Abstract
Purpose
In-transit disease afflicts approximately 10% of patients with extremity melanoma; no single treatment approach has been uniformly accepted as the most effective. We report long-term outcomes in patients with in-transit extremity melanoma who underwent isolated limb perfusion (ILP) in an era of increasingly accurate staging, uniform operative and treatment conditions, and regular long-term follow-up.
Patients and Methods
Between May 1992 and February 2005, 91 patients (median age, 57 years; 50 women, 41 men) underwent a 90-minute hyperthermic ILP (melphalan, 10 to 13 mg/L limb volume, tumor necrosis factor [TNF; n = 44], or interferon [n = 38]) using uniform operative technique and intraoperative leak monitoring. Patients were prospectively followed for response, in-field progression-free survival (PFS), and overall survival (OS). Parameters associated with in-field PFS and OS were analyzed by standard statistical methods.
Results
There was one operative death (1.1%). There were 62 complete responses (69%) and 23 partial responses (26%) in 90 assessable patients. At a median potential follow-up of 11 years, median in-field PFS was 12.4 months and median OS was 47.4 months; 5 and 10-year actuarial OS probabilities were 43% and 34%, respectively. Female sex and low tumor burden (≤ 20 lesions) were associated with prolonged in-field PFS (male:female hazard ratio [HR], 2.07; 95% CI, 1.27 to 3.38; 21+ v ≤ 20 tumors HR, 2.29; 95% CI, 1.21 to 4.34; P < .011 for both). Female sex was associated with improved OS (P = .027; male:female HR, 1.82; 95% CI, 1.07 to 3.09).
Conclusion
In appropriately selected patients, ILP has clinical benefit. The use of TNF was not associated with improved in-field PFS, while female sex was associated with better survival.
INTRODUCTION
An estimated 62,480 new cases of primary melanoma occurred in 2008.1 The proportion of extremity lesions is approximately 50%2 and, therefore, approximately 31,000 new cases of extremity melanoma occurred in the United States in 2008. Between 9% and 17% of patients who have extremity melanoma of intermediate or greater thickness will develop regional cutaneous disease in approximately a 2:1 ratio of in-transit metastases to satellite lesions indicating that a significant population of melanoma patients will develop regionally advanced melanoma of the extremity.
Isolated limb perfusion (ILP) is a surgical procedure for regional intravascular delivery of chemotherapeutic or biologic agents to a tumor-burdened extremity. ILP was first conceived and performed more than 50 years ago by Creech et al3 at Tulane University in New Orleans, LA. The higher drug concentrations achieved by ILP may improve the efficacy of treatment while limiting the systemic toxic effects of antineoplastic agents. Over the past 15 years there has been substantial interest in ILP because of the availability of recombinant tumor necrosis factor (TNF) for use in the treatment regimen.4 Clinical trials using this biologic agent have provided significant insights into the role of isolation perfusion in different clinical settings. Because of the severe systemic toxicity associated with even small perfusate to systemic leaks of TNF during isolation perfusion, uniform perfusion techniques, more consistent treatment parameters, and the routine use of leak monitoring were widely adopted.5–7
Currently, ILP has not been widely adopted as a treatment option for patients with in-transit melanoma because of an under appreciation of its treatment efficacy and limited data regarding long-term outcomes. In this report we summarize our institutional experience with ILP in carefully selected patients with in-transit extremity melanoma who were treated using uniform operative and treatment parameters and followed regularly thereafter.
PATIENTS AND METHODS
From May 1992 to February 2005, 91 patients with in-transit malignant melanoma of the extremity (all stage IIIB or IIIC, 2002 American Joint Committee on Cancer Staging System) underwent ILP. All patients were treated on one of several institutional review board–approved protocols after obtaining informed consent. Demographic data, disease presentation, treatment characteristics, and prior treatment history were retrieved from a prospectively maintained database. A complete preoperative evaluation was performed. The number of in-transit lesions and the surface area of the largest in-transit lesion were recorded for each patient.
