of OFQ/nociceptin and ORL-1 mRNA expression in the female rat limbic hypothalamic system in Society for Neuroscience Abstr. 2001 doi: 10.1002/cne.20949. [DOI] [PubMed] [Google Scholar]
- 79.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Micevych P, Sinchak K. Mini-reviews: synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology. 2008 doi: 10.1210/en.2008-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych PE. Membrane estradiol receptors interact with metabotropic glutamate receptors to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2008 doi: 10.1210/en.2008-0994. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 83.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 84.Koshimizu TA, Van Goor F, Tomic M, Wong AO, Tanoue A, Tsujimoto G, et al. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol Pharmacol. 2000;58:936–945. doi: 10.1124/mol.58.5.936. [DOI] [PubMed] [Google Scholar]
- 85.Chaban VV, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates ATP-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat DRG neurons in Society for Neuroscience. 2007 doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]