Abstract
Truncated BID (tBID), a proapoptotic BCL2 family protein, induces BAK/BAX-dependent release of cytochrome c and other mitochondrial intermembrane proteins to the cytosol to induce apoptosis. The voltage-dependent anion channels (VDACs) are the primary gates for solutes across the outer mitochondrial membrane (OMM); however, their role in apoptotic OMM permeabilization remains controversial. Here, we report that VDAC2−/− (V2−/−) mouse embryonic fibroblasts (MEFs) are virtually insensitive to tBID-induced OMM permeabilization and apoptosis, whereas VDAC1−/−, VDAC3−/− and VDAC1−/−/VDAC3−/− MEFs respond normally to tBID. V2−/− MEFs regain tBID sensitivity after VDAC2 expression. Furthermore, V2−/− MEFs are deficient in mitochondrial BAK despite normal tBID–mitochondrial binding and BAX/BAK expression. tBID sensitivity of BAK−/− MEFs is also reduced, although not to the same extent as V2−/− MEFs, which might result from their strong overexpression of BAX. Indeed, addition of recombinant BAX also sensitized V2−/− MEFs to tBID. Thus, VDAC2 acts as a crucial component in mitochondrial apoptosis by allowing the mitochondrial recruitment of BAK, thereby controlling tBID-induced OMM permeabilization and cell death.
Keywords: VDAC2, BAK, tBID, mitochondria, apoptosis
Introduction
The voltage-dependent, anion-selective channel (VDAC) proteins are the principal pathways for metabolite and ion flux through the outer mitochondrial membrane (OMM; Colombini, 2004; Craigen & Graham, 2008). VDACs have also been implicated in apoptosis, forming either an open pore that allows the release of cytochrome c (cyto c) and other proteins from the mitochondrial intermembrane space to the cytosol (Shimizu et al, 1999), or a closed pore that induces permeabilization of the OMM through total blockage of the flux of metabolites (Vander Heiden et al, 1999). VDAC1 has been involved in cisplatin-induced apoptosis, downstream from BAK and upstream from BAX (Tajeddine et al, 2008), in superoxide anion (Madesh & Hajnoczky, 2001) and in Ca2+-induced apoptosis (Roy et al, 2009). Furthermore, binding of hexokinase to VDAC1 has been attributed as an anti-apoptotic mechanism (Pastorino et al, 2002; Abu-Hamad et al, 2008). However, a recent study claimed that the VDACs are dispensable for both the BCL2 family member and mitochondrial permeability transition pore-dependent cell death (Baines et al, 2007).
VDAC isoforms can substitute for each other in the metabolite flux (Craigen & Graham, 2008), and they also have important non-redundant roles in cell function. For example, VDAC1−/− (V1−/−) and VDAC3−/− (V3−/−) mice are viable, whereas embryos with homozygous deletion of VDAC2 alleles die during development (Cheng et al, 2003). An indication of the specific role of VDAC2 comes from studies that described the association of VDAC2 with BAK, a proapoptotic BCL2 family member in the mitochondria (Cheng et al, 2003), and provided evidence for VDAC2 dependence of BAK targeting to the mitochondria (Setoguchi et al, 2006). BAK is an integral membrane protein of the OMM where it can mediate membrane permeabilization. BAX, another proapoptotic BCL2 family protein, is localized in the cytosol, but in response to some stress-factor-induced conformational changes, translocates to the OMM where it can also mediate membrane permeabilization (Nechushtan et al, 1999). Many apoptotic agents activate several BAK/BAX-dependent pathways and even engage parallel BAX/BAK-independent mechanisms to execute the OMM permeabilization (Wei et al, 2001). We reasoned that VDAC2 might be of importance for apoptotic pathways that target BAK in the mitochondria.