Patients underwent ILP as described4,8; 80 (88%) were via the iliac or femoral artery approach, three were via the axillary artery and eight were via the popliteal artery approach. For the iliac artery approach, the vessels were exposed with a standard lower-abdominal transplant incision and retroperitoneal dissection. The external iliac artery and vein were dissected distally, and all venous and arterial tributaries were ligated and divided. The external iliac artery and vein were then cannulated and a tourniquet was wrapped at the most proximal portion of the extremity. A heat exchanger and external warming blanket were used to maintain limb temperature between 38.5°C and 40.0°C. The pump oxygenator was primed with heparinized balanced salt solution and packed RBCs to maintain a hematocrit of approximately 25%. Continuous intraoperative monitoring was performed to assess for systemic perfusate leakage, and adjustments were made to avoid further leakage when this was discovered. All patients received a melphalan dose of 10 mg/L lower and 13 mg/L upper extremity volume. Thirty-seven patients (41%) received 0.2 mg of IFN-γ, 4 mg of TNF-α, and melphalan. Six patients received 6 mg of TNF-α in addition to melphalan. Perfusion of the lower extremity was performed for 90 minutes. The lower extremity was then flushed with 2 L of crystalloid solution and 1 L of 5% albumin. Data were collected intraoperatively on the various perfusion-related parameters, such as flow rate and perfusion pressure, achieved during ILP.
Patients were observed postoperatively at 4 to 6 weeks after ILP, every 3 months for the first year, every 4 months for the second year, and every 6 months thereafter. At each follow-up, each patient underwent physical examination, laboratory tests, and imaging studies (computed tomography of the chest, abdomen, and pelvis). Head magnetic resonance imaging was performed annually, and bone scan was performed when clinically indicated. Photographs were taken of all patients preoperatively and at multiple time points postoperatively to document responses. Some patients with deep lesions that were difficult or impossible to palpate and measure directly were evaluated by sequential extremity computed tomography or magnetic resonance imaging scans. The perfusion field was defined as the region of the limb below a line extending from the inguinal crease medially to the upper one third of the thigh laterally. After ILP, the perfusion field typically had mild chronic erythematous changes that aided in defining the upper extent of treatment. For pigmented lesions in which a tattoo remained after the substance of the lesion regressed, biopsies were performed on representative areas to confirm a complete pathologic response. Nonpigmented lesions were monitored clinically. If recurrence occurred within the perfusion field, it was defined as an in-field recurrence. If it occurred anywhere outside of the perfusion field, it was defined as out of field. Dates of these recurrences were recorded for each patient.
Statistical analyses consisted initially of individual actuarial analyses using Kaplan-Meier curves and two-tailed log-rank tests. They were performed on several patient-, tumor-, and treatment-related factors for the following two outcomes: survival (defined as the time from ILP date to last follow-up date) and in-field recurrence free survival (defined as the time from ILP date to either in-field recurrence date or last follow-up date for patients who have not yet experienced an in-field recurrence). For analysis of continuously distributed potential prognostic variables, four strata were initially created of roughly equal sized groups based on quartiles of the observed parameter distribution in order to identify if potential differences in overall or in-field PFS could be identified in one set of values versus another. The division in the data which yielded the greatest association with outcome was selected for further investigation. In view of the number of parameters examined and the techniques used to identify cut-points to form groups for analysis, only unadjusted P values lower than .01 were interpreted as being statistically significant. Any unadjusted P values > .01 and ≤ .05 were interpreted as indicative of a trend and would require independent confirmation to establish the importance of the parameter with respect to either outcome. Unadjusted P values > .05 and ≤ .10 suggested potentially weaker trends, but ones that may also be worthy of further investigation. After evaluation of the univariate results, Cox proportional hazards model analyses were performed for each outcome, initially incorporating those factors with unadjusted P values lower than .10 in the univariate analyses. A backward selection algorithm was used to determine the final model, which was restricted to parameters with individual P values of lower than .05.
RESULTS
Patient demographic data and tumor-related characteristics at presentation are shown in Table 1; the sex ratio was approximately equal and the age at treatment ranged from 24 to 84 years. Sixty-one patients (68%) presented with stage IIIA disease. The median depth of penetration of the primary lesion at initial diagnosis was 2.6 mm; although the median interval between treatment of the primary lesion and the development of in-transit metastases was 18.2 months, the range (0 to 273 months) highlights the remarkably variable biology of this condition. Similarly, the burden of disease was diverse as quantitated by number of lesions (reported in categories) and size of the largest lesion (Table 1).
