Abstract
Introduction
Extremely low gestational age newborns (ELGANs) are at increased risk of chronic lung disease (CLD) and of developmental delay. Some studies have suggested that CLD contributes to developmental delay.
Patients and Methods
We examined data collected prospectively on 915 infants born before the 28th week of gestation in 2002–2004 who were assessed at 24 months of age with the Bayley Scales of Infant Development-2nd Edition or the Vineland Adaptive Behavior Scales. We excluded infants who were not able to walk independently (Gross Motor Function Classification System score < 1) and, therefore, more likely to have functionally important fine motor impairments. We defined CLD as receipt of oxygen at 36 weeks' postmenstrual age and classified infants as either not receiving mechanical ventilation (MV) (CLD without MV) or receiving MV (CLD with MV).
Results
Forty-nine percent of ELGANs had CLD; of these, 14% were receiving MV at 36 weeks' postmenstrual age. ELGANs without CLD had the lowest risk of a Mental Developmental Index (MDI) or a Psychomotor Developmental Index (PDI) of <55, followed by ELGANs with CLD not receiving MV, and ELGANs with CLD receiving MV (9%, 12%, and 18% for the MDI and 7%, 10%, and 20% for the PDI, respectively). In time-oriented multivariate models, the risk of an MDI of <55 was associated with the following variables: gestational age of <25 weeks; single mother; late bacteremia; pneumothorax; and necrotizing enterocolitis. The risk of a PDI of <55 was associated with variables such as single mother, a complete course of antenatal corticosteroids, early and persistent pulmonary dysfunction, pulmonary deterioration during the second postnatal week, pneumothorax, and pulmonary interstitial emphysema. CLD, without or with MV, was not associated with the risk of either a low MDI or a low PDI. However, CLD with MV approached, but did not achieve, nominal statistical significance (odds ratio: 1.9 [95% confidence interval: 0.97–3.9]) for the association with a PDI of <55.
Conclusions
Among children without severe gross motor delays, risk factors for CLD account for the association between CLD and developmental delay. Once those factors are considered in time-oriented risk models, CLD does not seem to increase the risk of either a low MDI or a low PDI. However, severe CLD might increase the risk of a low PDI.
Keywords: lung disease, prematurity, preterm infant, neurodevelopmental outcome
Chronic lung disease (CLD) (also known as bronchopulmonary dysplasia) seems to place an infant at increased risk of early developmental delays,1–3 cognitive impairment,4–6 language impairment,7,8 and poor academic performance.9 Predictors and correlates of CLD, such as prolonged mechanical ventilation (MV), are also associated with an increased risk of cognitive impairment.10–12
CLD is associated with global dysfunction rather than specific neuropsychological impairments.13 The more severe the CLD, the greater the probability of developmental impairment.14–16 The putative causal pathways from antenatal events to the development of CLD involve multiple antecedents, effect modifiers, and confounders (chorioamnionitis, preterm birth, antenatal steroids, postnatal infection, patent ductus arteriosus [PDA], and fluid management, etc).17,18
Although 2 randomized, controlled, clinical trials have shown that medications that reduce the risk of CLD also reduce the risk of neurodevelopmental impairment (eg, caffeine19,20 and vitamin A21,22), the underlying mechanisms have not been identified.23 Thus, we still do not know to what extent CLD, its antecedents, or comorbid conditions contribute to neurodevelopmental impairment.
The Extremely Low Gestational Age Newborn (ELGAN) study assessed measures of pulmonary dysfunction as well as prenatal, antenatal, and postnatal exposures and events.24,25 The purpose of the analyses presented here was to explore to what extent CLD and its antecedents influence the risk of developmental delays at 24-months adjusted age, as assessed with the Bayley Scales of Infant Development-2nd Edition (BSID-II),26 among infants without gross motor function impairments.
Patients and Methods
The ELGAN Study
The ELGAN study identified characteristics and exposures that increase the risk of structural and functional neurologic disorders in ELGANs. During the years 2002-2004, women delivering before 28 weeks' gestation at 1 of 14 participating institutions were asked to enroll in the study. The enrollment and consent processes were approved by the individual institutional review boards. We limited the sample to children who were assessed at 24 months of age with the BSID-II or the Vineland Adaptive Behavior Scales (VABS). Fine motor impairments, which interfere with the Mental developmental index (MDI) and the Psychomotor developmental index (PDI) assessments, tend to accompany gross motor dysfunctions. To avoid attributing cognition and perception deficits to motor impairments, we limited our analyses to children who were able to walk independently (Gross Motor Function Classification System [GMFCS] < 1), and who, therefore, were unlikely to have functionally important fine motor impairments.
Demographic, Pregnancy, and Newborn Variables
The clinical circumstances that led to each maternal admission and ultimately to each preterm delivery were defined a priori.27 The gestational age estimates were based on a hierarchy of the quality of available information.24,25 The birth weight z score is the number of SDs the infant's birth weight is above or below the median weight of infants at the same gestational age in a standard data set.28 We collected the physiology, laboratory, and therapy data for the first 12 hours needed to calculate a Score for Neonatal Acute Physiology-II (SNAP-II).29
During the first week, details about the newborn were collected daily, then weekly thereafter through the first month (days 7, 14, 21, and 28). Infants were classified by their respiratory characteristics during the first 2 postnatal weeks as “persistently low fraction of inspired oxygen [Fio2]” (Fio2 consistently below 0.23 on all days between days 3 and 7 and Fio2 ≤ 0.25 on day 14), “pulmonary deterioration” (PD) (Fio2 < 0.23 on any day between days 3 and 7 and Fio2 > 0.25 on day 14), and “early and persistent pulmonary dysfunction” (EPPD) (Fio2 > 0.23 on all days between days 3 and 7 and Fio2 > 0.25 on day 14).24 Late bacteremia was defined as recovery of an organism from blood drawn during postnatal weeks 2, 3, or 4.
Pneumothorax, pulmonary interstitial emphysema (PIE), pulmonary hemorrhage, and PDA (clinical and echocardiographic) were recorded. Ophthalmologists examined the eyes of the infants according to protocol and completed forms specifically for the ELGAN study. Definitions of retinopathy of prematurity (ROP) were those definitions endorsed by the International Committee for Classification of Retinopathy of Prematurity.30
The diagnosis of CLD was made at 36 weeks' postmenstrual age (PMA). If an infant was receiving supplemental oxygen, the infant was classified as having CLD, and further classified on the level of respiratory support: CLD with MV (either conventional MV [CMV] or high-frequency ventilation) or CLD without MV. Necrotizing enterocolitis (NEC) was classified according to the modified Bell staging system.31
Protocol Ultrasound Scans
Cranial ultrasound scans were performed by technicians at all of the hospitals by using digitized high-frequency transducers (7.5 and 10 MHz) and included the standard views.32 The 3 sets of protocol scans were defined by the postnatal day on which they were obtained (days 1–4, 5–14, and ≥15). Reading procedures and efforts to reduce observer variability are presented elsewhere.33
24-Month Developmental Assessment
The assessment at 24-months' corrected age included the BSID-II,26 a neurologic examination,34 an assessment of gross motor function by using the GMFCS35 and, when necessary, a parent-reported assessment of adaptive development by using the VABS.36 Seventy-seven percent of the children had developmental assessments within the range of 23.5 to 27.9 months; of the other children, about half were assessed before 23.5 months and about half after 27.9 months.