‘Death' receptors (tumour necrosis factor receptor 1 (TNFR1)/Fas) engage caspase 8, whereas various stress conditions induce calpain, caspases, cathepsins or granzyme B to cleave and activate BID, a proapoptotic BH3-only BCL2 family member (Yin, 2006). Activation of BID is initiated in the cytosol, but it seems to be completed at the mitochondrial surface (Gonzalvez et al, 2008). Truncated BID (tBID) binds to the OMM to induce BAK/BAX-dependent release of the soluble intermembrane space proteins (Desagher et al, 1999; Wei et al, 2001). Whether tBID directly activates proapoptotic BAK/BAX (Kuwana et al, 2002; Letai et al, 2002) or antagonizes the function of their pro-survival BCL2 relatives (Willis et al, 2007) is under debate.
Here, we report that VDAC2 is essential for tBID-induced OMM permeabilization, whereas VDAC1 and VDAC3 are dispensable. We also show that the effect of VDAC2 is mediated through control of mitochondrial BAK and through BAK-dependent regulation of the expression of BAX. These data establish a new and specific role for VDAC2 in mitochondrial apoptosis.
Results And Discussion
Resistance of V2−/− MEFs to tBID-induced apoptosis
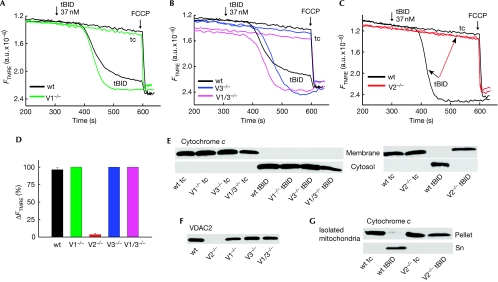
To study the role of each VDAC in tBID-induced OMM permeabilization, we performed fluorimetric measurements of ΔΨm in suspensions of permeabilized primary mouse embryonic fibroblasts (MEFs) lacking various VDAC isoforms (Wu et al, 1999). The addition of 37 nM recombinant tBID caused a rapid and complete loss of ΔΨm in wild-type (wt) and V1−/− MEFs (Fig 1A,D). Dissipation of the ΔΨm was probably due to OMM permeabilization and the ensuing loss of cyto c to the bulk assay medium, as the tBID-induced depolarization could be prevented if cyto c was added to the medium (Madesh et al, 2002). The depolarization started 2–3 min after the addition of tBID and reached completion by 5–6 min. Similar results were also observed for V3−/− and VDAC1 and VDAC3−/− (V1/3−/−) MEFs on tBID treatment (Figs 1B,D). The addition of 3.7 nM tBID also caused the complete dissipation of ΔΨm in wt, V1−/−, V3−/− and V1/3−/− MEFs, the effect being delayed and slower (supplementary Fig S1 online). Western blot analysis of the membrane and the cytosol fractions separated at the end of the ΔΨm measurements (Fig 1E; supplementary Fig S1 online) showed translocation of cyto c from mitochondria to the cytosol in cells treated with both 37 nM and 3.7 nM tBID. By contrast, VDAC2−/− (V2−/−) MEFs failed to show any tBID (37 nM)-induced depolarization (Fig 1C,D) and cyto c release (Fig 1E). To clarify whether the absence of VDAC2 caused the desensitization of V2−/− MEFs to tBID, immunoblot analysis of VDAC2 was performed. VDAC2 was present in wt, V1−/−, V3−/− and V1/3−/− MEFs but was undetectable in V2−/− MEFs (Fig 1F). The above results were a surprise in the light of previous work, which claimed that VDAC2 inhibits (Cheng et al, 2003) or is irrelevant for (Baines et al, 2007) mitochondrial apoptosis. In those studies, however, the effect of tBID on cyto c release or loss of ΔΨm was not evaluated for VDAC2-knockout cells. To evaluate further the VDAC2 dependence of tBID-induced OMM permeabilization, we isolated mitochondria (supplementary Fig S2 online) from wt and V2−/− MEFs. tBID (37 nM for 10 min) caused cyto c release only from wt mitochondria (Fig 1G).