Table 1.
Patient Demographics and Tumor-Related Characteristics at Presentation for Isolated Limb Perfusion
| Characteristic | No. | % |
|---|---|---|
| Median age, years | 57 | |
| Range | 24-84 | |
| Female: male | 50:41 | |
| AJCC stage | ||
| III B | 61 | 68 |
| III C | 29 | 32 |
| Median primary tumor | ||
| Thickness, mm | 2.6 | |
| Range | 0.4-10.0 | |
| Median time to in-transit | ||
| disease, months | 18.2 | |
| Range | 0-273 | |
| Primary tumor location | ||
| Arm | 3 | 3 |
| Thigh | 7 | 8 |
| Leg | 50 | 56 |
| Foot | 30 | 33 |
| Median No. in-transit | ||
| lesions | 6-10 | |
| Range | 1-> 99 | |
| Mean largest in-transit size, cm | 2.5 | |
| Range | 0.1-4.5 | |
Abbreviation: AJCC, American Joint Committee on Cancer.
Treatment parameters are presented in Table 2. All patients underwent a 90-minute ILP with a standard dose of melphalan with or without biologic agents. Melphalan was dosed based on measured limb volume as described in the methods, and the lower doses in the total melphalan dose range (32 to 181 mg) reflect the smaller limb volumes of the upper extremity. Almost half (48%) of patients received 4 (n = 37) or 6 mg (n = 6) of TNF-α; those who received 4 mg were also given 0.2 mg of IFN-γ during ILP. The administration of TNF-α or IFN-γ was determined by the clinical research trial under which that patient received treatment. Flow rates were generally adjusted upward until arterial line pressures approximated the mean arterial blood pressure and were adjusted slightly upward or downward depending on changes in the reservoir volume within the perfusion circuit or measured systemic leak of perfusate. Target tissue temperature was 38.5°C.
Table 2.
Treatment Characteristic
| Parameter | No. | % |
|---|---|---|
| TNF dose level, mg | ||
| 3-4 | 37 | 41 |
| 6 | 6 | 7 |
| IFN, 0.2 mg | 37 | 41 |
| Median melphalan dose, mg | 109 | |
| Range | 32-181 | |
| Median flow rate, ml/min | 641 | |
| Range | 325 to 1,050 | |
| Median temperature, °C | 39.3 | |
| Range | 36.9-40.7 | |
| Leak > 5% | 10 | 13 |
| Median percent leak | 0 | |
| Range | 0-16 | |
| Vessels accessed | ||
| Axillary | 3 | 3 |
| Iliac or common femoral | 79 | 88 |
| Popliteal | 8 | 9 |
Abbreviations: TNF, tumor necrosis factor; IFN, interferon.
Eighty patients (87%) had less than a 5% leak of perfusate as measured by continuous intraoperative leak monitoring; the maximum leak measured was 16%. There was one postoperative mortality (one of 91 or 1.1%) in a septuagenarian woman who had pulmonary complications after undergoing urgent reoperation to control retroperitoneal bleeding after ILP; this complication did not appear to be related to the use of TNF in the perfusate. Most patients experienced transient and mild regional toxicities; severe regional toxicities were rare. Two patients experienced transient grade 3 skin toxicity (erythema and blistering) and two patients experienced transient grade 4 nerve and muscle toxicity (pain and weakness) Most significant systemic toxicities were associated with the use of TNF; transient hypotension was the most common consistent with previously published reports.5,9 Eleven patients had transient grade 3 or 4 hypotension and three patients had transient grade 3 fever. Other systemic toxicities included grade 3 hepatic toxicity (n = 3), leucopenia (n = 1), and arrhythmia (n = 1).
