Abstract
Thioredoxin reductase 1 (TrxR1) is an important antioxidant enzyme that controls cellular redox homeostasis. By using a proteomic-based approach, here we identify TrxR1 as a caveolar membrane-resident protein. We show that caveolin 1, the structural protein component of caveolae, is a TrxR1-binding protein by demonstrating that the scaffolding domain of caveolin 1 (amino acids 82–101) binds directly to the caveolin-binding motif (CBM) of TrxR1 (amino acids 454–463). We also show that overexpression of caveolin 1 inhibits TrxR activity, whereas a lack of caveolin 1 activates TrxR, both in vitro and in vivo. Expression of a peptide corresponding to the caveolin 1 scaffolding domain is sufficient to inhibit TrxR activity. A TrxR1 mutant lacking the CBM, which fails to localize to caveolae and bind to caveolin 1, is constitutively active and inhibits oxidative-stress-mediated activation of the p53/p21Waf1/Cip1 pathway and induction of premature senescence. Finally, we show that caveolin 1 expression inhibits TrxR1-mediated cell transformation. Thus, caveolin 1 links free radicals to activation of the p53/p21Waf1/Cip1 pathway and induction of cellular senescence by acting as an endogenous inhibitor of TrxR1.
Keywords: caveolin, premature senescence, oxidative stress, thioredoxin reductase 1
Introduction
Excessive production of reactive oxygen species (ROS) is usually counteracted by antioxidants such as the thioredoxin–thioredoxin reductase (TrxR) and glutathione–glutaredoxin systems. A redox imbalance occurs when ROS production exceeds the antioxidant capacity of the cell. This leads to cellular dysfunctions such as senescence (Chen & Ames, 1994), which is considered to be an important defence mechanism that prevents the propagation of cells with damaged DNA and, therefore, of cells potentially carrying oncogenic mutations.
Thioredoxin reductase 1 (TrxR1) is an essential antioxidant enzyme that is expressed in all cell types and organs (Behne & Kyriakopoulos, 2001). It is known to reduce various cellular compounds, in addition to thioredoxin, by using nicotinamide adenine dinucleotide phosphate (Mustacich & Powis, 2000). TrxR1 is a homodimeric selenoenzyme with a flavin adenine dinucleotide, a functional disulphide/dithiol and a penultimate carboxy-terminal selenocysteine residue (Sandalova et al, 2001). Together with an adjacent cysteine, this selenocysteine residue forms a selenenylsulphide as the active site, which is maintained in a reduced state by a conserved sequence (Cys–Val–Asn–Val–Gly–Cys) at the amino-terminal of the other subunit within the dimer (Zhong & Holmgren, 2000).
Most cells cannot divide indefinitely because of a process termed cellular senescence. Several cellular stressors can induce senescence, including oxidative stress (Frippiat et al, 2001). Emerging data indicate that antioxidant enzymes might have an important role in the signalling pathways that link oxidative stress to cellular senescence: knockdown of superoxide dismutase 1 was reported to induce cellular senescence in human fibroblasts (Blander et al, 2003), and a lack of either glutathione peroxidase 1 (de Haan et al, 2004) or peroxiredoxin II (Han et al, 2005) was shown to promote senescent-like features in mouse embryonic fibroblasts. Thus, it seems that by preventing ROS-mediated signalling, antioxidant enzymes protect against cellular senescence.
Previous work from our laboratory indicates that caveolin 1, the structural protein component of caveolae, has an important role in stress-induced premature senescence (SIPS). We have shown that oxidative stress induces premature senescence by stimulating caveolin 1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two G+C-rich promoter elements (Dasari et al, 2006). In addition, we have shown that overexpression of caveolin 1 is sufficient to induce premature senescence (Volonte et al, 2002), and that a lack of caveolin 1 inhibits SIPS in mouse embryonic fibroblasts (MEFs; Bartholomew et al, 2009) and lung fibroblasts (Volonte et al, 2009). The molecular mechanisms downstream from caveolin 1, underlying caveolin-1-mediated SIPS, are yet to be clarified. Here, we identify caveolin 1 as an endogenous inhibitor of TrxR1 and show that inhibition of TrxR1 by caveolin 1 promotes oxidative SIPS.