Figure 1.
tBID-induced ΔΨm loss and cytochrome c release in permeabilized VDAC-knockout MEFs and in isolated mitochondria. ΔΨm was monitored in permeabilized wt and V1−/− MEFs (A), in wt, V3−/− and V1/3−/− MEFs (B) and in wt and V2−/− MEFs (C) treated with 37 nM tBID. Mock-treated samples are shown as time control (tc). FCCP (5 μM) was added at the end of each run to depolarize the entire mitochondrial population. (D) Cumulative data showing tBID-induced depolarization of wt, V1−/−, V3−/−, V1/3−/− and V2−/− MEFs. (E) Release of cytochrome c visualized by immunoblotting of rapidly separated pellets (membrane) and supernatants (cytosol). (F) Expression of VDAC2 as visualized by immunoblotting of membrane fractions of MEFs. (G) Isolated mitochondria from wt and V2−/− MEFs were treated with 37 nM tBID. Release of cytochrome c visualized by immunoblotting of rapidly separated mitochondrial supernatants (Sn) and pellets. FCCP, carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone; MEF, mouse embryonic fibroblast; tBID, truncated BID; V1−/−, VDAC1−/−; V2−/−, VDAC2−/−; V3−/−, VDAC3−/−; VDAC, voltage-dependent anion channel; wt, wild type.
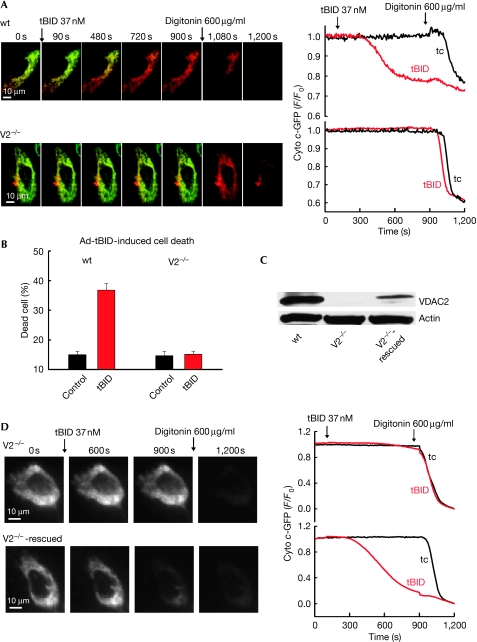
To visualize the effect of tBID in single cells, we performed real-time imaging studies of cyto c release in adherent permeabilized wt and V2−/− MEFs. For this purpose we transfected the MEFs with cyto c-green fluorescent protein (GFP) and a mitochondrial matrix-targeted DsRed (mtDsRed). Treatment of cells with 37 nM tBID caused progressive release of cyto c-GFP in wt MEFs, as shown by the loss of green fluorescence (91 out of 92 cells), whereas V2−/− MEFs failed to show any change (Fig 2A; 277 out of 286 cells showed no response and nine showed some release). As expected, the addition of digitonin (600 μg/ml) mobilized the entire cyto c-GFP store in V2−/− MEFs (Fig 2A). These results indicate that mitochondria of V2−/− MEFs are resistant to tBID-induced OMM permeabilization and cyto c release.
Figure 2.
VDAC2 dependence of tBID-induced cyto c-GFP release and cell death in permeabilized and intact MEFs. (A) Fluorescence time-lapse imaging of cyto c-GFP-expressing (green) and mtDsRed-expressing (red), permeabilized wt and V2−/− MEFs. tBID (37 nM) caused release of cyto c-GFP (green to red shift in the overlaid images) only in wt MEFs. At the end of the experiment, 600 μg/ml digitonin was added to discharge the entire mitochondrial pool. Graphs showing the means of tBID-induced cyto c-GFP release kinetics of the entire cell population in the imaging field (15–20 cells; 4–5 measurements). (B) tBID adenovirus (16 h of infection) induced cell death. Means±s.e. (n=3). (C) Immunoblot of cell lysates confirming the rescue of VDAC2 expression in VDAC2-transfected V2−/− MEFs. (D) Fluorescence time-lapse imaging of control and rescued V2−/− MEFs transfected with cyto c-GFP. Addition of 37 nM tBID caused progressive release of cyto c-GFP (shown in greyscale). Graphs showing the mean response of the entire cell population in the imaging field (15–20 cells; 4–5 measurements). The tBID-induced fluorescence change was normalized to the effect of digitonin. Ad-tBID, tBID adenovirus; cyto c, cytochrome c; GFP, green fluorescent protein; MEF, mouse embryonic fibroblast; tBID, truncated BID; V2−/−, VDAC2−/−; VDAC, voltage-dependent anion channel; wt, wild type.