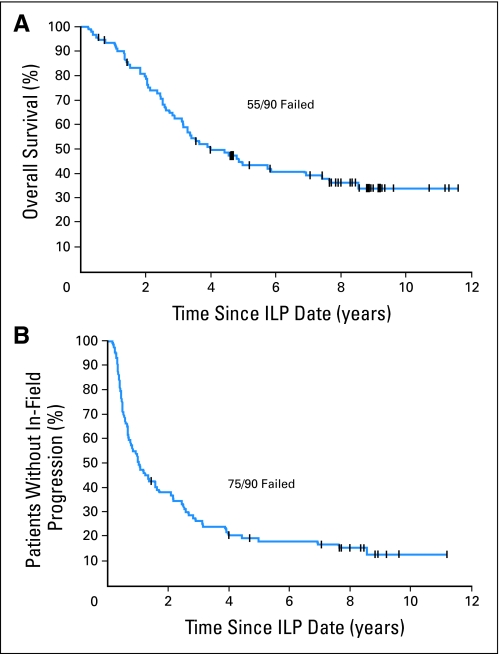
The overall response rate was 95% (85 of 90 evaluable); there were 62 patients (69%) who had a complete response (CR) and the overall median in-field progression-free survival (PFS) was 12.4 months. With a median potential follow-up time of 11 years, the median overall survival (OS) was 47.4 months and the 5- and 10-year actuarial OS probabilities were 43% and 34%, respectively (Fig 1).
Fig 1.
Actuarial (A) overall survival and (B) in-field progression-free survival in 90 patients after isolated limb perfusion (ILP) for melanoma.
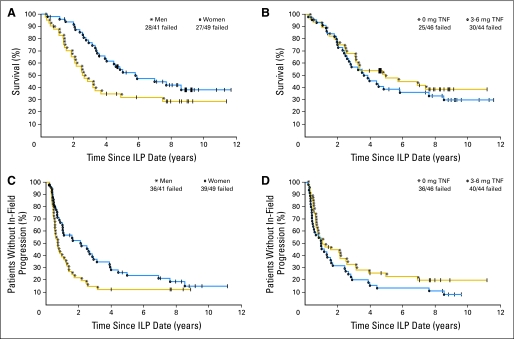
Individual actuarial analyses were performed on several clinical and pathological parameters for the two outcomes of survival and the development of in-field recurrence. Factors such that unadjusted univariate P values for their association with outcome were less than .10, along with the effect of TNF (for completeness) are presented in Table 3. In this exploratory analysis, a number of factors appeared worthy of further evaluation in a Cox model since the unadjusted P value was lower than .10. In the analysis of factors potentially associated with outcome, the backward selection algorithm for the Cox proportional hazards model identified ≤ 20 lesions and female sex as significantly and independently associated with prolonged in-field PFS, while only female sex was shown to be associated with OS (Table 4, Fig 2). Notably, the use of TNF had no influence on in-field PFS or OS while the presence of a higher disease burden as defined by more than 20 lesions was associated with a shorter time to in-field recurrence (Fig 2).
Table 3.
Factors Potentially Associated With In-Field Recurrence or Survival
| Outcome | Unadjusted P |
|---|---|
| In-field PFS | |
| Lesions, ≤ 20 v 21+ | .062 |
| TNF dose, 0 v 3-6 mg | .18 |
| Sex | .019 |
| Dx to in-transit melanoma, < 66 v 67+ months | .069 |
| Perfusion pressure, ≤ 85 v 86+ | .079 |
| Stage | .050 |
| Survival | |
| TNF 0 v 3-6 mg | .49 |
| Sex | .025 |
| Dx to intransit melanoma, ≤18 v > 18 months | .073 |
| Stage | .065 |
Abbreviations: PFS, progression-free survival; TNF, tumor necrosis factor; Dx, diagnosis of primary tumor.
Table 4.
Cox Proportional Hazards Model
| Variable | P | Hazard Ratio | 95% CI |
|---|---|---|---|
| In-field progression-free survival | |||
| Lesions (≤ 20 v 21+) | .011 | 2.29 | 1.21 to 4.34 |
| Sex (male v female) | .004 | 2.07 | 1.27 to 3.38 |
| Overall survival | |||
| Sex (male v female) | .027 | 1.82 | 1.07 to 3.09 |
Fig 2.
Actuarial overall survival and in-field progression-free survival in (A and C) men versus women (P = .025 and .019, respectively) and (B and D) with or without tumor necrosis factor (TNF) after isolated limb perfusion (ILP) in patients with melanoma.
