Abstract
Non-steroidal anti-inflammatory drugs such as aspirin are used for pain relief and chemoprevention against cancer, but frequently cause gastric mucosal injury. We examined whether combinations of aspirin and alpha-tocopherol (αT) or gamma-tocopherol (γT), two major forms of vitamin E, are better anti-inflammatories than aspirin alone and whether these combinations alleviate aspirin-associated side effects. In the carrageenan-induced air-pouch inflammation model in the rat, aspirin (150mg/kg) or a combination of aspirin and γT (33mg/kg) inhibited proinflammatory prostaglandin E2 (PGE2) by 70% (P<0.02) at the inflammation site at six hours after inflammation was initiated. But at 18 hours, only the combination decreased exudate volume (15%, P<0.05), and showed modest inhibition of PGE2 (40%, P<0.07) and lactate dehydrogenase activity (30%, P=0.07), in the fluid collected at the inflammation site. γT but not αT spared aspirin-caused reduction of food intake, partially reversed aspirin-depressed gastric PGE2 and attenuated stomach lesions. Surprisingly, the combination of aspirin and αT (33mg/kg) did not show more benefits than aspirin alone, but worsened gastric injury and food-intake reduction. Our study demonstrated that a combination of aspirin and γT, but not αT, has some advantage over aspirin alone in the anti-inflammatory effect and attenuation of the aspirin-caused adverse effect. This combination may be useful to complement aspirin in the treatment of chronic inflammatory conditions and cancer.
Keywords: Vitamin E, NSAIDs, cyclooxygenase, PGE2, inflammation
INTRODUCTION
Cyclooxygenase (COX)-catalyzed conversion of arachidonic acid to pro-inflammatory eicosanoids, including prostaglandin E2 (PGE2), plays a key role in regulation of the inflammatory response. During inflammation, the inducible COX-2 is primarily responsible for PGE2 generation at the inflammation site, in contrast to the constitutively expressed COX-1 which is found in many tissues including the stomach where prostaglandins are generated to help maintain physiological activities (1–3). Non-steroidal anti-inflammatory drugs (NSAIDs) targeting COX-1 and/or COX-2 have widely been used for the treatment of inflammatory diseases such as arthritis. However, NSAIDs including aspirin are known to have adverse effects including upper gastrointestinal (GI) injury such as ulcers (4, 5). Aspirin causes GI problems, in part, due to its irreversible inhibition of COX-1-mediated formation of gastric PGE2 which is important for cyto-protection (6, 7). In addition, the stomach damage caused by aspirin appears to involve oxidative stress (5, 8). Therefore, addition of antioxidants, such as vitamin E, has been proposed for alleviating the aspirin-associated side effect. The combination of aspirin and α-tocopherol (αT), the major form of Vitamin E in tissues, has been investigated in animals and clinical trails, but these studies have had varied outcomes (9–12).
Recent studies indicate that γ-tocopherol (γT), the predominant form of vitamin E in US diets, have unique bioactivities compared with αT (13–15). We have shown that γT, but not αT, has anti-inflammatory properties mediated through inhibition of COX-2 catalyzed pro-inflammatory PGE2 in cultured cells and in a rat inflammation model (16, 17). γT is better than αT in inhibiting lipid peroxidation and scavenging electrophilic products of inflammation such as peroxynitrite and other reactive nitrogen species (18–20). Because γT has both anti-inflammatory and antioxidant activity, we hypothesize that a combination of aspirin and γT may show a stronger anti-inflammatory effect and may alleviate aspirin-caused stomach injury. To test this hypothesis, we compared the anti-inflammatory action of a combination of aspirin and γT to aspirin alone in the rat in a carrageenan-injected air-pouch model. The effect of γT on attenuating aspirin-related stomach injury was also examined. For comparison, the combination of aspirin and αT was tested.
MATERIALS AND METHODS
Chemicals
αT (~99%) and γT (95%–97%) were purchased from Acros Organics (New Jersey, USA) or Sigma (St Louis, MO, USA). Carrageenan, aspirin, and all other chemicals were from Sigma.