V2−/− MEFs are insensitive to tBID-induced apoptosis
Next, we investigated the sensitivity of V2−/− MEFs to tBID-induced apoptosis. Flow cytometric analysis of annexin and propidium iodide fluorescence after 16 h of tBID adenovirus infection showed approximately 25% cell death in wt MEFs, whereas the viability of V2−/− MEFs was unchanged, similarly to the mock-treated controls (Fig 2B). Caspase activation and apoptosis induced by a FAS antibody (JO2) were also markedly suppressed in V2−/− MEFs (supplementary Fig S3 online). Thus, intact V2−/− MEFs are also resistant to FAS activation and BID-induced apoptosis.
Rescue of the tBID sensitivity in V2−/− MEFs by VDAC2
To confirm that resistance to tBID was due to the absence of VDAC2, a genetic rescue strategy was used. V2−/− MEFs were cotransfected with VDAC2 and cyto c-GFP or with cyto c-GFP only. Transfected cells were sorted on the basis of GFP fluorescence, and VDAC2 expression was confirmed by immunoblotting (Fig 2C). VDAC2 expression restored cyto c-GFP release in the V2−/− MEFs (Fig 2D; 134 out of 153 cells). Similarly to wt MEFs, tBID-induced cyto c-GFP release in the V2−/−-rescued MEFs was progressive and involved all of the mitochondria. These data confirmed that tBID-induced OMM permeabilization and cell death are dependent on VDAC2 expression. Previously, decreased sensitivity of V2−/− MEFs to tumour necrosis factor-α (TNFα)-induced cell killing has been shown (Cheng et al, 2003). This result is consistent with the present data, as TNFα-induced cell killing involves BID but it also recruits parallel pathways that effectively kill the cells even in the absence of BID (Yin et al, 1999; Chen et al, 2007). Interestingly, enhanced sensitivity of VDAC2-deficient MEFs to staurosporine and H2O2-induced cell killing has also been reported (Cheng et al, 2003; Baines et al, 2007). These agents do not rely on BID-induced OMM permeabilization (Yin et al, 1999). Thus, VDAC2 is required for tBID-mediated cell killing but in parallel lowers the activity of some alternative cell-killing mechanisms.
Reduction of mitochondrial BAX in V2−/− MEFs
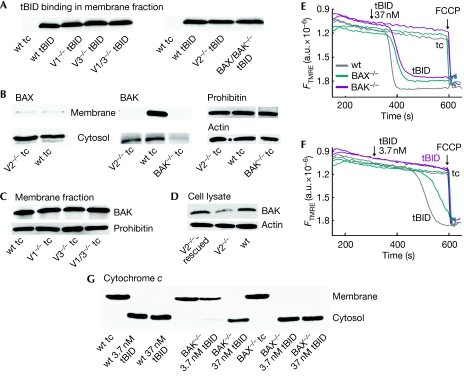
The binding of tBID to the mitochondria was similar in V2−/−, wt and in other VDAC-knockout MEFs (Fig 3A), indicating that attenuated tBID recruitment to the mitochondria could not account for the lack of tBID sensitivity in V2−/− MEFs.
Figure 3.