DISCUSSION
Although ILP has been clinically used for treatment of in-transit extremity melanoma for more than 50 years it has not gained widespread acceptance as an effective treatment option for this clinical condition for a number of reasons. For many years, the published reports on its use were largely retrospective institutional reviews in which patient selection criteria, treatment parameters such as type or dose of antineoplastic agents, duration of perfusion, use of hyperthermia, or outcomes such as response rates and survival were not consistent or rigorously measured making interpretation of the efficacy and benefit of ILP uncertain. Although short-term CR rates of up to 90% have been reported,10–12 long-term outcomes including limb PFS and OS have not been clearly defined. The current data show a median in-field PFS of approximately 1 year and median OS of 47.4 months. The 5- and 10-year actuarial OS were 43% and 34%, respectively. Female sex was associated with both prolonged PFS and OS. Interestingly, low tumor burden (≤ 20 lesions) was associated with prolonged in-field PFS suggesting that earlier intervention when tumor burden is minimal may be important in optimizing outcome after ILP. Successful management of regional extremity recurrence after ILP is important as most patients will survive for many years beyond their first recurrence. We have previously reported that regional recurrences after a CR to ILP are frequently limited in number and can be effectively managed with serial local excision.13 In patients who have experienced an initial partial response or CR and then recur with multifocal disease confined to the extremity, a repeat ILP can be performed.14 Together these data suggest that even though recurrence after ILP is common; the biology of the disease may be altered to one that is amenable to local excision.
There has been considerable interest in the use of TNF in ILP. The American College of Surgeons Oncology Group reported results of 133 patients with in-transit extremity melanoma randomly assigned to one of two treatment arms; the primary end point of the study was to assess CR rates at 3 months.15 No long-term follow-up data were reported from that trial; the CR rates were 25% and 26% for the melphalan alone and the combined TNF and melphalan treatments, respectively. These response rates are lower those reported by others and considerably lower than those in this report. The most likely explanation for this disparity is that responses in the American College of Surgeons Oncology Group study were scored based on measurements of residual pigmented lesions. Under these circumstances flat lesions were likely scored as stable disease although in many patients they may have actually been a CR with only residual melanin pigmentation at sites of previous in-transit disease. We routinely biopsy under these circumstances to confirm that the lesion represents dermal pigmentation with no residual melanoma; this was a standard practice for patients in our series. Notably, the response rates in our study are in line with other reports of similarly treated patients.11,16,12
Consistent with the findings of the American College of Surgeons Oncology Group trial, the use of TNF did not influence PFS or OS in this patient cohort and should not be used in the initial management of patients with melanoma undergoing ILP for multifocal metastases. There are some data suggesting that response to ILP for large bulky melanoma metastases may be improved with TNF.17,4 However, for most patients, melphalan alone is an effective regimen; the drug, an analog of tyrosine, is actively taken up by cells that use tyrosine for melanin synthesis.18
In summary, our data show that operative ILP results in high response rates and is associated with long-term survival in patients with in-transit melanoma. Factors associated with prolonged in-field PFS are lower tumor burden (reflected in tumor number fewer than 20) and female sex. The latter was also associated with longer OS. ILP with melphalan alone should be considered for patients with in-transit extremity melanoma.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: H. Richard Alexander Jr, Douglas L. Fraker, David L. Bartlett
Administrative support: H. Richard Alexander Jr, Steven K. Libutti, Perry Soriano, Tatiana Beresnev