Carrageenan-induced inflammation in the air-pouch model
The animal use protocol was approved by the animal care committee at Purdue University and strictly followed. Male Wistar rats (250–330g) (Charles River, CA, USA) were caged singly and routinely fed ad libitum with 2018 Teklad Global 18% Protein Rodent Maintenance Diet (Harlan Teklad, IN) with free access to the tap water. An air pouch was created by a subcutaneous injection of 12mL sterile air into the intrascapular area of the rat’s back. Thirty hours later, 2mL of 0.6% carrageenan or phosphate-buffered saline (PBS) (as non-inflamed controls) was injected into the air pouch. αT and γT was first dissolved in tocopherol-stripped corn oil (Dyets Inc., Bethlehem, PA, USA), and then aspirin was added. Prior to each feeding, the mixtures with aspirin were vigorously mixed. Animals were fed with aspirin (150mg/kg bw), aspirin plus γT (33 mg/kg) or αT (33 mg/kg) continuously for 3 days by gavage using 0.5mL tocopherol-stripped corn oil as the vehicle. Control animals received the same volume of tocopherol-stripped corn oil. Immediately after the third gavage, carrageenan was injected in the pouch to initiate inflammation. Six or eighteen hours later, rats were sacrificed and pouch fluid was collected by lavage with Hanks-buffered saline solution (HBSS) containing 0.004% heparin but no Ca2+/Mg2+. After a brief centrifugation, the supernatant was collected and frozen immediately for the measurement of PGE2, LTB4, and TNF-α etc. Total cells were counted by a hemocytometer.
Stomach lesion evaluation
After sacrificing, the stomach was removed, cut open along the greater curvature and rinsed in saline. Macroscopic lesions were assessed. Scores were given for different grade of injury: 0, no lesion; 1, slight injury (<1 gastric ulcer); 2, intermediate injury (1–2 gastric ulcers); 3, severe injury (>2 gastric ulcers). Ulcer lesion is defined as mucosal erosion equal or greater than 0.3 cm.
Measurement of αT and γT
Plasma and exudate αT and γT were extracted using a mixture of methanol/hexane (2:5, v/v) in the presence of 0.8 mM butylated hydroxytoluene (21, 22). After a brief centrifugation at 4°C, the top hexane layer was dried under N2, and the residue was re-suspended in ethanol. Tocopherols were separated on a 150 × 4.6mm, 5μm Supelcosil™ LC-18-DB column (Supelco, Bellefonte, PA, USA), and eluted with 95:5 (v/v) methanol/0.1M lithium acetate (final 25mM, pH 4.75) at a flow rate of 1.3 ml/min. Tocopherols were monitored by coulometric detection (Model Coulochem II, ESA Inc., Chelmsford, MA, USA) at 300 (upstream) and 500mV (downstream electrode) using a Model 5011 analytical cell.
Measurement of PGE2, LTB4, 8-isoprostane, LDH and TNF-α in the exudate fluid
The exudate fluid was mixed vigorously with 2 mL methanol to precipitate proteins and with 5 mL hexane to remove lipids. Following a brief centrifugation and aspiration of the hexane layer, the methanol layer was removed and evaporated under N2. PGE2, LTB4 and 8-isoprostane were measured using the corresponding ELISA kits from Cayman Chemicals (Ann Arbor, MI, USA). TNF-α and lactate dehydrogenase (LDH) in the exudate were measured directly using an ELISA kit from R&D (Minneapolis, MN, USA) and an analytical kit from Roche (Indianapolis, IN, USA), respectively.
Measurement of PGE2 and 8-isoprostane in stomach mucosa
Gastric mucosal samples were weighed and homogenized in 400μL methanol at 4 °C. After a brief centrifugation to remove unbroken tissues, the supernatant was collected and dried under N2. The residues were reconstituted in 200μL PBS. PGE2 and 8-isoprostane (pg/mg of tissue) were measured by ELISA assays (Cayman Chemicals).
Statistics
One-way ANOVA was performed in all the data analysis, and non-paired student t-tests were performed to compare two treatment groups. Data are expressed as mean ± SEM.
RESULTS
Effect of aspirin or combinations of aspirin and γT or αT on inflammatory response
In a carrageenan-induced inflammation model in the rat, a single injection of carrageenan causes potent localized inflammation, as indicated by a marked increase in white cell infiltration, eicosanoid formation and tissue damages (23). This model is known to mimic the pathological process occurring in joint diseases (23, 24) and has been used to evaluate the anti-inflammatory efficacy of NSAIDs. In the current study, aspirin (150mg/kg) or the combination of aspirin and γT or αT (33mg/kg) was administered by gavage using corn oil as the vehicle for three days. Aspirin at the similar dose is known to have anti-inflammatory effects and has been shown to cause stomach lesion. The dosage of γT represents a relatively-high supplementation dose, at which it exhibits anti-inflammatory effect in vivo (16).
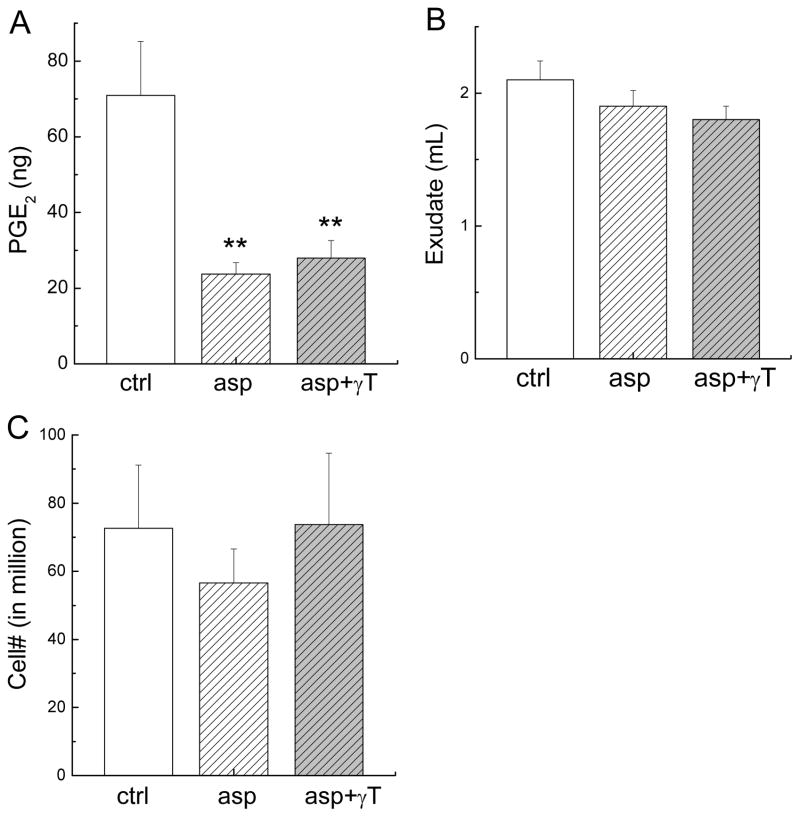
Six hours after carrageenan injection, there was a marked increase of proinflammatory eicosanoids in the pouch compared with non-inflamed controls, among which PGE2 (70.9 ± 14.3 ng/pouch versus 0.1 ± 0.06 ng/pouch) was quantitatively predominant compared with others such as LTB4 (0.33 ± 0.22 ng/pouch versus 0.05 ± 0.03 ng/pouch). Carrageenan treatment also caused a significant increase of TNF-a (4.7 ± 2.1 ng/pouch versus 0.3 ± 0.2 ng/pouch). Administration of aspirin or aspirin plus γT reduced PGE2 by 70% (P < 0.02) at the site of inflammation (Figure 1A), whereas neither showed significant effect on exudate volume (Figure 1B) or the number of immune cells infiltrating (Figure 1C). Aspirin alone or combination with γT did not have significant effect on LTB4 or TNF-α (data not shown).
Figure 1. The effect on PGE2, exude volume and the number of immune cell infiltration at the inflammation site at 6 hrs after carrageenan injection.
Male Wistar rats were fed with aspirin at 150 mg/kg (asp, n = 6) or combination of aspirin and γT at 33 mg/kg (asp+ γT, n = 6) by gavage using 0.5 mL tocopherol-stripped corn oil as the vehicle for 3 days before carrageenan injection. Control (ctrl, n = 6) animals received the same volume of tocopherol-stripped corn oil. Six hours after the induction of inflammation, the effect on PGE2 (A), exudate volume (B) and immune cell infiltration (in million) (C) were determined. Results are expressed as Mean ± SEM. **P < 0.02 indicates a statistically significant difference between asp or asp+γT and ctrl.
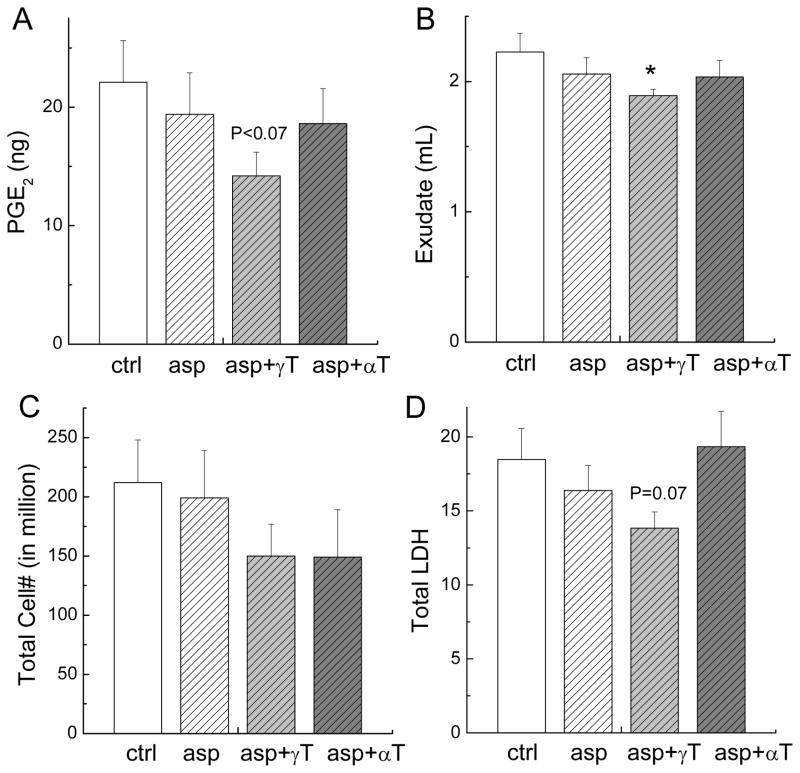
At eighteen hours after the initiation of inflammation, PGE2 levels at the inflammation site remained elevated, whereas LTB4 and TNFα in the pouch dropped to the basal level of non-inflamed controls (data not shown). Compared with corn-oil treated rats, animals fed the combination of aspirin and γT, but not aspirin alone or aspirin and αT, had 40% (P < 0.07) reduced PGE2 (Figure 2A) and 15% decreased (P < 0.05) exudate volume (Figure 2B). None of the treatment affected immune cell infiltration (Figure 2C). Inflammation-associated tissue damage was assayed by increased release of cytosol lactate dehydrogenase (LDH), a marker of cytotoxicity and tissue damage (23). The combination of aspirin and γT showed a tendency in decreasing (~30%, P = 0.07) LDH in the exudate fluid (Figure 2D).
Figure 2. The effect on PGE2, exude volume, immune cell infiltration and LDH at the inflammation site at 18 hrs after carrageenan injection.
Male Wistar rats were fed with aspirin at 150 mg/kg (asp, n = 11), combination of aspirin and γT at 33 mg/kg (asp+γT, n = 11), or combination of aspirin and αT at 33 mg/kg (asp+αT, n = 11) by gavage using 0.5 mL tocopherol-stripped corn oil as the vehicle for 3 days before carrageenan injection. Control (ctrl, n = 11) animals received the same volume of tocopherol-stripped corn oil. Eighteen hours after the induction of inflammation, the effect on PGE2 (A) exudate volume (B), cell infiltration (C) and LDH (D) in exudate fluid were evaluated. Results are expressed as Mean ± SEM. *P < 0.05 or P values indicate a statistic difference between treated rats and corn-oil controls.
Plasma and exudate concentrations of αT and γT
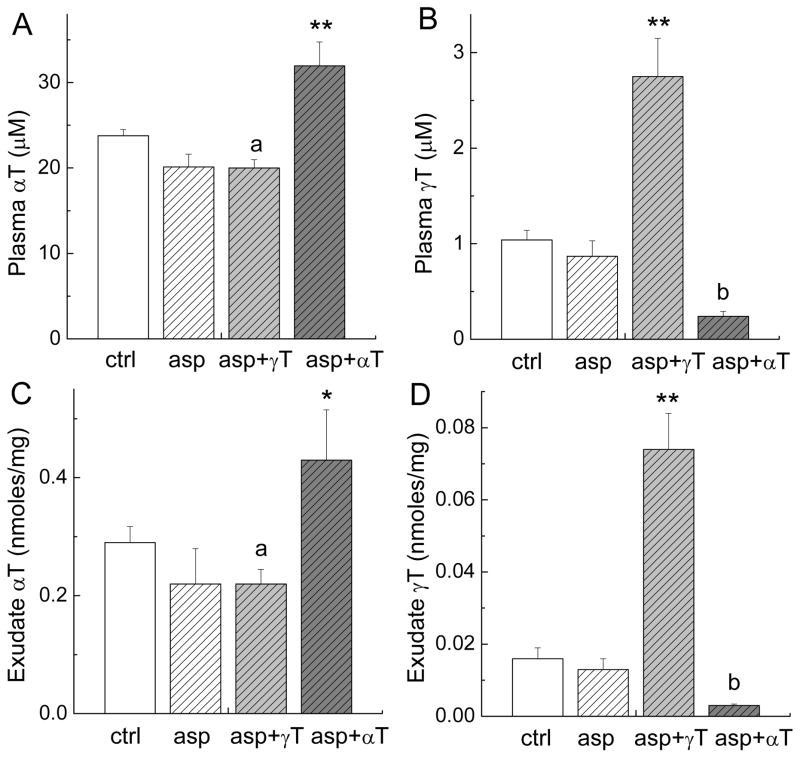
To evaluate the relative bio-availability of the administrated compounds, we measured concentrations of γT and αT in the plasma and exudate. Feeding of αT or γT led to significant increases in the corresponding tocopherols in the plasma. Compared with aspirin-fed rats, αT-administrated animals had 1.5-fold increase of this tocopherol and γT-fed rats had 3-fold elevation of γT in the plasma (Figure 3A and 3B). Similarly, rats administered with αT or γT had 2-fold (P < 0.05) or 5-fold (P < 0.01) increase of the respective tocopherol in the exudate fluid (Figure 3C and 3D). Like previous studies (25, 26), αT caused a significant decrease of γT in the plasma (73%, P < 0.01) and exudate (77%, P < 0.01). Aspirin alone non-significantly lowered αT, while aspirin plus γT significantly decreased αT in the plasma (16%, P < 0.05) and exudate (24%, P < 0.05), respectively. This reduction of αT seems to be associated with aspirin because the concentrations of αT were similar in rats fed aspirin and aspirin plus γT (Figure 3A and 3C), and γT has been shown to have no significant impact on or sometimes increase αT (25, 26).
Figure 3. Plasma and exudate concentrations of αT and γT.
The conditions of each treatment are the same as indicated in Fig. 2. *P < 0.05 or **P < 0.01 indicate a significant difference between the combinations and aspirin alone. a(P < 0.05) indicates a significant difference in αT concentrations between ctrl and asp+γT. b(P < 0.01) indicates a significant difference in γT concentrations between asp+αT and asp.
Effects on food intake, stomach lesion, and gastric PGE2 and 8-isoprostane
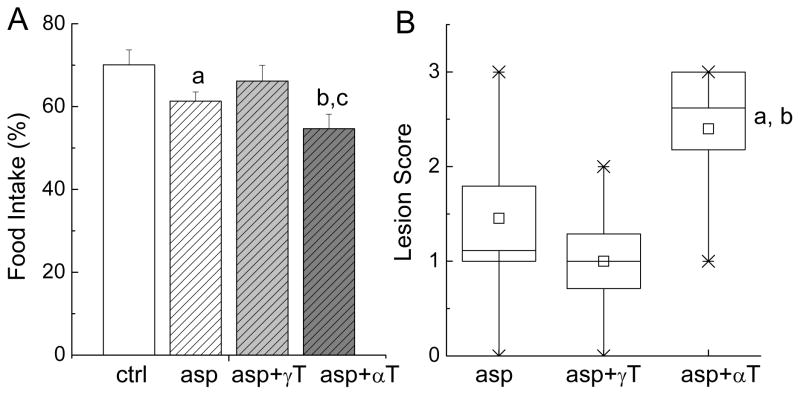
We previously showed that carrageenan treatment led to 30–40% reduction of food intake during the first twenty hours after inflammation was initiated (16). Similarly, in the current study, carrageenan injection caused 30% reduction of food intake (Figure 4A). Aspirin treatment led to a further and significant decrease (12.4%, P < 0.05) of food intake compared with corn-oil controls (Figure 4A). γT supplementation spared aspirin-caused decrease in food intake (Figure 4A). On the other hand, the combination of aspirin and αT appeared to aggravate aspirin-induced food reduction, as indicated by 22% (P<0.01), 11% (P = 0.12) and 18% (P<0.05) reduction of food consumption compared with corn-oil controls, aspirin alone and aspirin plus γT, respectively (Figure 4A).
Figure 4. Effect on food intake (A) and stomach injury (B).
The conditions of each treatment are the same as indicated in Figure 2. The food consumption (%) of individual animal (A) was the ratio of the food intake measured after and before the carrageenan injection. Results are expressed as Mean ± SEM. a(P < 0.05) and b(P < 0.01) indicate a statistically significant difference between the asp- or asp+αT-treated rats and the corn oil-administrated animals, respectively. c(P < 0.05) indicates significant difference between asp+γT and asp+αT administered rats. Stomach lesion (B) was expressed in a Box plot showing box as SE and whisker of 5–95%. a(P < 0.05) or b(P < 0.01) indicates a significant difference between the treatment of asp- and asp+αT, or asp+γT and asp+αT, respectively.
Consistently with reduced food intake, three-day administration of aspirin caused lesions in the stomach as indicated by macroscopic examination which showed gastric ulcers. The combination of aspirin and γT non-significantly alleviated aspirin-induced lesions (Figure 4B). In contrast, the combination of aspirin and αT showed more severe lesions compared with aspirin alone (P < 0.05) or the combination of aspirin and γT (P < 0.01) (Figure 4B).
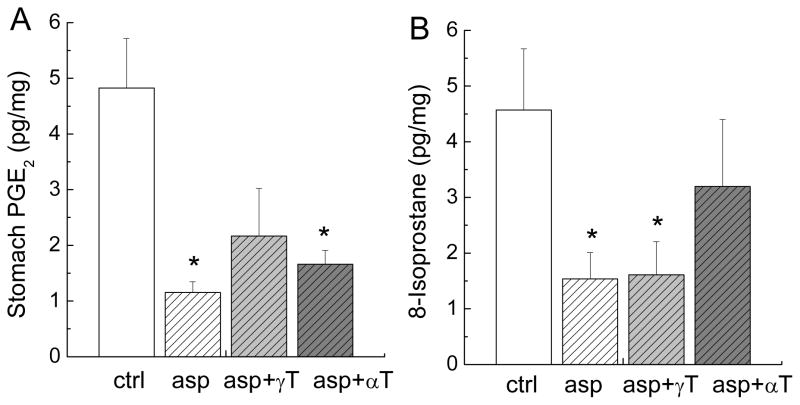
To explain the beneficial effect of γT on aspirin-induced food reduction and seemingly protection of stomach injury, we examined the drug effect on gastric PGE2, which is generated by COX-1-catalyzed reaction and is believed to provide mucosal cytoprotection (6, 7). Eighteen hours after the last dosing, rats fed aspirin and aspirin plus αT had more than 4-fold (P < 0.05) and 3-fold (P < 0.05) reduced PGE2, respectively, in the mucus of stomach (Figure 5A). In contrast, the combination of aspirin and γT partially attenuated aspirin-caused reduction of gastric PGE2. Because oxidative stress may play a role in aspirin-caused stomach injury (8, 10), we measured 8-isoprostane, a lipid peroxidation marker. Compared with corn-oil controls, treatment with aspirin or aspirin plus γT led to almost three-fold reduction of 8-isoprostane (P < 0.05) in the stomach mucus, whereas aspirin plus αT did not significantly affect 8-isoprostane (Figure 5B). We also measured gastric LTB4 which is also believed to contribute to gastric injury (27). The results indicated that LTB4 was not affected by aspirin or the combinations compared with corn-oil controls (data not shown).
Figure 5. Effect on gastric PGE2 and 8-isoprostane.
The conditions of each treatment are the same as indicated in Figure 2. Stomach PGE2 (A) and 8-isoprostane (B) were measured by ELISA. Results (pg/mg of tissue) are expressed as Mean ± SEM. *(P < 0.05) indicates a significant difference between corn-oil control rats and drug-treated animals.
DISCUSSION
The present study shows that a combination of aspirin and γT appears to have some advantage over aspirin alone in anti-inflammatory action and alleviation of aspirin-associated adverse effects. The combination of aspirin and γT, rather than aspirin alone or aspirin and αT, reduced exudate volume which is an index of inflammation and showed moderate inhibition of proinflammatory PGE2 and tissue damage during the prolonged inflammation period. We previously showed that γT is more effective than αT in inhibition of pro-inflammatory PGE2 in endotoxin-treated macrophages and IL-1β stimulated epithelial cells (17). We recently found that long-chain carboxychromanols, which are mainly generated from metabolism of non-alpha tocopherol forms of vitamin E such as γT (21, 28), are potent COX inhibitors (Jiang et al. unpublished results). Consistently, in the similar air-pouch inflammation model in rats, γT but not αT at the same dose reduces proinflammatory PGE2 and attenuated inflammation-associated damage (16). In the present study, at 18 h, the concentrations of γT in the exudate are 5-fold higher than those in corn-oil treated controls. Therefore, the anti-inflammatory activity of γT and its metabolites may account for the prolonged anti-inflammatory effect in the combination with aspirin. In contrast, aspirin alone only transiently reduced PGE2 at the site of inflammation as a result of the rapid clearance of this drug and its metabolite, salicylate (1/2 half life in the plasma is reported to be 20 min to 4.5 h). The prolonged anti-inflammatory effect was not observed with the combination of aspirin and αT.
Unlike the combination of aspirin and γT, aspirin alone or aspirin plus αT caused prolonged depression of gastric PGE2 and further reduction of food intake. It is well established that aspirin inhibits COX-1 in stomach mucus via acetylation of serine530 at the substrate binding site and therefore blunts gastric PGE2 which is believed to be important for the maintenance of mucosal function and defense mechanisms (6, 7). The irreversible inhibition of COX-1 is responsible for the prolonged depletion of gastric PGE2 even after aspirin is washed out from the stomach. Aspirin-associated reduction of food intake is likely caused by the stomach injury induced by this drug. γT spared aspirin-caused reduction of food intake and partially attenuated stomach lesions. The observed beneficial effect of γT can be explained, in part, by its partial counteraction of aspirin-related reduction of gastric PGE2, but the reason for this action is not clear. Besides depletion of gastric prostaglandins, other factors may contribute to stomach damage such as oxidative stress-associated increase of lipid peroxidation (10) and reduction of hydrophobicity of mucosal surface (29). Interestingly, both aspirin and its combination with γT significantly reduced 8-isoprostane, a lipid peroxidation marker. It is possible that the attenuation of PGE2 depletion together with inhibition of lipid peroxidation in the gastric mucus may account for the superior outcome from the combination of aspirin and γT, which is in sharp contrast to the effect of aspirin plus αT.
It is unexpected that addition of αT worsened aspirin-induced gastric injury, which is consistent with the accentuated decrease of food intake by this combination. Previous studies investigating potentially protective effect of αT supplementation on aspirin-induced damage revealed varied results. Fesharaki et al (30) showed that supplementation of αT reversed aspirin-induced acute gastric erosions and restored aspirin-caused decrease in superoxide dismutase activity and glutathione levels in the stomach. However, other studies indicated that αT fails to attenuate aspirin-caused stomach injury (11) or provides protection only limited to vitamin E (αT) deficient animals but not those with adequate vitamin E intake (10, 12). For instance, Sugimoto et al (12) reported that supplementation of αT (500mg/kg) did not reduce aspirin plus HCl-induced gastric mucosal injury compared with rats fed adequate αT (20mg/kg), although rats with adequate or supplemented αT have reduced lesions compared with animals fed a vitamin E deficient diet. Stickel et al (11) reported that supplementation of αT did not provide further protection of aspirin-induced gastric injury in 20-month old rats.
Although αT is believed to be safe across a broad range of intakes (31), the surprisingly adverse effect from addition of αT to aspirin in the current study is in line with the observation that individuals supplemented with αT and aspirin had increased gingival bleeding compared with aspirin alone, which is observed in a controlled clinical trial, the Alpha-Tocopherol, Beta-Carotene Cancer (ATBC) Prevention Study (9). The ATBC study also showed that αT supplementation was associated with an increased risk of death due to hemorrhagic stroke compared with placebo controls (32). The enhanced bleeding associated with αT supplementation may stem from its action on platelet aggregation via inhibition of protein kinase C activity (33) or modulation of vitamin K status (34). Whether these mechanisms account for the adverse effect of αT observed in the current study waits to be determined. We found that addition of αT did not have any impact on aspirin-caused reduction of gastric PGE2, which is consistent with the study by Stickel et al (11). On the other hand, αT appeared to reverse aspirin-related reduction of 8-isoprostane, but the significance and mechanism of this effect is not clear.
NSAIDs including aspirin are commonly used for pain relief and have consistently been shown to reduce the risk of cancers (35, 36). Low-dose aspirin is effective in reduction of cardiovascular disease risk because of its antithrombotic effect (37). However, the long-term use of aspirin and other NSAIDs has been hindered by their associated adverse effects including upper gastrointestinal bleeding and ulcers (37, 38). Our current study suggests that the combination of aspirin and γT may be superior to aspirin alone in treatment of chronic inflammatory diseases and inflammation-associated disorders, such as cancer, due to a prolonged anti-inflammatory action and protective effect on gastric injury as well as the anti-cancer effect of γT itself (39). The safety and efficacy of chronic use of this combination should be further tested in animal models and human studies. Further investigation is also needed for selecting the optimal dose of this combination. In addition, because of the varied outcome of the combination of aspirin and αT, cautions should be taken in the recommendation of this combination.
Acknowledgments
This study was supported by NIH R01AT001821.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 2.Vane JR. Prostaglandins as mediators of inflammation. Adv Prostaglandin Thromboxane Res. 1976;2:791–801. [PubMed] [Google Scholar]
- 3.Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. Int J Tissue React. 1998;20:3–15. [PubMed] [Google Scholar]
- 4.Dubois RW, Melmed GY, Henning JM, Bernal M. Risk of Upper Gastrointestinal Injury and Events in Patients Treated With Cyclooxygenase (COX)-1/COX-2 Nonsteroidal Antiinflammatory Drugs (NSAIDs), COX-2 Selective NSAIDs, and Gastroprotective Cotherapy: An Appraisal of the Literature. J Clin Rheumatol. 2004;10:178–189. doi: 10.1097/01.rhu.0000128851.12010.46. [DOI] [PubMed] [Google Scholar]
- 5.Scheiman JM. The impact of nonsteroidal anti-inflammatory drug-induced gastropathy. Am J Manag Care. 2001;7:S10–14. [PubMed] [Google Scholar]
- 6.Cryer B, Feldman M. Effects of nonsteroidal anti-inflammatory drugs on endogenous gastrointestinal prostaglandins and therapeutic strategies for prevention and treatment of nonsteroidal anti-inflammatory drug-induced damage. Arch Intern Med. 1992;152:1145–1155. [PubMed] [Google Scholar]
- 7.Schoen RT, Vender RJ. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastric damage. Am J Med. 1989;86:449–458. doi: 10.1016/0002-9343(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 8.Kusuhara H, Komatsu H, Sumichika H, Sugahara K. Reactive oxygen species are involved in the apoptosis induced by nonsteroidal anti-inflammatory drugs in cultured gastric cells. Eur J Pharmacol. 1999;383:331–337. doi: 10.1016/s0014-2999(99)00599-3. [DOI] [PubMed] [Google Scholar]