BAX and BAK dependence of tBID-induced ΔΨm loss and cytochrome c release. (A) Immunoblots of 30 μg proteins showing the binding of tBID in the membrane fractions of tBID-treated permeabilized MEFs. (B) The level of BAX and BAK in the membrane and the cytosolic fractions of permeabilized wt, V2−/− and BAK−/− MEFs. (C) The level of BAK in the membrane fractions of permeabilized MEFs and (D) the level of BAK in the whole cell lysates of wt, V2−/− and VDAC2-rescued V2−/− MEFs. ΔΨm was monitored for permeabilized wt, BAK−/− and BAX−/− MEFs treated with either 37 nM (E) or 3.7 nM (F) recombinant tBID for 5 min. (G) Immunoblot of rapidly separated pellets (membrane) and supernatants (cytosol) showing the release of cytochrome c on tBID treatment. FCCP, carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone; MEF, mouse embryonic fibroblast; tBID, truncated BID; V1−/−, VDAC1−/−; V1/3−/−, VDAC1−/− and VDAC3−/−; V2−/−, VDAC2−/−; V3−/−, VDAC3−/−; VDAC, voltage-dependent anion channel; wt, wild type.
To test whether BAX or BAK is important for the differential tBID sensitivity, we assessed the endogenous levels of BAX and BAK in wt and VDAC-knockout MEFs. Immunoblots of the isolated membrane and cytosolic fractions showed most of the BAX in the cytosol of MEFs and showed a small increase in V2−/− as compared with that in wt (Fig 3B). By contrast, BAK was found in the membrane fractions, with considerably less in V2−/− MEFs (Fig 3B; supplementary Fig S4A online). Notably, V1−/−, V3−/− and V1/3−/− MEFs showed a high level of BAK in the membrane fraction (Fig 3C). The small cytosolic fraction of BAK was similar in wt and V2−/− MEFs, indicating that the precursor of mitochondrial BAK was available in V2−/− MEFs (Fig 3B) and was not due to protein extraction by digitonin from the membrane (supplementary Fig S4B online). Remarkably, the VDAC2-transfected V2−/− MEFs showed restoration of the normal levels of BAK (Fig 3D; supplementary Fig S5A online). Thus, our data showed a greatly reduced level of BAK in the membranes of V2−/− MEFs and rescue of BAK on VDAC2 expression. Depletion of BAK could be due to decreased protein stability or decreased mitochondrial targeting. Protein synthesis and proteasomal inhibitors similarly affected BAK levels in V2−/− and wt MEFs (supplementary Fig S4C,D online). Notably, a recent VDAC2 silencing study has reported the suppression of the mitochondrial targeting of BAK (Setoguchi et al, 2006), which was confirmed here by studying the subcellular distribution of overexpressed BAK-GFP and its release on plasma membrane permeabilization in V2−/− and wt MEFs (supplementary Fig S4E online). Thus, ineffective targeting seems to be a more important reason for mitochondrial BAK depletion in the V2−/− MEFs than an increase in the susceptibility for proteolysis. Our data also indicate that a low level of VDAC2 expression seems to be sufficient for the mitochondrial targeting of BAK (see Fig 2C compared with Fig 3D). The rescue of mitochondrial BAK targeting by VDAC2 expression also supported tBID-induced BAK oligomerization, whereas neither BAX insertion nor BAX oligomerization occurred in permeabilized cells (supplementary Fig S5A online). Thus, the shortage of mitochondrial BAK could be the main reason for the insensitivity to tBID-induced OMM permeabilization in V2−/− MEFs.