Provision of study materials or patients: H. Richard Alexander Jr, Douglas L. Fraker, David L. Bartlett, Steven K. Libutti
Collection and assembly of data: H. Richard Alexander Jr, Douglas L. Fraker, David L. Bartlett, Steven K. Libutti, Seth M. Steinberg, Tatiana Beresnev
Data analysis and interpretation: H. Richard Alexander Jr, Douglas L. Fraker, David L. Bartlett, Seth M. Steinberg, Perry Soriano
Manuscript writing: H. Richard Alexander Jr, Seth M. Steinberg, Perry Soriano
Final approval of manuscript: H. Richard Alexander Jr, Seth M. Steinberg
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Slingluff CL, Flaherty K, Rosenberg SA, et al. Cutaneous Melanoma. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. ed 8. Philadelphia, PA: Lippincott; 2008. pp. 1897–1951. [Google Scholar]
- 3.Creech O, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: Regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–632. doi: 10.1097/00000658-195810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander HR. Isolation perfusion. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. ed 8. Philadelphia, PA: Lippincott; 2008. pp. 701–707. [Google Scholar]
- 5.Thom AK, Alexander HR, Andrich MP, et al. Cytokine levels and systemic toxicity in patients undergoing isolated limb perfusion (ILP) with high-dose TNF, interferon-gamma and melphalan. J Clin Oncol. 1995;13:264–273. doi: 10.1200/JCO.1995.13.1.264. [DOI] [PubMed] [Google Scholar]
- 6.Stam TC, Jongen-Lavrencic M, Eggermont AMM, et al. Effects of isolated limb perfusion with tumour necrosis factor-alpha on the function of monocytes and T lymphocytes in patients with cancer. Eur J Clin Invest. 1996;26:1085–1091. doi: 10.1046/j.1365-2362.1996.480599.x. [DOI] [PubMed] [Google Scholar]
- 7.Vrouenraets BC, Kroon BB, Ogilvie AC, et al. Absence of severe systemic toxicity after leakage-controlled isolated limb perfusion with tumor necrosis factor-alpha and melphalan. Ann Surg Oncol. 1999;6:405–412. doi: 10.1007/s10434-999-0405-9. [DOI] [PubMed] [Google Scholar]
- 8.Zogakis TG, Bartlett DL, Libutti SK, et al. Factors affecting survival after complete response to isolated limb perfusion in patients with in-transit melanoma. Ann Surg Oncol. 2001;8:771–778. doi: 10.1007/s10434-001-0771-4. [DOI] [PubMed] [Google Scholar]
- 9.Stam TC, Swaak AJG, de Vries MR, et al. Systemic toxicity and cytokine/acute phase protein levels in patients after isolated limb perfusion with tumor necrosis factor-α complicated by high leakage. Ann Surg Oncol. 2000;7:268–275. doi: 10.1007/s10434-000-0268-6. [DOI] [PubMed] [Google Scholar]
- 10.Lienard D, Ewalenko P, Delmotti JJ, et al. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 11.Knorr C, Meyer T, Janssen T, et al. Hyperthermic isolated limb perfusion (HILP) in malignant melanoma: Experience with 101 patients. Eur J Surg Oncol. 2006;32:224–227. doi: 10.1016/j.ejso.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Grunhagen DJ, de Wilt JH, van Geel AN, et al. TNF dose reduction in isolated limb perfusion. Eur J Surg Oncol. 2005;31:1011–1019. doi: 10.1016/j.ejso.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Libutti SK, Alexander HR, Bartlett DL, et al. Patterns of local failure in extremity melanoma after a complete response to isolated limb perfusion with melphalan with or without tumor necrosis factor. Proc Am Soc Clin Oncol. 1996;15:552. [Google Scholar]
- 14.Bartlett DL, Ma G, Alexander HR, et al. Isolated limb reperfusion with tumor necrosis factor and melphalan in patients with extremity melanoma after failure of isolated limb perfusion with chemotherapeutics. Cancer. 1997;80:2084–2090. doi: 10.1002/(sici)1097-0142(19971201)80:11<2084::aid-cncr7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group trial Z0020. J Clin Oncol. 2006;24:4196–4201. doi: 10.1200/JCO.2005.05.5152. [DOI] [PubMed] [Google Scholar]
- 16.Klaase JM, Kroon BBR, van Geel AN, et al. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion using melphalan. Surgery. 1994;115:39–45. [PubMed] [Google Scholar]
- 17.Fraker DL, Alexander HR, Andrich M, et al. Palliation of regional symptoms of advanced extremity melanoma by isolated limb perfusion with melphalan and high-dose tumor necrosis factor. Cancer J Sci Am. 1995;1:122–130. [PubMed] [Google Scholar]
- 18.Luck JM. Action of p-dichloroethyl amino-L-phenylalanine on Harding-Passey mouse melanoma. Science. 1956;123:984–985. doi: 10.1126/science.123.3205.984. [DOI] [PubMed] [Google Scholar]