- 9.Liede KE, Haukka JK, Saxen LM, Heinonen OP. Increased tendency towards gingival bleeding caused by joint effect of alpha-tocopherol supplementation and acetylsalicylic acid. Ann Med. 1998;30:542–546. [PubMed] [Google Scholar]
- 10.Nafeeza MI, Fauzee AM, Kamsiah J, Gapor MT. Comparative effects of a tocotrienol-rich fraction and tocopherol in aspirin-induced gastric lesions in rats. Asia Pac J Clin Nutr. 2002;11:309–313. doi: 10.1046/j.1440-6047.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- 11.Stickel F, Meydani M, Wu D, Bronson R, Martin A, Smith D, Meydani SN, Russell RM. Effect of vitamin E supplementation on prostaglandin concentrations in aspirin-induced acute gastric injury in aged rats. Am J Clin Nutr. 1997;66:1218–1223. doi: 10.1093/ajcn/66.5.1218. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto N, Yoshida N, Yoshikawa T, Nakamuara Y, Ichikawa H, Naito Y, Kondo M. Effect of vitamin E on aspirin-induced gastric mucosal injury in rats. Dig Dis Sci. 2000;45:599–605. doi: 10.1023/a:1005417929009. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich M, Traber MG, Jacques PF, Cross CE, Hu Y, Block G. Does gamma-tocopherol play a role in the primary prevention of heart disease and cancer? A review. J Am Coll Nutr. 2006;25:292–299. doi: 10.1080/07315724.2006.10719538. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Cooney RV. The Encyclopedia of Vitamin E. CABI Publishing; England: 2007. Chemical and biological properties of tocopherols and their relation to cancer incidence and progression; pp. 876–885. [Google Scholar]
- 16.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. Faseb J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha- tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci U S A. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, Wood LA, Cooney RV. Enhancement of intracellular gamma-tocopherol levels in cytokine-stimulated C3H 10T1/2 fibroblasts: relation to NO synthesis, isoprostane formation, and tocopherol oxidation. BMC Chem Biol. 2007;7:2. doi: 10.1186/1472-6769-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J Lipid Res. 2007;48:1221–1230. doi: 10.1194/jlr.D700001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 23.Sedgwick AD, Lees P. Studies of eicosanoid production in the air pouch model of synovial inflammation. Agents Actions. 1986;18:429–438. doi: 10.1007/BF01965008. [DOI] [PubMed] [Google Scholar]
- 24.Sedgwick AD, Sin YM, Edwards JC, Willoughby DA. Increased inflammatory reactivity in newly formed lining tissue. J Pathol. 1983;141:483–495. doi: 10.1002/path.1711410406. [DOI] [PubMed] [Google Scholar]
- 25.Clement M, Bourre JM. Graded dietary levels of RRR-gamma-tocopherol induce a marked increase in the concentrations of alpha- and gamma-tocopherol in nervous tissues, heart, liver and muscle of vitamin-E-deficient rats. Biochim Biophys Acta. 1997;1334:173–181. doi: 10.1016/s0304-4165(96)00090-6. [DOI] [PubMed] [Google Scholar]
- 26.Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol levels in humans. J Nutr. 1985;115:807–813. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- 27.Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut. 1993;34:742–747. doi: 10.1136/gut.34.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You CS, Sontag TJ, Swanson JE, Parker RS. Long-chain carboxychromanols are the major metabolites of tocopherols and tocotrienols in A549 lung epithelial cells but not HepG2 cells. J Nutr. 2005;135:227–232. doi: 10.1093/jn/135.2.227. [DOI] [PubMed] [Google Scholar]
- 29.Darling RL, Romero JJ, Dial EJ, Akunda JK, Langenbach R, Lichtenberger LM. The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, and prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology. 2004;127:94–104. doi: 10.1053/j.gastro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Fesharaki M, Nasimi A, Mokhtari S, Mokhtari R, Moradian R, Amirpoor N. Reactive oxygen metabolites and anti-oxidative defenses in aspirin-induced gastric damage in rats: Gastroprotection by Vitamin E. Pathophysiology. 2006;13:237–243. doi: 10.1016/j.pathophys.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, Johnston CS, Kelly FJ, Kraemer K, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–745. doi: 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- 32.Alpha-Tocopherol B-CCPSG. The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 33.Freedman JE, Farhat JH, Loscalzo J, Keaney JF., Jr alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation. 1996;94:2434–2440. doi: 10.1161/01.cir.94.10.2434. [DOI] [PubMed] [Google Scholar]
- 34.Booth SL, Golly I, Sacheck JM, Roubenoff R, Dallal GE, Hamada K, Blumberg JB. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. Am J Clin Nutr. 2004;80:143–148. doi: 10.1093/ajcn/80.1.143. [DOI] [PubMed] [Google Scholar]
- 35.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women [see comments] N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 37.Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 38.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134:21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]