BAX dominates tBID-induced apoptosis in MEFs
Although BAX was not present in permeabilized cell membranes (supplementary Fig S5A online), in intact cells tBID overexpression could evoke BAX membrane insertion (supplementary Fig S5B online). To assess the relative significance of BAK and BAX in the endogenous BID pathway, death-receptor-induced caspase 3 activation was studied in wt, BAX−/− and BAK−/− MEFs. The most severe suppression of the death receptor cascade occurred in BAK−/− MEFs (supplementary Fig S6A online), highlighting the relevance of BAK. To evaluate further the respective roles of BAK and BAX, we have studied the effect of tBID in suspensions of permeabilized BAX−/− and BAK−/− MEFs. The addition of 37 nM tBID caused rapid dissipation of ΔΨm in wt, BAX−/− and BAK−/− MEFs (Fig 3E). However, when less tBID was applied (3.7 nM) the tBID-induced depolarization was almost eliminated in BAK−/− MEFs, whereas it was still complete in wt and BAX−/− MEFs (Fig 3F). Immunoblots confirmed the bulk release of cyto c in all three MEFs treated with 37 nM tBID, but cyto c release evoked by 3.7 nM tBID was markedly suppressed in the BAK−/− MEFs (Fig 3G). The decreased sensitivity towards tBID-induced cyto c release and depolarization in BAK−/− MEFs, and the preservation of the tBID effect in BAX−/− MEFs emphasize the importance of BAK in the tBID-induced OMM permeabilization. Thus, depletion of mitochondrial BAK in V2−/− MEFs can contribute to the lack of tBID sensitivity. Nevertheless, some other factors also have to be involved, since partial depletion of BAK in the V2−/− MEFs caused more complete inhibition of the tBID effect than the knockout of BAK.
Distinct BAX levels and function in V2−/− and BAK−/− MEFs
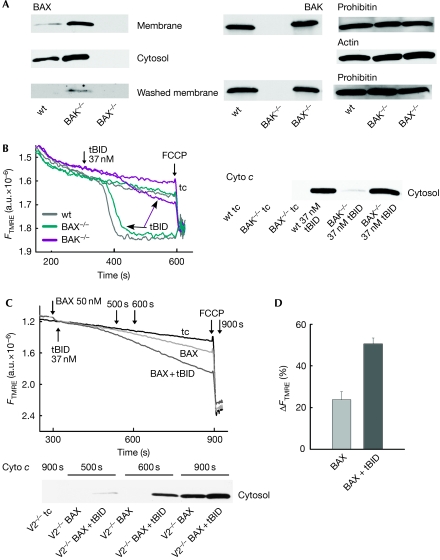
To isolate these factors, we studied the endogenous level of VDAC2, BAX and BAK in wt, BAX−/− and BAK−/− MEFs. VDAC2 was unaltered in both BAX−/− and BAK−/− MEFs (supplementary Fig S6B online). Notably, the BAX level was much higher in both cytosol and membrane of the BAK−/− MEFs than in wt MEFs (Fig 4A, upper left; cell lysates shown in supplementary Fig S6C online). At the same time, there were no alterations of BAK levels in the membrane fractions of wt and BAX−/− MEFs (Fig 4A, upper right) or in the whole-cell lysates (supplementary Fig S6C online). The higher level of BAX in BAK−/− MEFs might have adequately supported the tBID-induced OMM permeabilization (Figs 3E,G). We reasoned that washout of the cytoplasmic and the loosely membrane-associated factors might help to test this possibility. Washing of the permeabilized MEFs resulted in less BAX in wt and BAK−/− MEFs, whereas no change was found in the BAK level of wt and BAX−/− MEFs (Fig 4A, lower panels; supplementary Fig S6D online). The addition of 37 nM tBID caused rapid and complete loss of ΔΨm in washed wt, and BAX−/− MEFs, whereas the effect of tBID was greatly reduced in washed BAK−/− MEFs (Fig 4B). Immunoblots confirmed the bulk release of cyto c in wt, and BAX−/− MEFs, but cyto c release was almost abolished in the BAK−/− MEFs (Fig 4B). Thus, washing removed BAX seems to partly compensate for the loss of BAK in BAK−/− MEFs. The compensation might be absent in V2−/− MEFs because they fail to upregulate BAX, unlike BAK−/− MEFs. This might be because V2−/− MEFs maintain some low-level BAK content.
Figure 4.