Certified examiners administered and scored the BSID-II. All examiners had previous experience with the BSID-II and attended a 1-day workshop at which the published guidelines for test administration and videotaped examinations were viewed and discussed. Examiners were aware of the infant's enrollment in the ELGAN study but were not informed of any specifics of the child's medical history. Examiners were told the child's corrected age.
As suggested by the test manual, we defined a significant delay for the BSID-II Mental and Motor scales as a score <70, a score that is more than 2 SDs below the mean for the standardization sample. We also evaluated associations with MDIs and PDIs of <55, which are more than 3 SDs below the mean for the standardization sample. When a child's impairments precluded administration of the BSID-II, or >2 items were omitted or judged to be “unscoreable,”the child was classified as nontestable on that scale. The Adaptive Behavioral Composite of the VABS was obtained for 26 of 33 children who were considered nontestable with the BSID-II Mental scale. For these infants, the Adaptive Behavioral Composite was used as an approximation of the MDI. Among infants unscoreable with the BSID-II Motor scale, 32 were assessed in the Motor Skills Domain of the VABS and that score was used as an approximation of the PDI.
Data Analysis
Data analysis was oriented to test the hypothesis that antecedents of CLD, and not CLD itself, contribute to suboptimal performance on the BSID-II. We assessed associations between antecedents (antenatal and postnatal variables and CLD) and low MDIs and PDIs. Relationships were assessed with Pearson's χ2, and variables associated with both CLD and a low BSID-II at a P value of ≤.30 were considered candidates for logistic regression analyses.37
Because postnatal phenomena, such as the need for ventilation, can be influenced by antepartum phenomena, we created logistic regression models in which risk factors were ordered in a temporal pattern, so that the earliest occurring predictors/covariates of an outcome (eg, MDI < 55) were entered first and were not displaced by later occurring covariates.11,38–42 For these time-oriented risk models (TORMs), we categorized sets of antecedents/covariates by the time they occurred or were identified. Each set is called an epoch. We used a step down procedure seeking a parsimonious solution without interaction terms. To account for the possibility that infants born at a particular hospital are more like each other than like infants born at other hospitals, a hospital cluster term was included in all models.43
Results
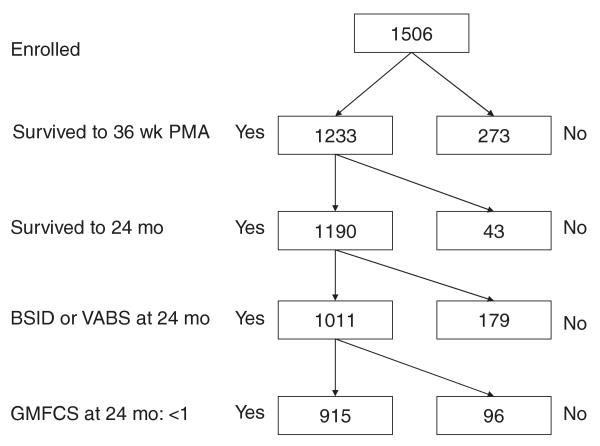
The cohort comprised 915 children whose CLD status at 36 weeks was known and were able to walk independently (GMFCS < 1) at the 24-month follow-up assessment (Fig 1). Ninety-six infants had a GMFCS ≥1 and were excluded. The 450 children who were treated with oxygen at 36 weeks' PMA (ie, had CLD) were more likely than their 465 peers who were not oxygen dependent to have an MDI in the severely delayed range (<55:13% vs 9%), although the 2 groups did not differ in the percentage of infants in the less severely delayed range (55–69: 10% vs 11%) (Table 1). MDIs in the 55 to 69 range tended to be modestly overrepresented among infants who had CLD with MV (15% vs 10% in the CLD without MV group versus 11% in the no CLD group). MDIs of <55 were more prominently overrepresented among children who had CLD with MV (18% vs 12% in the CLD without MV group versus 9% in the no CLD group).
FIGURE 1.
Description of the study sample.
TABLE 1.
MDI and PDI Stratified According to Presence of CLD (Oxygen Dependence at 36 Weeks' PMA) and Severity of CLD (CLD Without MV [CMV or High Frequency] or CLD With MV)
| MDI, % | PDI, % | N | |||
|---|---|---|---|---|---|
| <55 | 55–69 | <55 | 55–69 | ||
| CLD | |||||
| No | 9 | 11 | 7 | 14 | 465 |
| Yes | 13 | 10 | 12 | 17 | 450 |
| Maximum n | 103 | 99 | 86 | 143 | 915 |
| Severity of CLD | |||||
| Without MV | 12 | 10 | 10 | 17 | 385 |
| With MV | 18 | 15 | 20 | 18 | 65 |
Univariate Associations With CLD and BSID-II Scores
We chose to compare children with CLD to those children with no CLD without additional classification into CLD with or without MV because only 65 children were receiving MV at 36 weeks' PMA. A number of associations were observed for an increased risk of CLD and an increased risk of both an MDI and a PDI of <55, including lower birth weight z scores, higher SNAP-IIs, and lower Pao2 values (lowest quartiles) at the end of each of the first 2 weeks (Table 2). Children who had late bacteremia were at an increased risk of CLD, an MDI of <55, and a PDI between 55 and 69. Placental abruption was not associated with CLD, but was associated with a lower risk of an MDI and a PDI of <55.
TABLE 2.
Selected Prenatal and Postnatal Factors Associated With CLD and MDI or PDI at 24 Months' Postterm Equivalent
| Postnatal Factors | CLD, % | MDI, % | PDI, % | N | ||
|---|---|---|---|---|---|---|
| <55 | 55–69 | <55 | 55–69 | |||
| Marital status | ||||||
| Single | 47 | 16 | 14 | 12 | 16 | 375 |
| Not single | 50 | 8 | 9 | 8 | 16 | 540 |
| Antenatal corticosteroida | ||||||
| Complete | 47 | 12 | 11 | 12 | 15 | 588 |
| Incomplete | 54 | 9 | 12 | 6 | 17 | 234 |
| None | 48 | 11 | 9 | 4 | 15 | 93 |
| Cesarean delivery | ||||||
| Yes | 49 | 10 | 10 | 9 | 15 | 611 |
| No | 49 | 13 | 12 | 10 | 16 | 304 |
| Initiator of preterm delivery | ||||||
| Preterm labor | 46 | 9 | 11 | 8 | 18 | 411 |
| Preterm, prolonged rupture of membranes | 47 | 14 | 9 | 10 | 13 | 201 |
| Preeclampsia | 58 | 16 | 11 | 18 | 9 | 120 |
| Abruption | 55 | 5 | 11 | 2 | 16 | 100 |
| Cervical insufficiency | 44 | 14 | 10 | 15 | 15 | 48 |
| Fetal indicationb | 63 | 17 | 20 | 9 | 20 | 35 |
| Gestational age, wk | ||||||
| 23 | 78 | 13 | 13 | 9 | 28 | 32 |
| 24 | 77 | 16 | 10 | 9 | 14 | 130 |
| 25 | 61 | 11 | 13 | 11 | 18 | 184 |
| 26 | 44 | 12 | 8 | 10 | 14 | 250 |
| 27 | 32 | 8 | 12 | 8 | 14 | 319 |
| Birth weight z score | ||||||
| Less than − 1 | 71 | 17 | 13 | 16 | 18 | 174 |
| −1 to 0 | 52 | 11 | 11 | 7 | 16 | 342 |
| 0 or higher | 37 | 9 | 10 | 9 | 14 | 399 |
| SNAP-II | ||||||
| <20 | 38 | 9 | 9 | 8 | 14 | 489 |
| 20–29 | 57 | 13 | 13 | 10 | 18 | 222 |
| ≥30 | 69 | 16 | 12 | 12 | 19 | 187 |
| Pao2 week 1 | ||||||
| <42 (lowest quartile) | 71 | 19 | 13 | 18 | 13 | 79 |
| ≥42 | 63 | 13 | 12 | 11 | 18 | 321 |
| Missing | 37 | 9 | 10 | 7 | 14 | 515 |
| Pao2 week 2 | ||||||
| <41 (lowest quartile) | 73 | 25 | 12 | 24 | 20 | 51 |
| ≥41 | 66 | 15 | 15 | 12 | 23 | 165 |
| Missing | 43 | 9 | 10 | 8 | 14 | 699 |
| Early bacteremia, week 1 | ||||||
| None | 47 | 9 | 10 | 10 | 16 | 532 |
| Presumed | 52 | 13 | 12 | 5 | 14 | 325 |
| Definite | 51 | 19 | 16 | 9 | 23 | 57 |
| Late bacteremia, week 2–4 | ||||||
| None | 44 | 9 | 10 | 8 | 14 | 549 |
| Presumed | 58 | 9 | 12 | 12 | 16 | 139 |
| Definite | 55 | 18 | 13 | 11 | 21 | 224 |
| Maximum n | 450 | 103 | 99 | 86 | 143 | 915 |
CLD included infants with and without MV (CMV or high-frequency ventilation).
A course of antenatal corticosteroids given to enhance fetal lung maturation was considered complete if the mother received 2 doses of betamethasone 24 hours apart or 4 doses of dexamethasone at 12-hour intervals and delivered at least 48 hours after the first dose.
Fetal indication includes nonreassuring fetal assessments, oligohydramnios, Doppler abnormalities of umbilical cord blood flow, and severe intrauterine growth restriction.
Aggressive modes of ventilation on days 7, 14, 21, and 28 were associated with a greater risk of CLD, as well as a greater risk of an MDI of <55and a PDI of <55 (Table 3). Treatment at 36 weeks' PMA with any form of respiratory support (continuous positive airway pressure [CPAP], CMV, or high-frequency ventilation) was associated with an increased risk of an MDI and a PDI of <55.
TABLE 3.
Mode of Ventilation on Days 0, 7, 14, 21, and 28 and MDIs and PDIs at 24 Months' Postterm Equivalent
| Mode of Ventilation | CLD, % | MDI, % | PDI, % | N | ||
|---|---|---|---|---|---|---|
| <55 | 55–69 | <55 | 55–69 | |||
| Day 0 | ||||||
| No support | 0 | 0 | 0 | 0 | 0 | 0 |
| Hood/cannula | 50 | 0 | 50 | 0 | 0 | 2 |
| CPAP | 20 | 4 | 13 | 1 | 18 | 71 |
| CMV | 49 | 12 | 11 | 10 | 15 | 712 |
| High frequency | 66 | 12 | 11 | 13 | 20 | 130 |
| Day 7 | ||||||
| No support | 7 | 11 | 15 | 5 | 17 | 46 |
| Hood/cannula | 31 | 12 | 9 | 6 | 11 | 95 |
| CPAP | 26 | 6 | 8 | 8 | 17 | 236 |
| CMV | 68 | 12 | 13 | 9 | 14 | 437 |
| High frequency | 73 | 20 | 9 | 18 | 25 | 101 |
| Day 14 | ||||||
| No support | 7 | 11 | 11 | 9 | 13 | 46 |
| Hood/cannula | 25 | 9 | 7 | 5 | 15 | 135 |
| CPAP | 26 | 7 | 11 | 8 | 14 | 217 |
| CMV | 67 | 11 | 11 | 9 | 14 | 395 |
| High frequency | 75 | 23 | 14 | 18 | 25 | 119 |
| Day 21 | ||||||
| No support | 2 | 8 | 10 | 4 | 14 | 49 |
| Hood/cannula | 22 | 7 | 11 | 7 | 16 | 169 |
| CPAP | 29 | 19 | 11 | 9 | 14 | 130 |
| CMV | 68 | 11 | 12 | 9 | 15 | 422 |
| High frequency | 81 | 26 | 11 | 21 | 24 | 80 |
| Day 28 | ||||||
| No support | 2 | 8 | 11 | 6 | 15 | 62 |
| Hood/cannula | 26 | 7 | 12 | 8 | 12 | 216 |
| CPAP | 38 | 11 | 9 | 10 | 16 | 203 |
| CMV | 74 | 12 | 12 | 10 | 16 | 354 |
| High frequency | 84 | 25 | 8 | 16 | 27 | 63 |
CLD included infants with and without MV (CMV or high-frequency ventilation).
Receipt of a methylxanthine for more than 2 weeks was associated with a reduced risk of CLD, an MDI of <55, and a PDI of 55 to 69 (Table 4). Conversely, an increased risk of CLD and an increased risk of MDI and a PDI <55 were associated with receipt of hydrocortisone and with PDA ligation. Children who received an analgesic, a sedative, or multiple transfusions of packed red blood cells were at higher risk than others of CLD and a low MDI. Treatment with dexamethasone was associated with an increased risk of CLD, an MDI of <70, and a PDI of <70.
TABLE 4.
Medication and Treatment Associated With CLD and MDIs and PDIs at 24 Months' Postterm Equivalent
| Medications and Therapies | CLD, % | MDI, % | PDI, % | N | ||
|---|---|---|---|---|---|---|
| <55 | 55–69 | <55 | 55–69 | |||
| Surfactant, first week | ||||||
| Yes | 53 | 11 | 11 | 10 | 16 | 809 |
| No | 24 | 11 | 11 | 7 | 12 | 106 |
| Methylxanthine | ||||||
| Yes | 43 | 11 | 11 | 9 | 15 | 787 |
| No | 85 | 15 | 12 | 11 | 22 | 128 |
| Hydrocortisone | ||||||
| Yes | 67 | 15 | 8 | 18 | 16 | 85 |
| No | 47 | 11 | 11 | 9 | 16 | 830 |
| Dexamethasone | ||||||
| Yes | 71 | 4 | 21 | 8 | 25 | 24 |
| No | 49 | 11 | 11 | 9 | 15 | 821 |
| Analgesics | ||||||
| Yes | 56 | 12 | 10 | 11 | 15 | 579 |
| No | 37 | 10 | 12 | 7 | 17 | 336 |
| Sedative | ||||||
| Yes | 57 | 18 | 15 | 8 | 20 | 200 |
| No | 47 | 10 | 10 | 10 | 14 | 716 |
| PDA treatmenta | ||||||
| Ligation | 69 | 16 | 10 | 11 | 21 | 127 |
| Indomethacin | 53 | 10 | 12 | 11 | 15 | 349 |
| Neither | 46 | 11 | 13 | 8 | 10 | 130 |
| PDA treatmentb | ||||||
| Yes | 58 | 12 | 12 | 10 | 17 | 544 |
| No | 37 | 10 | 10 | 8 | 14 | 371 |
| Transfusionc | ||||||
| Yes | 52 | 12 | 11 | 8 | 16 | 842 |
| No | 14 | 8 | 11 | 10 | 11 | 72 |
CLD included infants with and without MV (CMV or high-frequency ventilation).
Among those with a clinical or echocardiographic PDA diagnosis.
Among all infants.
Packed red blood cells during 3 or 4 separate weeks in the first postnatal month.
Pneumothorax, PIE, PD, EPPD, and higher stages of NEC and ROP were associated with an increased risk of CLD and a low MDI and a low PDI (Table 5). The diagnosis of PDA was associated with an increased risk of CLD, but not a low MDI or a low PDI. Ventriculomegaly and an echolucent lesion were not associated with an increased risk for CLD, but were minimally associated with a low MDI, and modestly with a low PDI.
TABLE 5.
Diagnostic and Classification Entities Associated With MDIs and PDIs at 24 Months' Postterm Equivalent
| Diagnosis | CLD, % | MDI, % | PDI, % | N | ||
|---|---|---|---|---|---|---|
| <55 | 55–69 | <55 | 55–69 | |||
| PDA | ||||||
| No | 38 | 11 | 8 | 8 | 16 | 309 |
| Yes | 55 | 11 | 12 | 10 | 15 | 606 |
| Pneumothorax | ||||||
| No | 47 | 10 | 11 | 9 | 16 | 843 |
| Yes | 71 | 28 | 7 | 18 | 15 | 72 |
| PIE | ||||||
| No | 45 | 10 | 11 | 8 | 14 | 782 |
| Yes | 77 | 19 | 12 | 16 | 26 | 133 |
| Pulmonary hemorrhage | ||||||
| No | 49 | 11 | 11 | 9 | 16 | 889 |
| Yes | 50 | 19 | 8 | 12 | 12 | 26 |
| Respiratory group classification | ||||||
| Low Fio2 | 14 | 6 | 9 | 3 | 13 | 182 |
| PD | 49 | 13 | 10 | 11 | 16 | 341 |
| EPPD | 68 | 12 | 12 | 10 | 17 | 363 |
| NEC | ||||||
| None | 47 | 9 | 10 | 9 | 14 | 569 |
| Watch | 48 | 13 | 14 | 10 | 20 | 147 |
| Stage I | 48 | 13 | 11 | 6 | 13 | 101 |
| Stage II | 68 | 19 | 14 | 8 | 17 | 36 |
| Stage IIIa | 60 | 10 | 10 | 10 | 20 | 10 |
| Stage IIIb | 62 | 28 | 10 | 17 | 31 | 29 |
| Isolated perforation | 70 | 22 | 4 | 17 | 9 | 23 |
| ROP | ||||||
| None | 28 | 10 | 10 | 9 | 15 | 252 |
| Stage 1 | 40 | 6 | 12 | 7 | 12 | 204 |
| Stage 2 | 58 | 11 | 10 | 9 | 15 | 205 |
| Stage 3 | 72 | 17 | 12 | 12 | 21 | 229 |
| Stage 4/5 | 93 | 7 | 4 | 7 | 4 | 14 |
| Ventriculomegaly | ||||||
| No | 49 | 11 | 11 | 15 | 9 | 844 |
| Yes | 50 | 14 | 11 | 21 | 14 | 70 |
| Echolucent lesion | ||||||
| No | 49 | 11 | 11 | 9 | 15 | 871 |
| Yes | 53 | 14 | 14 | 19 | 21 | 43 |
CLD included infants with and without MV (CMV or high-frequency ventilation).
Time-Oriented Risk Models
Because the associations between CLD categories (no CLD, CLD without MV, and CLD with MV) with both the MDI and the PDI were most evident for scores <55, we limited our models to these outcomes. We created 3 epoch models of the MDI and the PDI at <55. We grouped prenatal and birth characteristics and exposures into the antenatal epoch, all exposures and characteristics during the first 28 days into the neonatal epoch, and exposures and characteristics occurring or reported between 28 days and 36 weeks' PMA into the 36-weeks' PMA epoch.
In the antenatal epoch of the TORM for an MDI of <55, gestational age of 23 or 24 weeks, single mother, and the composite variable “preeclampsia or fetal indication” were associated with an increased risk of an MDI of <55 (Table 6). In the neonatal epoch, late bacteremia, pneumothorax, and NEC of Bell stage II or higher were also associated with an MDI of <55. Neither CLD without MV nor CLD with MV, added in the last epoch, achieved statistical significance for an MDI of <55 after we accounted for the antenatal and neonatal epoch variables.
TABLE 6.
ORs and 95% CIs Obtained With TORMs of MDI <55
| Epoch | Epoch, OR (95% CI) | ||
|---|---|---|---|
| Antenatal | Neonatal (First 28 d) | 36 wk PMA | |
| Antenatal | |||
| Gestational age 23–24 wk | 1.7 (1.00–3.1) | 1.4 (0.6–3.0) | 1.3 (0.7–2.8) |
| Single mother | 2.3 (1.5–3.5) | 2.3 (1.5–3.5) | 2.3 (1.5–3.6) |
| Preeclampsia or fetal indication | 2.0 (1.01–3.8) | 1.9 (0.96–3.8) | 1.9 (0.9–3.8) |
| Neonatal (first 28 d) | |||
| Late bacteremia | — | 1.8 (1.3–2.5) | 1.8 (1.3–2.5) |
| Pneumothorax | — | 3.7 (2.6–5.4) | 3.6 (2.5–5.4) |
| NEC ≥ stage II | — | 2.2 (1.3–3.7) | 2.1 (1.2–3.7) |
| 36 wk PMA | |||
| CLD without MV | — | — | 1.1 (0.8–1.4) |
| CLD with MV | — | — | 1.2 (0.7–2.3) |
These analyses include only those children without a severe gross motor impairment (GMFCS score < 1). Antenatal epoch includes the following variables: gestational age of 23–24 weeks; gestational age of 25–26 weeks; single mother; complete course of antenatal steroids; cesarean delivery; and delivery for preeclampsia or fetal indications. Neonatal epoch includes the following variables: SNAP-II in the top quartile; MV or high-frequency ventilation at 7 days; late bacteremia; pneumothorax; PIE; Pao2 in the lowest quartile (week 1); Pao2 missing (week 1); transfusions (packed red blood cells); PD; EPPD; ventriculomegaly; echolucent lesion; echodense lesion; NEC stage II or worse; methylxanthine; PDA; and PDA ligation. Thirty-six weeks' PMA epoch includes the following variables: CLD without MV (CMV or high-frequency ventilation) at 36 weeks and CLD with MV.
In the antenatal epoch of the TORM for a PDI <55, single mother, a complete course of antenatal steroids, and the composite variable “preeclampsia or fetal indication” were associated with an increased risk of a PDI <55 (Table 7). In the neonatal epoch, pneumothorax, PIE, PD, and EPPD were also associated with a PDI <55. Neither CLD without MV nor CLD with MV achieved statistical significance for a PDI <55 after we accounted for the antenatal and neonatal epoch variables. However, CLD with MV approached, but did not achieve, nominal statistical significance (odds ratio [OR]: 1.9 [95% confidence interval (CI): 0.97–3.9]) for the association with a PDI <55.
TABLE 7.
ORs and 95% CIs Obtained With TORMs of PDI <55
| Epoch | Epoch, OR (95% CI) | ||
|---|---|---|---|
| Antenatal | Neonatal (First 28 d) | 36 wk PMA | |
| Antenatal | |||
| Single mother | 1.8 (1.02–3.0) | 1.8 (0.95–3.5) | 1.8 (0.9–3.5) |
| Complete antenatal corticosteroids | 2.3 (1.5–3.5) | 2.4 (1.5–3.9) | 2.4 (1.5–3.8) |
| Preeclampsia or fetal indication | 2.2 (1.5–3.1) | 2.3 (1.7–3.3) | 2.2 (1.5–3.2) |
| Neonatal (first 28 d) | |||
| Pneumothorax | — | 1.9 (1.1–3.5) | 1.9 (1.1–3.3) |
| PIE | — | 2.2 (1.04–4.7) | 2.0 (0.99–4.2) |
| PD | — | 3.6 (1.2–7.6) | 2.7 (1.1–6.5) |
| EPPD | — | 3.0 (1.2–7.6) | 2.7 (1.1–6.5) |
| 36 wk PMA | |||
| CLD without MV | — | — | 1.1 (0.6–2.0) |
| CLD with MV | — | — | 1.9 (0.97–3.9) |
These analyses include only those children without a severe gross motor impairment (GMFCS score < 1). Antenatal epoch includes the following variables: gestational age of 23–24 weeks; gestational age of 25–26 weeks; single mother; complete course of antenatal corticosteroids; cesarean delivery; and delivery for preeclampsia or fetal indications. Neonatal epoch includes the following variables: SNAP-II in the top quartile; MV or high-frequency ventilation at 7 days; late bacteremia; pneumothorax; PIE; Pao2 in the lowest quartile (week 1); Pao2 missing (week 1); transfusions (packed red blood cells); PD; EPPD; ventriculomegaly; echolucent lesion; echodense lesion; NEC stage II or worse; methylxanthine; PDA; and PDA ligation. Thirty-six weeks' PMA epoch includes the following variables: CLD without MV (CMV or high-frequency ventilation) at 36 weeks and CLD with MV.
Discussion
After adjusting for confounders and other antecedents, we found that CLD, even when adjusted for severity, was not associated with an increased risk of delayed development at 24-months adjusted age. On the other hand, antecedents of CLD seemed to be associated with impaired development. Several potential reasons might explain our observations: (1) brain injury occurs as a consequence of antecedents of CLD (eg, infection, pneumothorax, respiratory, and other therapies), rather than CLD itself; (2) brain injury is caused by CLD; however, this association was masked in the multivariate model by previous adjustment for factors in the causal chain leading to CLD; or (3) lung dysfunctions, and associated therapies, are indicators of maturation-associated vulnerabilities of lung and brain.
In reports of developmental delay identified with the BSID-II, study participants are often classified by whether their scores are <70, which according to the test developers indicates “significantly delayed performance.”26 However, studies of extremely low birth weight infants indicate that an appreciable proportion of infants with an MDI of <70 do not have mental retardation when assessed at school age.44,45 By dichotomizing BSID-II scores at 55, we increased the positive predictive value of the definition of developmental delay used in this study. We also restricted our analyses to children who were free from moderate or severe gross, and presumably fine, motor function impairments, which were almost always because of cerebral palsy. The present study estimates the strength of association between CLD and developmental delay at 24 adjusted months only in children who do not have cerebral palsy. Significant fine motor impairment precludes valid assessment of cognitive functioning with the MDI portion of the BSID-II.
However, cerebral palsy is associated with mental retardation.46 If CLD contributes causally to the outcome of cerebral palsy with mental retardation, then our exclusion of infants with cerebral palsy results in an understatement of the strength of the CLD/MDI < 55 relationship. On the other hand, this sample restriction provides a more informative analysis of the relationship of CLD to delayed cognitive and perceptual motor development.
Previous studies attribute much of the increased risk of delayed development to antecedents of CLD. For example, late bacteremia might prolong the need for MV, and prolonged MV is associated with developmental delay.47 In another example, prolonged ventilation and hypocarbia, antecedents of CLD, are associated with ultrasonographically detectable cerebral white matter damage,48 which is, in turn, associated with developmental delay.49,50 We also found that a number of our variables were highly correlated. For example, EPPD and PD predict a low PDI. It is possible that each of these variables conveys information about immaturity of the brain as well as the lung, along with information about exposures that might be in the causal chain leading to a low PDI. Our analyses suggest that it is not CLD, but rather risk factors for CLD, that are more closely linked to developmental delay at 24-months adjusted age. Once these risk factors are considered, CLD imparts no significant additional risk.
Traditionally, risk factors for both a low MDI and a low PDI have been considered together. In the analyses presented here, a low MDI and a low PDI shared antecedents, but some risk factors were antecedents of one but not of the other. This observation suggests that the pathogenesis of developmental delay differs for mental and motor domains.50 Both late bacteremia51 and higher grade NEC52,53 have been identified as antecedents of a low MDI. What they have in common is a systemic inflammatory response that might lead to cerebral white matter injury,54 which predicts low MDI and low PDI scores.50 An inflammatory response, initiated in the intestinal compartment, may become systemic and lead to brain injury through a variety of inflammatory mediators such as tumor necrosis factor α, interleukin 6, and platelet activating factor.55,56 Interventions that have been associated with a lower risk of NEC, such as probiotics57 and human milk feeding58 might improve developmental outcome, as observed in recent studies of human milk and developmental outcome.59
Low PDI seems to be related to factors associated with pulmonary disease (eg, pneumothorax, PIE, PD, and EPPD). Two previous studies15,60 found that the association of CLD and developmental delay arises primarily from the group of infants with severe CLD. Pulmonary inflammation, either initiated or exacerbated by oxygen toxicity and injury from MV, plays a major role in the pathogenesis of CLD. Although lung inflammation may be confined within the lungs, under certain conditions, it may also be accompanied by a systemic inflammatory response.61 It is possible that a systemic inflammatory response associated with prolonged MV (ie, among infants with CLD with MV) might increase the likelihood of neonatal brain injury and subsequent disability. Our inability to demonstrate this relationship conclusively may have resulted from the relatively small number of infants with CLD with MV in our sample. Alternatively, the need for ventilator assistance and/or ventilator-associated lung injuries might be markers for immaturity-related brain vulnerability, and might not be causally related. It is also possible that by excluding infants with significantly impaired gross motor function, we may have understated the strength of association between severe CLD and development delay.
Compared with the infants of married mothers, infants of single mothers were at higher risk for delayed cognitive development. “Single mother” may be a variable that summarizes disadvantaged social class correlates. Social disadvantage has repeatedly been found as a predictor of impaired development in children born preterm.1–3,62–64 Explanations include potential contributions of genetics and limited access to resources that enhance development.
Delayed development was more frequent among children whose mothers were treated with antenatal steroids than among children not exposed in utero to exogenous steroids. An explanation for the apparent adverse effect of antenatal steroids is that antenatal steroids were not randomly assigned, so confounding by indication is likely to have occurred.65 For example, deliveries in response to maternal and fetal indication tend to have higher rates of antenatal steroids than “spontaneous” deliveries. Another explanation is that exposure to antenatal steroids might have improved survival, which may come at the expense of greater risk of impairment.66
Our study offers several advantages over previous studies. First, we selected infants on the basis of gestational age, not birth weight, to minimize confounding because of factors related to fetal growth restriction.67 Second, examiners were not aware of the medical histories of the children they examined, thereby minimizing “diagnostic suspicion bias.”68 Third, high quality ultrasonographic data describing brain damage were available to us as a result of rigorous efforts to minimize variability in sonologists' interpretations.33
Conclusions
Antecedents and correlates of CLD are associated strongly with the risk of delayed mental and motor development. Infants with CLD who were receiving MV at 36 weeks' adjusted age might have an increased risk of neurodevelopmental impairment. This observation will inform future investigations of the biological mechanisms underlying the association between CLD and later neurodevelopmental impairments affecting former preterm infants.
What's Known on This Subject: Some studies suggest that CLD in ELGANs is associated with neurodevelopmental impairments.
What This Study Adds: Among children without severe gross motor delays, risk factors for CLD account for the association between CLD and developmental delay. However, severe CLD might increase the risk of lower motor scores.
Acknowledgments
This study was supported by a cooperative agreement (5U01NS040069-04) with the National Institute of Neurological Disorders and Stroke. Dr Bose was supported by the Thrasher Research Fund.
We thank our ELGAN study colleagues: Tufts Medical Center (Boston, MA): Olaf Dammann and John Fiascone; Baystate Medical Center (Springfield, MA): Bhavesh L. Shah; Beth Israel Deaconess Medical Center (Boston, MA): Camilia Martin; Brigham and Women's Hospital (Boston, MA): Robert Insoft; Boston Medical Center (Boston, MA): Karl Kuban; UMass Memorial Health Center (Worcester, MA): Francis Bednarek; Yale University School of Medicine (New Haven, CT): Richard A. Ehrenkranz; Wake Forest University/Baptist Medical Center (Winston-Salem, NC): T. Michael O'Shea; University Health Systems of Eastern Carolina (Greenville, NC): Stephen C. Engelke; University of North Carolina (Chapel Hill, NC): Carl Bose; DeVos Children's Hospital (Grand Rapids, MI): Mariel Poortenga, Ed Beaumont; Sparrow Hospital (Lansing, MI): Nigel Paneth; University of Chicago Hospital (Chicago, IL): Michael D. Schreiber; William Beaumont Hospital (Royal Oak, MI): Daniel Batton; Frontier Science and Technology Research Foundation (Amherst, NY): Greg Pavlov; and Deborah Hirtz (project officer).
We thank our ELGAN study follow-up colleagues: Baystate Medical Center (Springfield, MA): Susan McQuiston, Herbert Gilmore, and Karen Christianson; Beth Israel Deaconess Medical Center (Boston, MA): AK Morgan, Haim Bassan, Cecil Hahn, Samantha Butler, Adre Duplessis, and Colleen Hallisey; MA General Hospital (Boston, MA): Kalpathy Krishnamoorthy and Maureen Quill; Floating Hospital for Children at Tufts Medical Center: Cecilia Keller, Karen Miller, Page Church, and Caitlyn Hurley; UMass Memorial Health Center (Worcester, MA): Robin Adair, Alice Miller, Rick Bream, Albert Scheiner, and Beth Powers; Yale University School of Medicine (New Haven, CT): Elaine Romano, Nancy Close, and Joanne Williams; East Carolina University: Kathyrn Kerkering, Steve Engelke, Lynn Whitley, Rebecca Helms, and Peter Resnik; Wake Forest University Baptist Medical Center (Winston-Salem, NC): Gail Hounshell, Don Goldstein, Lisa Washburn, Cherrie Heller, Robert Dillard, Debbie Hiatt, and Deborah Allred; University of North Carolina (Chapel Hill, NC): Diane Marshall, Lisa Bostic, Janice Wereszczak, Mandy Taylor, Carol Torres, Kristi Milowic, and Gennie Bose; DeVos Children's Hospital (Grand Rapids, MI): Lynn Fagerman, Steve Pasynrnak, Victoria Caine, Wendy Burdo-Hartman, and Dianah Sutton; Michigan State University: Nicholas Olomu, Padu Karna, Victoria Caine, Joan Price, and Karen Miras; University of Chicago: Sunila O'Connor, Michael Msall, Susan Plesha-Troyke, Leslie Caldarelli, and Grace Yoon; and William Beaumont Hospital (Royal Oak, MI): Karen Brooklier, Katie Solomon, Dan Batton, and Melisa Oca, Beth Kring.
Abbreviations
- CLD
chronic lung disease
- MV
mechanical ventilation
- PDA
patent ductus arteriosus
- ELGAN
extremely low gestational age newborn
- BSID-II
Bayley Scales of Infant Development-2nd Edition
- VABS
Vineland Adaptive Behavioral Scales
- MDI
Mental developmental index
- PDI
Psychomotor developmental index
- GMFCS
Gross Motor Function Classification System
- SNAP-II
Score for Neonatal Acute Physiology-II
- Fio2
fraction of inspired oxygen
- PD
pulmonary deterioration
- EPPD
early and persistent pulmonary dysfunction
- PIE
pulmonary interstitial emphysema
- ROP
retinopathy of prematurity
- PMA
postmenstrual age
- CMV
conventional mechanical ventilation
- NEC
necrotizing enterocolitis
- TORM
time-oriented risk model
- CPAP
continuous positive airway pressure
- OR
odds ratio
- CI
confidence interval
Footnotes
Financial Disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105(6):1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 2.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F134–F140. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fily A, Pierrat V, Delporte V, Breart G, Truffert P. Factors associated with neurodevelopmental outcome at 2 years after very preterm birth: the population-based Nord-Pas-de-Calais EPIPAGE cohort. Pediatrics. 2006;117(2):357–366. doi: 10.1542/peds.2005-0236. [DOI] [PubMed] [Google Scholar]
- 4.Singer L, Yamashita T, Lilien L, Collin M, Baley J. A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight. Pediatrics. 1997;100(6):987–993. doi: 10.1542/peds.100.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes CA, O'Gorman LA, Shyr Y, Schork MA, Bozynski ME, McCormick MC. Cognitive performance at school age of very low birth weight infants with bronchopulmonary dysplasia. J Dev Behav Pediatr. 1999;20(1):1–8. doi: 10.1097/00004703-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Lewis BA, Singer LT, Fulton S, et al. Speech and language outcomes of children with bronchopulmonary dysplasia. J Commun Disord. 2002;35(5):393–406. doi: 10.1016/s0021-9924(02)00085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer LT, Siegel AC, Lewis B, Hawkins S, Yamashita T, Baley J. Preschool language outcomes of children with history of bronchopulmonary dysplasia and very low birth weight. J Dev Behav Pediatr. 2001;22(1):19–26. doi: 10.1097/00004703-200102000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray PH, O'Callaghan MJ, Rogers YM. Psychoeducational outcome at school age of preterm infants with bronchopulmonary dysplasia. J Paediatr Child Health. 2004;40(3):114–120. doi: 10.1111/j.1440-1754.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 9.Short EJ, Klein NK, Lewis BA, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112(5) doi: 10.1542/peds.112.5.e359. Available at: www.pediatrics.org/cgi/content/full/112/5/e359. [DOI] [PMC free article] [PubMed]
- 10.Bozynski ME, Nelson MN, Matalon TA, et al. Prolonged mechanical ventilation and intracranial hemorrhage: impact on developmental progress through 18 months in infants weighing 1 200 grams or less at birth. Pediatrics. 1987;79(5):670–676. [PubMed] [Google Scholar]
- 11.Laptook AR, O'Shea TM, Shankaran S, Bhaskar B, NICHD Neonatal Network Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115(3):673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 12.Robertson CM, Etches PC, Goldson E, Kyle JM. Eight-year school performance, neurodevelopmental, and growth outcome of neonates with bronchopulmonary dysplasia: a comparative study. Pediatrics. 1992;89(3):365–372. [PubMed] [Google Scholar]
- 13.Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):227–232. doi: 10.1053/j.semperi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 15.Short EJ, Kirchner HL, Asaad GR, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Arch Pediatr Adolesc Med. 2007;161(11):1082–1087. doi: 10.1001/archpedi.161.11.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeng SF, Hsu CH, Tsao PN. Bronchopulmonary dysplasia predicts adverse developmental and clinical outcomes in very-low-birth weight infants. Dev Med Child Neurol. 2008;50(1):51–57. doi: 10.1111/j.1469-8749.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 17.Marshall DD, Kotelchuck M, Young TE, et al. Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. Pediatrics. 1999;104(6):1345–1350. doi: 10.1542/peds.104.6.1345. [DOI] [PubMed] [Google Scholar]
- 18.Van Marter LJ, Dammann O, Allred EN, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140(2):171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt B. Methylxanthine therapy for apnea of prematurity: evaluation of treatment benefits and risks at age 5 years in the international Caffeine for Apnea of Prematurity (CAP) trial. Biol Neonate. 2005;88(3):208–213. doi: 10.1159/000087584. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt B, Roberts RS, Davis P, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 21.Tyson JE, Wright LL, Oh W, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340(25):1962–1968. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 22.Ambalavanan N, Tyson JE, Kennedy KA, et al. Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics. 2005;115(3) doi: 10.1542/peds.2004-1812. Available at: www.pediatrics.org/cgi/content/full/115/3/e249. [DOI] [PubMed]
- 23.Ritchie K, Carriere I, de Mendonca A, et al. The neuroprotective effects of caffeine: a prospective population study (the Three City Study) Neurology. 2007;69(6):536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- 24.Laughon M, Allred EN, Bose C, et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics. 2009;123(4):1124–1131. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laughon M, Bose C, Allred E, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119(2):273–280. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayley N. Bayley Scales of Infant Development. 2nd. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 27.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birth weight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 29.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 30.Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102(8):1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 31.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17(4):213–288. doi: 10.1016/0045-9380(87)90031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teele R, Share J. Ultrasonography of Infants and Children. Philadelphia, PA: Saunders; 1991. [Google Scholar]
- 33.Kuban K, Adler I, Allred EN, et al. Observer variability assessing US scans of the preterm brain: the ELGAN study. Pediatr Radiol. 2007;37(12):1201–1208. doi: 10.1007/s00247-007-0605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuban KC, O'Shea M, Allred E, et al. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. J Child Neurol. 2005;20(10):829–831. doi: 10.1177/08830738050200101001. [DOI] [PubMed] [Google Scholar]
- 35.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 36.Sparrow S, Balla D, Cicchetti D. Vineland Adaptive Behavioral Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- 37.Dales LG, Ury HK. An improper use of statistical significance testing in studying covariables. Int J Epidemiol. 1978;7(4):373–375. doi: 10.1093/ije/7.4.373. [DOI] [PubMed] [Google Scholar]
- 38.Leviton A, Pagano M, Kuban KC, Krishnamoorthy KS, Sullivan KF, Allred EN. The epidemiology of germinal matrix hemorrhage during the first half-day of life. Dev Med Child Neurol. 1991;33(2):138–145. doi: 10.1111/j.1469-8749.1991.tb05092.x. [DOI] [PubMed] [Google Scholar]
- 39.Leviton A, Kuban KC, Van Marter L, Pagano M, Allred EN. Antenatal corticosteroids appear to reduce the risk of postnatal germinal matrix hemorrhage in intubated low birth weight newborns. Pediatrics. 1993;91(6):1083–1088. [PubMed] [Google Scholar]
- 40.Leviton A, Paneth N, Reuss ML, et al. Hypothyroxinemia of prematurity and the risk of cerebral white matter damage. J Pediatr. 1999;134(6):706–711. doi: 10.1016/s0022-3476(99)70285-4. [DOI] [PubMed] [Google Scholar]
- 41.Leviton A, Dammann O, Allred EN, et al. Antenatal corticosteroids and cranial ultrasound abnormalities. Am J Obstet Gynecol. 1999;181(4):1007–1017. doi: 10.1016/s0002-9378(99)70344-3. [DOI] [PubMed] [Google Scholar]
- 42.Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Pediatr Res. 1999;46(5):566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22(16):2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- 44.Kitchen WH, Rickards AL, Ford GW, Doyle LW, Kelly E, Ryan MM. Selective improvement in cognitive test scores of extremely low birth weight infants aged between 2 and 5 years. Aust Paediatr J. 1989;25(5):288–291. doi: 10.1111/j.1440-1754.1989.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 45.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 46.Rumeau-Rouquette C, Grandjean H, Cans C, du Mazaubrun C, Verrier A. Prevalence and time trends of disabilities in school-aged children. Int J Epidemiol. 1997;26(1):137–145. doi: 10.1093/ije/26.1.137. [DOI] [PubMed] [Google Scholar]
- 47.Walsh MC, Morris BH, Wrage LA, et al. Extremely low birth weight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146(6):798–804. doi: 10.1016/j.jpeds.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 48.Dammann O, Allred EN, Van Marter LJ, Dammann CE, Leviton A. Bronchopulmonary dysplasia is not associated with ultrasound-defined cerebral white matter damage in preterm newborns. Pediatr Res. 2004;55(2):319–325. doi: 10.1203/01.PDR.0000100906.09524.88. [DOI] [PubMed] [Google Scholar]
- 49.Hintz SR, O'Shea M. Neuroimaging and neurodevelopmental outcomes in preterm infants. Semin Perinatol. 2008;32(1):11–19. doi: 10.1053/j.semperi.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 50.O'Shea TM, Kuban KC, Allred EN, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122(3) doi: 10.1542/peds.2008-0594. Available at: www.pediatrics.org/cgi/content/full/122/3/e662. [DOI] [PMC free article] [PubMed]
- 51.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 52.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 53.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153(2):170–175. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 54.Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122(2):299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 55.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997;32(2):275–282. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 56.Amer MD, Hedlund E, Rochester J, Caplan MS. Platelet-activating factor concentration in the stool of human newborns: effects of enteral feeding and neonatal necrotizing enterocolitis. Biol Neonate. 2004;85(3):159–166. doi: 10.1159/000075306. [DOI] [PubMed] [Google Scholar]
- 57.Lin HC, Hsu CH, Chen HL, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122(4):693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 58.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O'Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. 2007;27(7):428–433. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- 59.Vohr BR, Poindexter BB, Dusick AM, et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118(1) doi: 10.1542/peds.2005-2382. Available at: www.pediatrics.org/cgi/content/full/118/1/e115. [DOI] [PubMed]
- 60.Grégoire MC, Lefebvre F, Glorieux J. Health and developmental outcomes at 18 months in very preterm infants with bronchopulmonary dysplasia. Pediatrics. 1998;101(5):856–860. doi: 10.1542/peds.101.5.856. [DOI] [PubMed] [Google Scholar]
- 61.Kramer BW, Ikegami M, Jobe AH. Intratracheal endotoxin causes systemic inflammation in ventilated preterm lambs. Am J Respir Crit Care Med. 2002;165(4):463–469. doi: 10.1164/ajrccm.165.4.2011118. [DOI] [PubMed] [Google Scholar]
- 62.Leonard CH, Clyman RI, Piecuch RE, Juster RP, Ballard RA, Behle MB. Effect of medical and social risk factors on outcome of prematurity and very low birth weight. J Pediatr. 1990;116(4):620–626. doi: 10.1016/s0022-3476(05)81616-6. [DOI] [PubMed] [Google Scholar]
- 63.Weisglas-Kuperus N, Baerts W, Smrkovsky M, Sauer PJ. Effects of biological and social factors on the cognitive development of very low birth weight children. Pediatrics. 1993;92(5):658–665. [PubMed] [Google Scholar]
- 64.Wang LW, Wang ST, Huang CC. Preterm infants of educated mothers have better outcome. Acta Paediatr. 2008;97(5):568–573. doi: 10.1111/j.1651-2227.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 65.Walker AM. Confounding by indication. Epidemiology. 1996;7(4):335–336. [PubMed] [Google Scholar]
- 66.Lorenz JM, Paneth N, Jetton JR, den Ouden L, Tyson JE. Comparison of management strategies for extreme prematurity in New Jersey and the Netherlands: outcomes and resource expenditure. Pediatrics. 2001;108(6):1269–1274. doi: 10.1542/peds.108.6.1269. [DOI] [PubMed] [Google Scholar]
- 67.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134(6):604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 68.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32(1–2):51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]