Results And Discussion
TrxR1 localizes in caveolar membranes
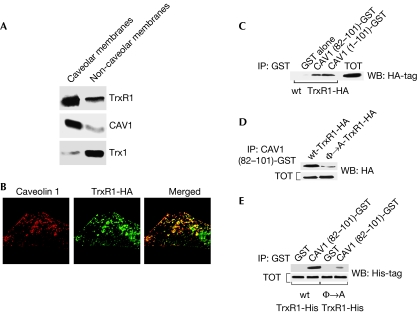
To identify the molecular mechanism downstream from caveolin 1 that mediates caveolin-1-dependent SIPS, we subjected caveolar membranes isolated from WI-38 human diploid fibroblasts to proteomic analysis. TrxR1 was among the proteins identified by mass spectrometry (data not shown). The presence of TrxR1 in caveolar membranes was confirmed by immunoblotting analysis using an antibody probe specific for TrxR1 (Fig 1A).
Figure 1.
TrxR1 binds to caveolin 1. (A) Caveolar membranes were isolated from WI-38 cells by equilibrium sucrose density gradient centrifugation. Immunoblotting with an antibody probe specific for TrxR1 was used to determine its caveolar localization. (B) Cellular localization of TrxR1-HA was investigated by immunofluorescence through double staining with monoclonal HA and polyclonal caveolin 1 antibodies. (C) In vitro reconstitution of TrxR1 binding to caveolin 1. GST fusion protein (prepared as shown in supplementary Fig S1C online) pulldown assays were performed using cell lysates from NIH 3T3 cells transiently transfected with TrxR1-HA. (D) In vitro reconstitution of the Φ → A-TrxR1 mutant binding to the caveolin 1 scaffolding domain. The experiments were performed as described in (C), with the exception that the Φ → A-TrxR1-HA mutant was transfected in addition to wt-TrxR1-HA and cell lysates were incubated with only affinity-purified GST–CAV1 (82–101). Total expression of wt-TrxR1-HA and φ → A-TrxR1-HA is shown in the lower panel. (E) The scaffolding domain of caveolin 1 directly binds to the caveolin-binding motif of TrxR1. Pulldown assays between either purified wt-TrxR1-His or φ → A-TrxR1-His and affinity-purified GST alone or CAV1 (82–101)-GST were performed. Total expression of wt-TrxR1-His and φ → A-TrxR1-His is shown in the lower panel. CAV1, caveolin 1; GST, glutathione S-transferase; HA, haemagglutinin; TrxR1, thioredoxin reductase 1; wt-TrxR1-HA, HA-tagged wild type TrxR1; wt, wild type.
To investigate further the nature of localization of TrxR1 in caveolar membranes, we subjected WI-38 cell lysates to co-immunoprecipitation studies. Caveolin 1 successfully co-immunoprecipitated TrxR1 (supplementary Fig S1A online), indicating that they are both part of the same multiprotein complex. In support of these data, we show that exogenously expressed haemagglutinin (HA)-tagged TrxR1 co-immunolocalized with caveolin 1 in NIH 3T3 fibroblasts (Fig 1B; supplementary Fig S1B online).
Caveolin 1 interacts with TrxR1
The caveolin scaffolding domain of caveolin 1 (residues 82–101) is sufficient for binding to TrxR1 (Fig 1C; supplementary Fig S1C online). We generated and expressed a TrxR1 mutant (Φ → A-TrxR1) in which the five aromatic residues of the putative caveolin-binding motif (CBM) were mutated to alanines (supplementary Fig S1D,E online). Cell lysates from cells expressing wild-type (wt)-TrxR1-HA and Φ → A-TrxR1-HA were then used for glutathione S-transferase (GST) pulldown assays. wt-TrxR1-HA, but not Φ → A-TrxR1-HA, bound to the caveolin 1 scaffolding domain (Fig 1D). Finally, we performed GST pulldown assays using purified wt-TrxR1-His and Φ → A-TrxR1-His. Only wt-TrxR1-His bound strongly to caveolin 1 (residues 82–101)–GST (Fig 1E). From these data, we conclude that the caveolin 1 scaffolding domain binds directly to the CBM (amino acids 454–463) of TrxR1.
Caveolin 1 is an inhibitor of TrxR activity
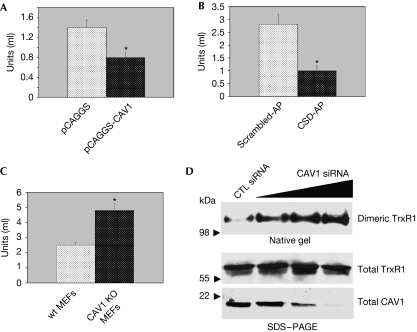
To investigate the functional nature of the interaction of caveolin 1 with TrxR1, we overexpressed caveolin 1 in NIH 3T3 fibroblasts and caveolin-1-negative MCF7 breast cancer epithelial cells, and we assessed TrxR activity. We found that overexpression of caveolin-1 inhibited TrxR activity by about 50% (Fig 2A; supplementary Fig S2A–E online). In addition, expression of cell-permeant peptide cavtratin—consisting of the homeodomain of the Drosophila transcription factor antennapedia (penetratin)—coupled to the caveolin 1 scaffolding domain (Lin et al, 2007) inhibited TrxR1 activity. The control peptide antennapedia coupled to a scrambled version of the caveolin scaffolding domain did not inhibit TrxR1 activity (Fig 2B). The fact that the degree of overexpression of caveolin 1 in both NIH 3T3 and MCF7 cells is higher than the degree of inhibition of TrxR activity indicates that accessory proteins and/or accessibility of TrxR1 by caveolin 1 might have an important role in caveolin-1-mediated inhibition of TrxR1. A lack of caveolin 1 in caveolin-1-null MEFs increased TrxR activity by approximately twofold (Fig 2C). Consistent with these data, we show that disruption of caveolar membranes by using methyl-β-cyclodextrin activated TrxR activity by approximately 50% (supplementary Fig S2F online). These data indicate that caveolin 1 is an inhibitor of TrxR activity. We also found that caveolin 1 inhibits TrxR1 activity by preventing the formation of TrxR1 homodimers, as shown by increased levels of dimeric TrxR1 in cells in which endogenous caveolin 1 expression was reduced by short interfering RNA (siRNA; Fig 2D).
Figure 2.
Caveolin 1 inhibits TrxR activity. (A–C) TrxR activity assay. (A) NIH 3T3 cells were transiently transfected with pCAGGS-CAV1 and pCAGGS alone. (B) WI-38 fibroblasts were incubated with either the peptide cavtratin (10 μM), containing the CSD-AP, or the control peptide AP fused to a scrambled version of the CSD (Scrambled-AP; 10 μM) for 2 days. (C) MEFs were derived from wt and caveolin-1-null mice. (A–C) Cells were collected and TrxR activity measured as described in Methods. Values are means±s.e.m. *P<0.001. (D) Caveolin 1 prevents the formation of dimeric TrxR1. WI-38 cells were transfected with scrambled siRNA (CTL siRNA) or increasing concentrations of caveolin 1 siRNA. Cells were collected and cell lysates subjected to either native gel electrophoresis or SDS–PAGE. Dimeric TrxR1 is shown in the upper panel; total TrxR1 and caveolin 1 are shown in the two lower panels. AP, antennapedia; CAV1, caveolin 1; CSD, caveolin scaffolding domain; MEFs, mouse embryonic fibroblasts; SDS–PAGE, SDS–polyacrylamide gel electrophoresis; siRNA, short interfering RNA; TrxR, thioredoxin reductase; wt, wild type.
Increased or reduced interaction with caveolin 1 modulates TrxR1 enzymatic activity in vivo, as shown by inhibition and enhancement of TrxR activity in lung tissues from caveolin 1 transgenic and null mice, respectively (Galbiati et al, 2001; Razani et al, 2001; supplementary Fig S3A,B online).
Localization of TrxR1 is redox sensitive
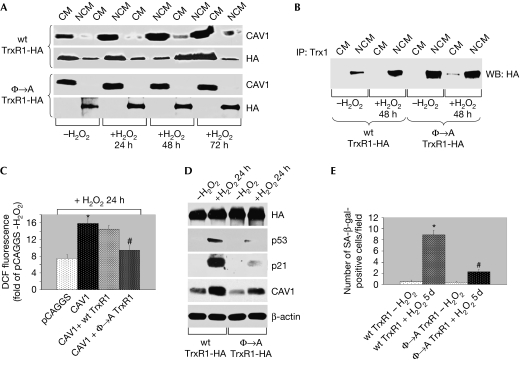
To examine whether the targeting of TrxR1 to caveolar membranes was dependent on the redox status of the cell, we subjected WI-38 human diploid fibroblasts to sub-cytotoxic oxidative stress (150 μM for 2 h). Cells were then washed and cultured in normal medium for different periods of time. During the first 24 h after sub-cytotoxic oxidative stress, TrxR activity did not increase and TrxR1 remained in caveolar membranes (supplementary Fig S4A,B online). We also found that oxidative stress promoted the exit of TrxR1 from caveolar membranes and the stimulation of TrxR activity 48 h after the insult (supplementary Fig S4A,B online). At 72 h after oxidative stress, TrxR1 only partly returned to caveolar membranes and TrxR activity remained elevated compared with untreated cells (supplementary Fig S4A,B online). Quantification of TrxR1 localization in caveolar and non-caveolar membranes is shown in supplementary Table S1 online. In contrast to exogenously expressed wt-TrxR1-HA, which behaved like its endogenous counterpart, a mutated form of TrxR1 (Φ → A-TrxR1-HA) that fails to bind to caveolin 1 was excluded from caveolar membranes before and after oxidative stress (Fig 3A), and was constitutively active (supplementary Fig S5A online). In support of these data, we show that oxidative stress stimulated the interaction between wt-TrxR1 expressed in the cytoplasmic fractions—but not wt-TrxR1 found in caveolar fractions—and Trx1, which is a main cellular target of TrxR1 (Fig 3B). By contrast, the interaction between the cytoplasmic fraction of Φ → A-TrxR1 and Trx1 was elevated before and after oxidative stress (Fig 3B), consistent with hyperactivation of this TrxR1 mutant. Finally, we show that overexpression of caveolin 1 increased ROS levels within the cells and that Φ → A-TrxR1, but not wt-TrxR1, reverted the caveolin-1-dependent production of ROS (Fig 3C). We conclude that caveolin 1 inhibits TrxR activity by binding directly to TrxR1 when TrxR1 is localized in caveolar membranes.
Figure 3.
Inhibition of TrxR1 activity by caveolin 1 is required for stress-induced activation of the p53/p21Waf1/Cip1 pathway and premature senescence. (A–E) HA-tagged wt and Φ → A mutant TrxR1 were stably expressed in NIH 3T3 cells. Cells were treated with 150 μM H2O2 for 2 h, washed and cultured in normal medium for the indicated period of time. (A) Caveolar membranes were isolated by equilibrium sucrose density gradient centrifugation. Immunoblotting with an antibody probe specific for HA was used to determine the caveolar localization of TrxR1. (B) Trx1 was immunoprecipitated from either CM or NCM with a polyclonal antibody specific for Trx1 and immunoprecipitates subjected to immunoblotting analysis using monoclonal HA antibodies. (C) 3T3 cells were transiently transfected with vector alone, caveolin 1 alone and caveolin 1 together with either wt-TrxR1 or Φ → A TrxR1. Cells were then treated with or without 150 μM H2O2 for 2 h and DCF fluorescence was measured by FACS analysis. (D) Cells were subjected to immunoblotting analysis using antibody probes specific for HA, p53, p21Waf1/Cip1 and caveolin 1. Immunoblotting with anti-β-actin IgGs was performed to show equal loading. (E) Cells were subjected to senescence-associated β-galactosidase activity assay. The number of positive cells per field was adjusted for the total cell number. Values are means±s.e.m. *,#P<0.001. CM, caveolar membrane; DCF, 2′,7′-dichlorofluorescein; FACS, fluorescence-activated cell sorting; HA, haemagglutinin; NCM, non-caveolar membrane; TrxR1, thioredoxin reductase-1; WB, western blot; wt, wild type.
Expression of Φ → A-TrxR1 prevents SIPS
Does the inhibition of TrxR1 by caveolin 1 during the first 24 h after oxidative stress have any functional consequences? Sub-cytotoxic oxidative stress is known to induce premature senescence (Chen & Ames, 1994) by activating the p53 pathway, which occurs 12–24 h after oxidative stress (Bartholomew et al, 2009). As antioxidant enzymes seem to have an important role in preventing ROS-induced premature senescence (Blander et al, 2003; de Haan et al, 2004; Han et al, 2005), and our published data indicate that caveolin 1 promotes SIPS (Bartholomew et al, 2009), we asked whether inhibition of TrxR1 by caveolin 1 immediately after oxidative stress was required for activation of the p53 pathway and induction of premature senescence.
To address this question directly, we subjected NIH 3T3 cells stably expressing either wt-TrxR1-HA or Φ → A-TrxR1-HA to subcytotoxic oxidative stress. We found that expression of the constitutively active Φ → A-TrxR1-HA inhibited ROS-induced upregulation of p53, expression of p21Waf1/Cip1 protein and activation of a p53 responsive element 24 h after oxidative stress, as compared with wt-TrxR1-HA (Fig 3D; supplementary Fig S5B online). We also show that expression of Φ → A-TrxR1-HA inhibited SIPS in fibroblasts, as shown by the lesser number of senescence-associated β-galactosidase-positive cells after oxidative stress as compared with wt-TrxR1-HA-expressing cells (Fig 3E; supplementary Fig S5C online).
Evidence indicates that p53 might represent a redox-dependent transcription factor, the expression and activity of which might be regulated by the TrxR system. In fact, downregulation of TrxR expression increased p53 messenger RNA (Gan et al, 2005) and protein levels, and enhanced the DNA-binding activity of p53 (Seemann & Hainaut, 2005). Thus, through inhibition of TrxR1 immediately after sub-cytotoxic oxidative stress, caveolin 1 might stimulate p53-dependent signalling and promote SIPS. As the p53 pathway remains activated in fibroblasts 48 h after oxidative stress (Bartholomew et al, 2009), at a time when TrxR1 exits caveolar membranes and TrxR activity is upregulated (supplementary Fig S4A,B online), it might be that caveolin-1-dependent inhibition of TrxR1 has a central role in the acute, but not chronic, activation of the p53 pathway under conditions of oxidative stress that lead to premature senescence.
Caveolin 1 inhibits TrxR1-mediated cell transformation
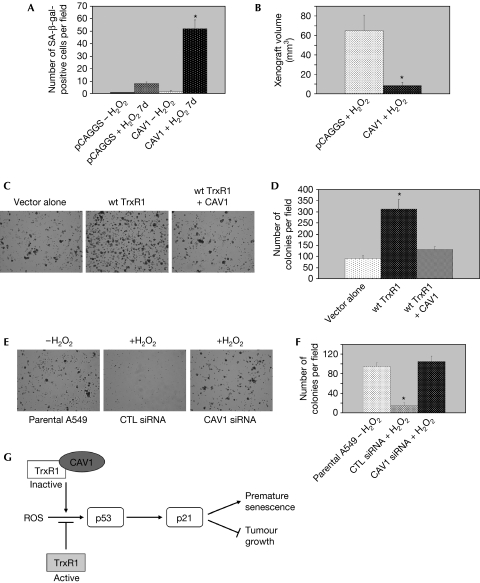
In normal cells, TrxR1 is a key regulator of redox homeostasis and protects against oxidative damage and potential DNA mutations. However, upon cell transformation, TrxR1 seems to support tumour cell growth. In fact, many tumour cells have elevated TxrR protein levels and activity (Berggren et al, 1996). Compounds acting as inhibitors of TrxR1 have been developed as anti-cancer drugs. Here, we show that caveolin 1 is an endogenous inhibitor of TrxR1. As caveolin 1 expression is reduced or lost in several human cancers, it is possible that low levels of caveolin 1 might prevent free-radical-induced premature senescence of cancer cells and contribute to their transformed phenotype by increasing TrxR activity. To address this possibility, we investigated the effect of caveolin 1 overexpression on the induction of SIPS and the transformed phenotype of A549 human lung carcinoma epithelial cells, in which caveolin 1 expression is reduced but not lost (Yang et al, 1998). We show that overexpression of caveolin 1 markedly enhanced SIPS of A549 cells (Fig 4A; supplementary Fig S6A online). Consistent with these data, we show that tumour formation in nude mice was significantly inhibited in hydrogen-peroxide-treated A549 cells overexpressing caveolin 1 (Fig 4B). We also found that the increased transformed phenotype of A549 cells stably overexpressing TrxR1 was inhibited when caveolin 1 was stably co-expressed with TrxR1 (Fig 4C,D).
Figure 4.
Caveolin 1 regulates the transformed phenotype of A549 human lung carcinoma epithelial cells. (A,B) A549 cells were stably transfected with vector alone (pCAGGS) or CAV1. They were then treated with 150 μM H2O2 for 2 h. (A) Cells were washed, cultured in normal medium for 7 days and subjected to senescence-associated β-galactosidase activity assay. Untreated cells were used as the control. The number of positive cells per field was adjusted for the total cell number. Values are means±s.e.m. *P<0.001. (B) Cells were washed and subcutaneously injected into the dorsal flap of nude (nu/nu) mice. Mice were killed 4 weeks later and the tumour size was measured. Values are means±s.e.m. *P<0.001. (C–F) A549 cells were stably transfected with vector alone (pCAGGS), wt-TrxR1, or stably co-transfected with wt-TrxR1 and caveolin 1 (wt-TrxR1+Cav-1; C,D). A549 cells were transfected with scrambled siRNA (CTL siRNA) or CAV1 siRNA. At 48 h after transfection, cells were treated with 150 μM H2O2 for 2 h. Untreated parental A549 cells were used as the control (E,F). The transformed phenotype of these cells was determined by growth in soft agar for 10 days. Representative images are shown in (C,E); quantification of growth is shown in (D,F). Values are means±s.e.m. *P<0.001. (G) Schematic diagram summarizing the role of caveolin-1-mediated inhibition of TrxR1 in SIPS. See text for details. CAV1, caveolin 1; SIPS, stress-induced premature senescence; siRNA, short interfering RNA; TrxR1, thioredoxin reductase 1; wt, wild type.
We then reasoned that downregulation of caveolin 1 in A549 cells at the time of oxidative stress might activate TrxR1 and, as a consequence, inhibit SIPS in these cells and maintain their transformed phenotype. To test our hypothesis, we analysed the transformed phenotype of A549 cells that were subjected to sub-cytotoxic oxidative stress 48 h after transient transfection with caveolin 1 siRNA, when caveolin 1 expression was downregulated by about 90% (supplementary Fig S6B online). We found that A549 cells maintained their tumorigenic potential, as assessed by growth in soft agar, when caveolin 1 expression was transiently downregulated by siRNA at the time of oxidative stress, in contrast to control (CTL) siRNA-transfected A549 cells, which formed only a few small foci in soft agar assays (Fig 4E,F). As cellular senescence is anti-tumorigenic, we propose a model in which inhibition of TrxR1 by caveolin 1 activates the p53/p21Waf1/Cip1 pathway, induces premature senescence and acts as a tumour-suppressor mechanism (Fig 4G). Thus, caveolin 1 replacement therapy in caveolin-1-negative and TrxR-overexpressing cancer cells, through inhibition of TrxR activity, might represent a valid and alternative therapeutic approach.
Methods
Cloning of TrxR1. The entire coding region with the SECIS element and the first three of the six A+U-rich elements located in the 3′-untranslated region of human TrxR1 were amplified by PCR by using the following primers: forward 5′-ATGAACGGCCCTGAAGATCTTCCC-3′ and reverse 5′-TGAGGCAGAGCCCTGTGGGGG-3′. The amplified DNA was sequenced to rule out the introduction of unwanted mutations. The PCR fragment was digested to create unique EcoRI and XhoI ends and subcloned into the pCMV-HA expression vector (wt-TrxR1-HA). The Φ → A mutant of TrxR1 (Φ → A-TrxR1-HA) was generated by replacement of the aromatic residues within the CBM of TrxR1 (amino acids 454–463) with alanines by PCR amplification by using appropriate internal primers and subcloned into the pCMV-HA expression vector.
TrxR activity assay. Cells were washed twice with cold PBS, collected and resuspended in lysis buffer (0.5% Triton X-100, 0.5% deoxycholate, 150 mM NaCl, 10 mM Tris–HCl pH 7.5, 5 mM EDTA + proteinase inhibitors). Samples were incubated for 45 min at 4°C on rotation before being centrifuged at 13,000g for 10 min at 4°C and the supernatant saved. Samples were normalized by bicinchoninic acid protein assay and 60 μl of each was put in an Eppendorf tube on ice. The following reagents were then added: 6 μl of 1.6 M Hepes (pH 7.5); 6 μl of 120 mM EDTA (pH 8.0); 24 μl of 10 mg/ml insulin; 12 μl of 100 μM Escherichia coli thioredoxin; 12 μl of 9 mg/ml nicotinamide adenine dinucleotide phosphate. Samples were incubated at 37°C for 20 min and the reaction was terminated by adding 500 μl of 0.4 mg/ml 5,5′-dithio-bis(2-nitrobenzoic acid). The absorbance at 412 nm was determined. The activity is represented by the absorbance at 412 mm (A412), where 1 mol of nicotinamide adenine dinucleotide phosphate reduces 1 mol of disulphide, giving rise to 2 mol of free 2-nitro-5-thiobenzoic acid, with the extinction coefficient being 13.6 per mM/cm.
Growth in soft agar. A549 cells (5 × 104) were suspended in 3 ml of RPMI containing 10% fetal bovine serum and 0.33% SeaPlaque low-melting temperature agarose. These cells were plated over a 2 ml layer of solidified RPMI containing 10% fetal bovine serum and 0.5% agarose, and were allowed to settle at the interface between these layers at 37°C. After 20 min, the plates were allowed to harden at room temperature (20–25°C) for 30 min before returning to 37°C. The plates were fed every 3 days by overlaying with 2 ml of medium containing 0.33% agarose. After 10 days, colonies were photographed under low magnification ( × 5). The number of colonies in 10 random fields at the same magnification was counted for quantitation purposes.
Tumorigenesis assays. The animal protocol described in this article was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Nude (nu/nu) mice (5–6 weeks old from Charles River, Wilmington, MA, USA) were injected subcutaneously into the dorsal flap with 5 × 106 A549 cells stably transfected with either vector alone (pCAGGS) or caveolin 1 and treated with 150 μM H2O2 for 2 h (100 μl volume). Four weeks after the injection, the x and y axes of each tumour were measured to determine tumour volume (size) according to the formula, volume=0.5 × width2 × length. Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figs 1–6
Acknowledgments
This work was supported by grants R01-AG022548 and R01-AG030636 from the National Institute on Aging (to F.G.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bartholomew JN, Volonte D, Galbiati F (2009) Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res 69: 2878–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behne D, Kyriakopoulos A (2001) Mammalian selenium-containing proteins. Annu Rev Nutr 21: 453–473 [DOI] [PubMed] [Google Scholar]
- Berggren M, Gallegos A, Gasdaska JR, Gasdaska PY, Warneke J, Powis G (1996) Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res 16: 3459–3466 [PubMed] [Google Scholar]
- Blander G, de Oliveira RM, Conboy CM, Haigis M, Guarente L (2003) Superoxide dismutase 1 knock-down induces senescence in human fibroblasts. J Biol Chem 278: 38966–38969 [DOI] [PubMed] [Google Scholar]
- Chen Q, Ames BN (1994) Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci USA 91: 4130–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari A, Bartholomew JN, Volonte D, Galbiati F (2006) Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res 66: 10805–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan JB, Bladier C, Lotfi-Miri M, Taylor J, Hutchinson P, Crack PJ, Hertzog P, Kola I (2004) Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic Biol Med 36: 53–64 [DOI] [PubMed] [Google Scholar]
- Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O (2001) Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem 276: 2531–2537 [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Liu J, Capozza F, Frank PG, Zhu L, Pestell RG, Lisanti MP (2001) Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell 12: 2229–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Yang XL, Liu Q, Xu HB (2005) Inhibitory effects of thioredoxin reductase antisense RNA on the growth of human hepatocellular carcinoma cells. J Cell Biochem 96: 653–664 [DOI] [PubMed] [Google Scholar]
- Han YH, Kim HS, Kim JM, Kim SK, Yu DY, Moon EY (2005) Inhibitory role of peroxiredoxin II (Prx II) on cellular senescence. FEBS Lett 579: 4897–4902 [DOI] [PubMed] [Google Scholar]
- Lin MI, Yu J, Murata T, Sessa WC (2007) Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res 67: 2849–2856 [DOI] [PubMed] [Google Scholar]
- Mustacich D, Powis G (2000) Thioredoxin reductase. Biochem J 346: 1–8 [PMC free article] [PubMed] [Google Scholar]
- Razani B et al. (2001) Caveolin-1 null mice are viable, but show evidence of hyper-proliferative and vascular abnormalities. J Biol Chem 276: 38121–38138 [DOI] [PubMed] [Google Scholar]
- Sandalova T, Zhong L, Lindqvist Y, Holmgren A, Schneider G (2001) Three-dimensional structure of a mammalian thioredoxin reductase: implications for mechanism and evolution of a selenocysteine-dependent enzyme. Proc Natl Acad Sci USA 98: 9533–9538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann S, Hainaut P (2005) Roles of thioredoxin reductase 1 and APE/Ref-1 in the control of basal p53 stability and activity. Oncogene 24: 3853–3863 [DOI] [PubMed] [Google Scholar]
- Volonte D, Zhang K, Lisanti MP, Galbiati F (2002) Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell 13: 2502–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte D, Kahkonen B, Shapiro S, Di Y, Galbiati F (2009) Caveolin-1 expression is required for the development of pulmonary emphysema through activation of the ATM-p53-p21 pathway. J Biol Chem 284: 5462–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-P, Galbiati F, Volonte D, Horwitz SB, Lisanti MP (1998) Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett 439: 368–372 [DOI] [PubMed] [Google Scholar]
- Zhong L, Holmgren A (2000) Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J Biol Chem 275: 18121–18128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs 1–6