BAX-dependent tBID-induced ΔΨm loss and cyto c release in permeabilized BAK−/− and V2−/− MEFs. (A) Immunoblot showing the presence of BAX in membrane and cytosol (upper left panels) and BAK in membrane (upper right panel) of permeabilized wt, BAK−/− and BAX−/− MEFs. Membranes from the permeabilized, washed cells were immunoblotted for BAX and BAK (lower two panels). (B) ΔΨm was monitored for permeabilized and washed wt, BAK−/− and BAX−/− MEFs treated with 37 nM recombinant tBID. Immunoblot of rapidly separated supernatants (cytosol) showed the release of cyto c. (C) ΔΨm was monitored for permeabilized V2−/− MEFs treated either with 50 nM oligomeric BAX only (light grey trace) or with 50 nM BAX+37 nM tBID (dark grey trace). The supernatants (cytosol) were rapidly separated at different time points (indicated by arrows) and immunoblotted for cyto c. (D) Cumulative data showing BAX and BAX+tBID induced depolarization of V2−/− MEFs. Cyto c, cytochrome c; FCCP, carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone; MEF, mouse embryonic fibroblast; tBID, truncated BID; V2−/−, VDAC2−/−; wt, wild type.
To test whether elevated BAX would also allow tBID-induced OMM permeabilization in V2−/− MEFs, we supplemented the cells with recombinant BAX. The addition of BAX caused slow mitochondrial depolarization in suspensions of permeabilized V2−/− MEFs (Fig 4C,D, light grey trace). When tBID was added together with BAX the depolarization was accelerated (Fig 4C,D, dark grey trace). Immunoblots showed that BAX alone induced a slow release of cyto c, whereas the combination of BAX and tBID accelerated the release (Fig 4C). Thus, both the ΔΨm and cyto c release results indicate that BAX can help to regain some tBID-induced OMM permeabilization in V2−/− MEFs.
We discovered that VDAC2 is essential for tBID-mediated mitochondrial apoptosis, contributing to cell death induced by tBID generation. VDAC2 is needed for recruiting BAK, a main downstream effector of tBID in OMM permeabilization, to the OMM. Furthermore, selective mitochondrial depletion of BAK in V2−/− MEFs does not initiate massive upregulation of BAX, preventing substitution of BAK by BAX in tBID-induced OMM permeabilization. Collectively, these findings strongly indicate that VDAC2 is needed for cell death pathways, not necessarily as a membrane pore but as a crucial factor in the recruitment of proapoptotic BCL2 family proteins to the OMM. Thus, the present study proposes a revision of the current models (Cheng et al, 2003; Baines et al, 2007) on the contribution of VDAC2 to BID-dependent mitochondrial apoptosis (see scheme in supplementary Fig S7 online). However, it also indicates that apoptotic mechanisms that do not directly engage mitochondrial BAK can be fully functional in the absence of VDAC2. The differential regulation of the BID-dependent and other cell death mechanisms by VDAC2 explains why its proapoptotic role remained unnoticed in previous studies.
Methods
Methods are described in more detail in the supplementary information online.
Cell culture, transfection and infection. MEFs were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum as described (Roy et al, 2009). Cells were transfected with plasmid DNA (cyto c-GFP, VDAC2 and mtDsRed) by means of electroporation. tBID adenoviruses were used as described (Sarig et al, 2003).
ΔΨm and cyto c release assay in suspension of permeabilized cells. Measurement of ΔΨm in permeabilized MEFs was performed as described (Madesh et al, 2002; Roy et al, 2009). In this assay, mitochondrial cyto c depletion causes ΔΨm loss. At the end of the fluorimetric measurements of ΔΨm, cytosol was separated from the membranes by centrifugation (10,000g for 5 min) or by rapid filtration (Madesh et al, 2002).
Fluorescence imaging. Fluorescence imaging of cyto c-GFP- expressing and mtDsRed-expressing permeabilized MEFs was performed alternating between optical filter sets for fluorescein (cyto c-GFP) and for rhodamine (mtDsRed) with a 6 s acquisition delay as described (Madesh et al, 2002).
Cell fractionation and mitochondrial cyto c release assay. Mitochondria were isolated and incubated with tBID for 10 min as described (Roy et al, 2009). After the assay, supernatant was separated from the pellet by centrifugation (10,000 g for 5 min).
Immunoblot analysis. Lysates from membrane fractions and whole cells were made by using radioimmunoprecipitation buffer. Western blot analysis was performed as described (Roy et al, 2009). Primary antibodies were anti-cyto c (Pharmingen, San Jose, CA, USA), anti-BID (R&D Systems, Minneapolis, MN, USA), anti-BAK NT (Upstate, Bedford, MA, USA), anti-BAX N20 (Santa Cruz, Santa Cruz, CA, USA) and anti-VDAC2 (Abcam, Cambridge, MA, USA). Anti-prohibitin (Abcam) and anti-actin (BD Transduction, San Jose, CA, USA) were used as loading controls for membrane and for cytosolic as well as for cell lysates, respectively.
Flow cytometry. To assess cell death, the MEFs (both attached and detached) were harvested and incubated with Annexin-V Alexa Fluor 488 conjugate (1:40 dilution) and propidium iodide (2.5 μg/ml). Samples were analysed within 1 h (488 and 568 nm excitation).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary methods
Acknowledgments
We thank Dr Atan Gross for his comments on the paper. This work was supported by a National Institutes of Health grant (GM59419) to G.H.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abu-Hamad S, Zaid H, Israelson A, Nahon E, Shoshan-Barmatz V (2008) Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J Biol Chem 283: 13482–13490 [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9: 550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ding WX, Ni HM, Gao W, Shi YH, Gambotto AA, Fan J, Beg AA, Yin XM (2007) Bid-independent mitochondrial activation in tumor necrosis factor alpha-induced apoptosis and liver injury. Mol Cell Biol 27: 541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301: 513–517 [DOI] [PubMed] [Google Scholar]
- Colombini M (2004) VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem 256–257: 107–115 [DOI] [PubMed] [Google Scholar]
- Craigen WJ, Graham BH (2008) Genetic strategies for dissecting mammalian and Drosophila voltage-dependent anion channel functions. J Bioenerg Biomembr 40: 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol 144: 891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, Petit PX, Vaz FM, Gottlieb E (2008) Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol 183: 681–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD (2002) Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111: 331–342 [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2: 183–192 [DOI] [PubMed] [Google Scholar]
- Madesh M, Antonsson B, Srinivasula SM, Alnemri ES, Hajnoczky G (2002) Rapid kinetics of tBid-induced cytochrome c and Smac/DIABLO release and mitochondrial depolarization. J Biol Chem 277: 5651–5659 [DOI] [PubMed] [Google Scholar]
- Madesh M, Hajnoczky G (2001) VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol 155: 1003–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18: 2330–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino JG, Shulga N, Hoek JB (2002) Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277: 7610–7618 [DOI] [PubMed] [Google Scholar]
- Roy SS, Madesh M, Davies E, Antonsson B, Danial N, Hajnoczky G (2009) Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell 33: 377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarig R, Zaltsman Y, Marcellus RC, Flavell R, Mak TW, Gross A (2003) BID-D59A is a potent inducer of apoptosis in primary embryonic fibroblasts. J Biol Chem 278: 10707–10715 [DOI] [PubMed] [Google Scholar]
- Setoguchi K, Otera H, Mihara K (2006) Cytosolic factor- and TOM-independent import of C-tail-anchored mitochondrial outer membrane proteins. EMBO J 25: 5635–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487 [DOI] [PubMed] [Google Scholar]
- Tajeddine N et al. (2008) Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene 27: 4221–4232 [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB (1999) Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell 3: 159–167 [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN et al. (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315: 856–859 [DOI] [PubMed] [Google Scholar]
- Wu S, Sampson MJ, Decker WK, Craigen WJ (1999) Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochim Biophys Acta 1452: 68–78 [DOI] [PubMed] [Google Scholar]
- Yin XM (2006) Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene 369: 7–19 [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ (1999) Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400: 886–891 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods