Abstract
Purpose
Ixabepilone (BMS-247550) is a microtubule-stabilizing epothilone B analog with activity in taxane-resistant metastatic breast cancer. The Gynecologic Oncology Group conducted a phase II evaluation of the efficacy and safety of ixabepilone in patients with recurrent or persistent platinum- and taxane-resistant primary ovarian or peritoneal carcinoma.
Patients and Methods
Patients with measurable platinum- and taxane-resistant ovarian or peritoneal carcinoma, defined as progression during or within 6 months of one prior course of treatment with each agent, received intravenous ixabepilone 20 mg/m2 administered over 1 hour on days 1, 8, and 15 of a 28-day cycle.
Results
Of 51 patients entered, 49 were eligible. The objective response rate was 14.3% (95% CI, 5.9% to 27.2%), with three complete and four partial responses. Twenty patients (40.8%) had stable disease, whereas sixteen (32.7%) had increasing disease. The median time to progression was 4.4 months (95% CI, 0.8 to 32.6+ months); median survival was 14.8 months (95% CI, 0.8 to 50.0) months. Patients received a median of two treatment cycles (range, 1 to 29 cycles), and 18.4% of patients received ≥ six cycles. Adverse effects included peripheral grade 2 (28.5%) and grade 3 (6.1%) neuropathy, grades 3 to 4 neutropenia (20.4%), grade 3 fatigue (14.3%), grade 3 nausea/emesis (22%), grade 3 diarrhea (10%), and grade 3 mucositis (4%).
Conclusion
Ixabepilone 20 mg/m2 over 1 hour on days 1, 8, and 15 of a 28-day cycle demonstrates antitumor activity and acceptable safety in patients with platinum- and taxane-resistant recurrent or persistent ovarian or primary peritoneal carcinoma.
INTRODUCTION
Taxanes are among the most active cytotoxic agents in epithelial ovarian cancer and have antitumor activity in platinum- and paclitaxel-resistant ovarian cancer.1,2 Taxane-based therapies are complicated by problems with formulations and hypersensitivity reactions, neuropathy, bone marrow suppression, and the development of resistance. Efforts to overcome some of these problems have led to the emergence of the epothilones, a novel class of microtubule-stabilizing agents.3,4 Epothilones are similar to taxanes in targeting microtubules and inducing mitotic arrest by inhibiting depolymerization of the microtubules, but there are important differences. Epothilones are structurally unrelated to taxanes and are less susceptible than taxanes to overexpression of P-glycoprotein, the presence of certain tubulin isoforms (class III β-tubulin), and tubulin mutations, all of which have been implicated in taxane resistance.5–7 Ixabepilone (previously known as aza-epothilone B [BMS-247550]) is a semisynthetic analog of the natural product epothilone B with improved in vitro metabolic stability and protein binding. Ixabepilone binds to β-tubulin, stabilizes microtubules, and induces G2-M cell cycle arrest and apoptosis.4
Ixabepilone demonstrated significant antitumor activity in taxane-resistant Pat-7 and A2780Tax human ovarian carcinoma xenografts; A2780Tax has a β-tubulin mutation.4
Phase I studies of ixabepilone have used different schedules: every 3 weeks, weekly, and daily for 3 to 5 days every 21 days.8 Antitumor responses have been observed in all of the phase I trials.8 Dose-limiting toxicities (DLT) with the every 3 weeks schedule were prolonged neutropenia and sensory neuropathy.9,10 Other preliminary phase I data available at the design stage of the study presented here suggested less neurotoxicity and neutropenia with weekly infusions.10–12
The Gynecologic Oncology Group (GOG) conducted a multicenter, phase II trial of ixabepilone in patients with platinum- and taxane-resistant recurrent ovarian or primary peritoneal cancer. This report focuses on the efficacy and safety of ixabepilone at a dose of 20 mg/m2 administered intravenously over 1 hour on days 1, 8, and 15 of a 28-day cycle.
PATIENTS AND METHODS
Eligibility
Eligible patients had recurrent or persistent epithelial ovarian or primary peritoneal carcinoma with histologic confirmation of the primary tumor by central GOG pathology review. All patients had to have disease that was considered resistant or refractory to both platinum and a taxane. Platinum/taxane-resistant disease was defined as occurrence of disease progression within 6 months after completing therapy with a taxane and platinum, either alone or in combination. Platinum/taxane-refractory disease was defined as disease progression during treatment with a taxane and platinum, alone or in combination. Patients who were initially treated with platinum and a taxane were restricted to one prior cytotoxic regimen. If the initial platinum regimen did not include a taxane, a second regimen including paclitaxel or docetaxel was allowed. Patients had to have measurable disease and at least one “target lesion” as defined by Response Evaluation Criteria in Solid Tumors (RECIST). Patients had to satisfy all of the following to be eligible: GOG performance status 0 to 2; recovered from recent surgery, radiotherapy, or chemotherapy, and free of active infection; absolute neutrophil count (ANC) ≥ 1,500/mL; platelets ≥ 100,000/mL; normal serum creatinine, bilirubin, AST, and alkaline phosphatase; neuropathy (sensory and motor) ≤ grade 1 by National Cancer Institute Common Toxicity Criteria (CTC; version 2.0). The study protocol was approved by the institutional review boards of participating institutions. All patients provided written informed consent.
Patients were ineligible for participation in this trial if they had a history of other invasive malignancies (excluding nonmelanoma skin cancer) or previous therapy with ixabepilone. Patients with a documented ≥ grade 2 hypersensitivity reaction to paclitaxel or polyoxyethylated castor oil, patients with active brain metastases, and patients taking food supplements such as St John's wort were also excluded.
Treatment
Patients were treated with ixabepilone 20 mg/m2 administered intravenously over 1 hour on days 1, 8, and 15 of a 28-day cycle. All patients received premedication with diphenhydramine and an H2 blocker. Dexamethasone was not used, except before rechallenge in patients who had experienced either recurrent CTC grade 2 (urticaria, drug-related fever ≥ 38°C despite slowing infusion) or CTC grade 3 or 4 hypersensitivity reactions. Concomitant administration of ixabepilone with inducers or inhibitors of CYP3A4/5 was to be avoided.13 Patients were removed from the study if they were unable to tolerate the lowest dose level (15 mg/m2) or if there was progression of disease.
The initial dose level of ixabepilone was 20 mg/m2, and one dose reduction was allowed to 15 mg/m2. If the ANC or platelet count was insufficient for treatment, the dose for that week was omitted and the next dose was administered on the original schedule. Subsequent cycles of therapy were not given until the ANC was ≥ 1,500/mL and the platelet count was ≥ 100,000/mL. Patients who did not have adequate counts after a maximum delay of 2 weeks were removed from the study. Patients who were unable to receive three successive weekly doses of 20 mg/m2 were treated with 15 mg/m2 the next cycle, with no subsequent dose escalation. One dose reduction was allowed for febrile neutropenia, persistent grade 3 neutropenia, and grade 4 thrombocytopenia. The use of granulocyte growth factors was limited to patients who experienced recurrent neutropenic complications despite dose reduction. The dose was reduced to 15 mg/m2 in patients who experienced ≥ grade 2 peripheral neuropathy, ≥ grade 2 renal toxicity, ≥ grade 2 hepatic toxicity, or persistent ≥ grade 3 gastrointestinal toxicity.
Toxicity Assessment
At baseline and before each cycle, a physical examination, including neurologic assessment, was performed. A CBC was obtained weekly, and serum chemistry and hematology were evaluated before each cycle. Adverse events (AEs) were evaluated continuously and graded according to CTC version 2.0.
Response Assessment
The primary objective of the study was to evaluate the objective response rate (ORR) to ixabepilone. Tumor measurements were collected by the investigators and reported before each cycle for clinical measurements and after every second cycle for radiographic measurements. Response was defined by investigators according to RECIST criteria. The best overall ORR was recorded; complete response (CR) and partial responses (PR) not confirmed at ≥ 4 weeks were classified as stable disease (SD).
Statistical Design and Methodology
This study used an optimal but flexible two-stage design with early stopping guidelines intended to limit patient accrual to inactive treatment.14 In the first stage of study, an accrual of 19 to 26 evaluable patients was planned. If there were more than two of 19 to 25 or three of 26 patients responding (CR or PR), accrual to the second stage of the study was to be initiated. Otherwise, the study would be stopped, and the treatment regimen would be classified as clinically uninteresting for future development. If the study advanced to the second stage, an overall accrual of 44 to 51 evaluable patients was targeted. If seven or more of 44 to 45 patients or eight or more of 46 to 51 patients had a response, the regimen would be considered worthy of additional investigation within the GOG. If the true response rate was 10%, the study design limited the average probability of incorrectly designating the treatment as active to 10%. On the other hand, if the true response rate was 25%, then the average probability of correctly classifying the treatment as active was 90%.14 Progression-free survival (PFS) is the period from entry until disease progression, death, or date of last contact. Overall survival (OS) is the observed length of life from entry into the study to death or the date of last contact.
RESULTS
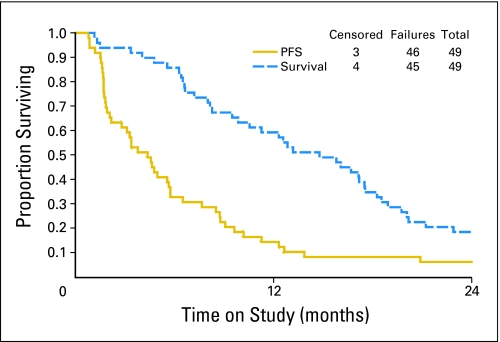
A total of 51 patients entered this multi-institutional phase II trial. Two patients were deemed ineligible on central pathology review; one had a second primary, the other did not have ovarian carcinoma. Response could not be determined in six (12.2%) of 49 eligible patients. All 49 eligible patients were evaluable for toxicity. Their characteristics are presented in Table 1. The majority of patients had primary ovarian carcinoma, GOG performance status 0, serous histology, and poorly differentiated tumors. The progression-free interval from prior taxane/platinum was ≥ 3 months in 29 patients (59%). A median of two courses (range, one to 29 courses) of therapy were administered; 18.4% of patients received six or more cycles of ixabepilone. Responses are presented in Table 2. The ORR for all 49 patients was 14.3% (95% CI, 5.9% to 27.2%). There were three CRs (6.1%) and four PRs (8.2%). Responses were observed after a median of four cycles (range, two to eight cycles). Twenty patients (40.8%) had SD as their best response. There were no responses in patients with platinum- and taxane-refractory disease. In responding patients, the median progression-free interval before treatment with ixabepilone was 5 months (range, 3 to 5.25 months). OS and PFS are shown in Figure 1. The median PFS was 4.4 months (95% CI, 0.8 to 32.6+ months). The median OS was 14.8 months (95% CI, 0.8 to 50.0 months).
Table 1.
Patient Characteristics
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| < 40 | 1 | 2 |
| 40-49 | 6 | 12 |
| 50-59 | 13 | 27 |
| 60-69 | 15 | 30 |
| 70-79 | 13 | 27 |
| ≥ 80 | 1 | 2 |
| Performance status | ||
| 0 | 31 | 63 |
| 1 | 18 | 37 |
| Primary site | ||
| Ovary | 40 | 82 |
| Peritoneum | 9 | 18 |
| Cell type | ||
| Serous | 38 | 78 |
| Clear cell | 4 | 8 |
| Mixed epithelial | 3 | 6 |
| Mucinous | 1 | 2 |
| Endometrioid | 2 | 4 |
| Adenocarcinoma, unspecified | 1 | 2 |
| Grade | ||
| 1 | 2 | 4 |
| 2 | 19 | 39 |
| 3 | 28 | 57 |
| Prior chemotherapy | 49 | 100 |
| Prior radiotherapy | 1 | 2 |
| Site of disease | ||
| Pelvic | 16 | 33 |
| Extrapelvic | 33 | 67 |
| Progression-free interval after platinum/taxane | ||
| Refractory | 4 | 8 |
| < 3 months | 16 | 33 |
| 3-6 months | 29 | 59 |
Table 2.
Response
| Response Category | Patients |
|
|---|---|---|
| No. | % | |
| Complete response | 3 | 6.1 |
| Partial response | 4 | 8.2 |
| Stable disease | 20 | 40.8 |
| Increasing disease | 16 | 32.7 |
| Unevaluable | 6 | 12.2 |
| Total | 49 | 100.0 |
Fig 1.
Progression-free survival (PFS) and overall survival.
AEs in Table 3 are presented as the worst grade per patient. There were no treatment-related deaths. Hematologic events consisted primarily of leukopenia and neutropenia, with grade 3 neutropenia in nine patients (18.4%) and grade 4 neutropenia in one patient (2%). Febrile neutropenia occurred in one 80-year-old patient, one patient required treatment with granulocyte growth factors, and in one patient, treatment was discontinued after the first cycle because of persistent neutropenia. Six patients (12.2%) required a dose reduction for neutropenia after the first course of treatment and did not experience recurrent neutropenia. Thrombocytopenia and anemia were mostly grade 1 and 2; four patients (8%) had grade 3 anemia; there was no grade 3 or 4 thrombocytopenia.
Table 3.
Adverse Events for Patients (N = 49)
| Adverse Event | Grade |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Leukopenia | 13 | 9 | 20 | 6 | 1 |
| Thrombocytopenia | 39 | 10 | 0 | 0 | 0 |
| Neutropenia | 17 | 10 | 12 | 9 | 1 |
| Anemia | 9 | 23 | 13 | 4 | 0 |
| Hematologic | 43 | 0 | 3 | 3 | 0 |
| Allergy | 46 | 1 | 2 | 0 | 0 |
| Cardiovascular | 45 | 3 | 0 | 1 | 0 |
| Coagulation | 47 | 0 | 0 | 2 | 0 |
| Fatigue | 11 | 16 | 15 | 6 | 1 |
| Dermatologic | 23 | 13 | 13 | 0 | 0 |
| Endocrine | 48 | 1 | 0 | 0 | 0 |
| Gastrointestinal | 10 | 15 | 14 | 9 | 1 |
| Genitourinary/renal | 41 | 6 | 1 | 1 | 0 |
| Hemorrhage | 48 | 1 | 0 | 0 | 0 |
| Hepatic | 36 | 10 | 2 | 1 | 0 |
| Infection | 43 | 2 | 2 | 2 | 0 |
| Metabolic | 36 | 6 | 3 | 4 | 0 |
| Neurologic | 20 | 12 | 14 | 3 | 0 |
| Ocular | 48 | 0 | 1 | 0 | 0 |
| Pain | 29 | 10 | 8 | 1 | 1 |
| Pulmonary | 37 | 1 | 9 | 2 | 0 |
Neurologic AEs consisted primarily of peripheral sensory neuropathy and were cumulative. Neuropathy was mild (grade 1) in 24.5% of patients. Grade 2 peripheral neuropathy was experienced by 14 patients (28.6%), and grade 3 peripheral neuropathy was reported in three patients (6.1%). In seven patients (14%), treatment was discontinued because of grade ≥ 2 neuropathy persisting beyond 2 weeks. Of the three patients requiring a dose reduction secondary to sensory neuropathy, one received 16 cycles of ixabepilone at 15 mg/m2, with improvement of neuropathy, whereas the two others received two cycles and one cycle, with recurrent neuropathy. Sensory neuropathy was observed after a median of three cycles (range, one to eight cycles) in the entire cohort, and after a median of five cycles (range, four to six cycles) in patients with an objective tumor response to ixabepilone.
Gastrointestinal AEs were grade 1 or 2 in 59% of patients and grade 3 or 4 in 20.4%. Grade 3 mucositis occurred in two patients, grade 3 nausea occurred in seven patients, grade 3 emesis occurred in four patients, grade 3 diarrhea occurred in five patients, grade 3 anorexia occurred in three patients, and grade 3 constipation occurred in one patient. In one patient, treatment was discontinued because of persistent grade 3 emesis. Hepatic and renal toxicity was usually mild. In one patient, grade 3 hepatotoxicity after the first course resolved completely before the second course. One patient had grade 3 nephrotoxicity. Eleven patients (22.4%) had grade 1 alopecia, and nine patients (18.4%) had grade 2 alopecia. One patient experienced grade 4 pain with the last two courses and grade 1 or 2 pain with subsequent courses. There were no severe hypersensitivity reactions.
Dose reductions were necessary in 14 patients (28.5%): neutropenia, six patients (12.2%); peripheral neuropathy, three patients (6.1%); grade 3 nausea/emesis/diarrhea, two patients; and grade 3 hypoxia, urinary incontinence, nephrotoxicity, one patient each.
Ixabepilone was discontinued because of toxicity in 10 patients (20.4%): six for sensory neuropathy, one for neutropenia, and one for intestinal obstruction causing delay of treatment. One patient experienced hypoxia (in retrospect secondary to fluid overload and pneumonia), and one patient had blurred vision and was later found to have glaucoma and cataract. Treatment with ixabepilone was stopped in three patients (6.1%) by the physician and in eight patients (16.3%) per patient request.
DISCUSSION
Ixabepilone seems to be an active cytotoxic agent in patients with recurrent platinum- and taxane-resistant ovarian or primary peritoneal carcinoma. The ORR was 14.3%, with median PFS of 4.4 months. SD was achieved in 40.8% of patients. A report in abstract form of a phase II trial of ixabepilone 40 mg/m2 administered every 21 days describes one PR in 14 patients with ovarian cancer (ORR, 7%).15 In patients with metastatic breast cancer resistant to taxanes, ORRs of 12% to 18.3% have been reported with ixabepilone 40 mg/m2 every 3 weeks.16,17 Ixabepilone (Ixempra) has received approval by the US Food and Drug Administration as monotherapy for the treatment of metastatic or locally advanced breast cancer resistant to taxanes, anthracyclines, and capecitabine and in combination with capecitabine in patients with metastatic breast cancer resistant to treatment with a taxane and an anthracycline.18
We used a weekly infusion schedule to minimize the neutropenia and sensory neuropathy seen with every-21-days dosing.9 Indeed, in a phase I trial of weekly ixabepilone in patients with advanced malignancies, grade 3 fatigue was the DLT at 30 mg/m2, and no DLT occurred at 20 mg/m2.11 In our study, peripheral sensory neuropathy remained the clinically most significant AE of ixabepilone. The frequency of ≥ grade 2 neuropathy (36%) is comparable to the 41% to 45% incidence of ≥ grade 2 neuropathy reported in two phase II trials of ixabepilone 40 mg/m2 administered every 3 weeks in metastatic breast cancer.16,17 In phase II trials including smaller proportions of taxane-resistant patients, the frequency of peripheral neuropathy tends to be lower.19–21 Although direct comparisons between these trials is not possible, it is likely that both the frequency and the severity of peripheral neuropathy are related to cumulative ixabepilone dose, baseline neuropathy, and previous exposure to neurotoxic agents.22 In patients with metastatic breast cancer not pretreated with taxanes, a median time to response of two cycles and a median time to grade 2 or 3 neurotoxicity of six cycles has been reported.21 We did not observe such a favorable therapeutic ratio in patients with taxane-resistant ovarian cancer. Neutropenia was the most common reason for dose reduction, but most patients could subsequently be treated at the reduced dose level. Gastrointestinal toxicity was similar to that of other studies with ixabepilone, but seems higher than with weekly paclitaxel.2,16 No severe hypersensitivity reactions were recorded, and patients in this trial were not premedicated with corticosteroids, a distinct advantage of ixabepilone over paclitaxel and docetaxel.
Although ixabepilone is active in patients with platinum- and taxane-resistant ovarian carcinoma, further study will be necessary to understand how to use ixabepilone in a patient population for whom there is a growing list of cytotoxic and noncytotoxic agents with antitumor activity. Even taxanes have demonstrated activity in patients with recurrent ovarian cancer who were considered platinum/taxane resistant. In a phase II trial of weekly paclitaxel (80 mg/m2), an ORR of 20.9% was observed; rates of grade 2 and 3 neuropathy were 21% and 4%, respectively.2 In another phase II trial of docetaxel 100 mg/m2 every 21 days, the response rate was 22.4%.(1) The principal adverse effect was grade 4 neutropenia (75%) associated with neutropenic fever (30%), and there was one treatment-related death.(1) There are no trials using a molecular definition of taxane resistance. Differences may exist between taxane-resistant tumors responding to re-treatment with taxanes and tumors responding to ixabepilone. Epothilones remain cytotoxic when P-glycoprotein is overexpressed or in the presence of tubulin mutations, both of which have been linked to taxane resistance in vitro.4,5 However, it is not clear that tubulin mutations play a role in the development of resistance to taxanes or epothilones in vivo.23 Overexpression of class III β-tubulin, rather than P-glycoprotein overexpression or α/β tubulin gene mutations, has been reported as a clinically more relevant mechanism of taxane resistance in ovarian cancer.8,24 Cells resistant to taxanes secondary to altered expression of class III β-tubulin remain sensitive to ixabepilone.7 It is also possible that resistance to taxanes and epothilones may involve identical mechanisms, such as abrogation of a functional spindle assembly checkpoint in metaphase, necessary for an efficient cytotoxic response to microtubule-stabilizing agents.25,26 Further refinement of the definition of taxane resistance is desirable to promote understanding of the respective roles of taxanes, ixabepilone, and other microtubule-stabilizing agents. There are currently no established biomarkers of tumor response to microtubule-stabilizing agents. Acetylation and detyrosination of α-tubulin are two of several different posttranslational modifications that only occur after assembly into stable microtubules. Increasing levels of detyrosinated and/or acetylated α-tubulin have been described in tumors of patients treated with ixabepilone and could be measured to show target engagement by ixabepilone.27 There is no consensus on the significance of the expression of the microtubule-associated protein tau-1 and response to ixabepilone.21 In a recent phase II genomics study of ixabepilone as neoadjuvant treatment for breast cancer, both estrogen receptor expression and tau expression were identified as predictors of response to ixabepilone.28 A better understanding of molecular determinants of risk for developing neuropathy would also be helpful. It has been suggested that P-glycoprotein polymorphisms may be relevant to the development of neuropathy with paclitaxel.29 Similar studies could be extended to ixabepilone and microtubule-stabilizing agents in general.
A large number of tubulin-targeting agents are currently in clinical development for cancer chemotherapy, including natural and synthetic microtubule-stabilizing and -destabilizing compounds.30 Among the microtubule-stabilizing agents are novel taxanes, the epothilones, and a large number of diverse new agents. Other epothilones in clinical trials are patupilone, KOS-862 (epothilone-D), ZK-EPO (sagopilone), BMS-310705, and KOS-1584.31 In a phase I/II trial of patupilone in patients with platinum-resistant ovarian cancer, a preliminary ORR of 25% has been reported.32
In this crowded field, ixabepilone has emerged as an active and relatively well-tolerated drug in taxane-resistant tumors and has received approval for the treatment of patients with taxane-resistant metastatic breast cancer. The results presented here show that ixabepilone is active in clinically defined taxane- and platinum and taxane–resistant ovarian cancer. It is not clear whether this offers an advantage over re-treatment with paclitaxel or docetaxel using a weekly schedule in this setting. Further study of ixabepilone as monotherapy or in combination therapy in ovarian carcinoma is warranted, with a focus on the identification of molecular markers of resistance to microtubule-stabilizing agents, including taxanes.
Appendix
The following Gynecologic Oncology Group member institutions participated in this study: Walter Reed Army Medical Center, University of Cincinnati, University of Iowa Hospitals and Clinics, Indiana University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, University of New Mexico, Columbus Cancer Council, University of Massachusetts Medical School, University of Virginia Health Sciences Center, University of Chicago, Tampa Bay Cancer Consortium, University of Texas at Galveston, and Central Connecticut Cancer Consortium.
Footnotes
Supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (Grant No. CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (Grant No. CA 37517).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00025155.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: John A. Blessing
Administrative support: William E. Richards
Provision of study materials or patients: Koen De Geest, Robert T. Morris, S. Diane Yamada, Bradley J. Monk, Susan L. Zweizig, Daniela Matei
Collection and assembly of data: Koen De Geest, S. Diane Yamada, Carolyn Y. Muller, William E. Richards
Data analysis and interpretation: Koen De Geest, John A. Blessing
Manuscript writing: Koen De Geest, John A. Blessing, Robert T. Morris, Daniela Matei
Final approval of manuscript: Koen De Geest, John A. Blessing, Robert T. Morris, S. Diane Yamada, Bradley J. Monk, Susan L. Zweizig, Daniela Matei, Carolyn Y. Muller, William E. Richards
REFERENCES
- 1.Rose PG, Blessing JA, Ball HG, et al. A phase II study of docetaxel in paclitaxel-resistant ovarian and peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:130–135. doi: 10.1016/s0090-8258(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 2.Markman M, Blessing J, Rubin SC, et al. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: A Gynecologic Oncology Group study. Gynecol Oncol. 2006;101:436–440. doi: 10.1016/j.ygyno.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 3.He L, Orr GA, Horwitz SB. Novel molecules that interact with microtubules and have functional activity similar to Taxol. Drug Discov Today. 2001;6:1153–1164. doi: 10.1016/s1359-6446(01)02038-4. [DOI] [PubMed] [Google Scholar]
- 4.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: A novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 5.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol) J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 6.Jordan MA, Miller H, Ni L, et al. The Pat-21 breast cancer model derived from a patient with primary Taxol resistance recapitulates the phenotype of its origin, has altered beta-tubulin expression and is sensitive to ixabepilone. Proc Am Assoc Cancer Res. 2006;47:73. abstr LB-280. [Google Scholar]
- 7.Mozzetti S, Ferlini C, Concolino P, et al. Class III β-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 8.Goodin S, Kane MP, Rubin EH. Epothilones: Mechanism of action and biologic activity. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Aghajanian C, Burris HA, 3rd, Jones S, et al. Phase I study of the novel epothilone analog ixabepilone (BMS-247550) in patients with advanced solid tumors and lymphomas. J Clin Oncol. 2007;25:1082–1088. doi: 10.1200/JCO.2006.08.7304. [DOI] [PubMed] [Google Scholar]
- 10.Mani S, McDaid H, Hamilton A, et al. Phase I clinical and pharmacokinetic study of BMS-247550, a novel derivative of epothilone B, in solid tumors. Clin Cancer Res. 2004;10:1289–1298. doi: 10.1158/1078-0432.ccr-0919-03. [DOI] [PubMed] [Google Scholar]
- 11.Awada A, Piccart MJ, Jones SF, et al. Phase I dose escalation study of weekly ixabepilone, an epothilone analog, in patients with advanced solid tumors who have failed standard therapy. Cancer Chemother Pharmacol. 2009;63:417–425. doi: 10.1007/s00280-008-0751-5. [DOI] [PubMed] [Google Scholar]
- 12.Hao D, Hammond LA, deBono JS, et al. Continuous weekly administration of the epothilone-B derivative, BMS-247550 (NSC710428): A phase I and pharmacokinetic (PK) study. Proc Am Soc Clin Oncol. 2002;21:103a. abstr 411. [Google Scholar]
- 13.Goel S, Cohen M, Comezoglu SN, et al. The effect of ketoconazole on the pharmacokinetics and pharmacodynamics of ixabepilone: A first in class epothilone B analogue in late-phase clinical development. Clin Cancer Res. 2008;14:2701–2709. doi: 10.1158/1078-0432.CCR-07-4151. [DOI] [PubMed] [Google Scholar]
- 14.Chen TT, Ng T. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–2312. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Molina A, Moore S, et al. Epothilone B analog (BMS-247550) at the recommended phase II dose in patients with gynecologic and breast cancers. J Clin Oncol. 2004;22(suppl):155s. abstr 2115. [Google Scholar]
- 16.Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25:3399–3406. doi: 10.1200/JCO.2006.08.9102. [DOI] [PubMed] [Google Scholar]
- 17.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 18.Vahdat LT, Thomas E, Li R, et al. Phase III trial of ixabepilone plus capecitabine compared to capecitabine alone in patients with metastatic breast cancer (MBC) previously treated or resistant to an anthracycline and resistant to taxanes. J Clin Oncol. 2007;25(suppl):33s. abstr 1006. [Google Scholar]
- 19.Vansteenkiste JF, Lara PN, Jr, Le Chevalier T, et al. Phase II clinical trial of the epothilone B analog, ixabepilone, in patients with non–small-cell lung cancer whose tumors have failed first-line platinum-based chemotherapy. J Clin Oncol. 2007;25:3448–3455. doi: 10.1200/JCO.2006.09.7097. [DOI] [PubMed] [Google Scholar]
- 20.Roché H, Yelle L, Cognetti F, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol. 2007;25:3415–3420. doi: 10.1200/JCO.2006.09.7535. [DOI] [PubMed] [Google Scholar]
- 21.Denduluri N, Low JA, Lee JJ, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol. 2007;25:3421–3427. doi: 10.1200/JCO.2006.10.0784. [DOI] [PubMed] [Google Scholar]
- 22.Goel S, Goldberg GL, Kuo DY, et al. Novel neurosensory testing in cancer patients treated with the epothilone B analog, ixabepilone. Ann Oncol. 2008;19:2048–2052. doi: 10.1093/annonc/mdn420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrieman H, Lind M, Cawkwell L. Do beta-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004;5:158–164. doi: 10.1016/S1470-2045(04)01411-1. [DOI] [PubMed] [Google Scholar]
- 24.Ferrandina G, Zannoni GF, Martinelli E, et al. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res. 2006;12:2774–2779. doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 25.Sudo T, Nitta M, Saya H, et al. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–2508. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- 26.Swanton C, Tomlinson I, Downward J. Chromosomal instability, colorectal cancer and taxane resistance. Cell Cycle. 2006;5:818–823. doi: 10.4161/cc.5.8.2682. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang SH, Hung YE, Hung L, et al. Evidence for microtubule target engagement in tumors of patients receiving ixabepilone. Clin Cancer Res. 2007;13:7480–7486. doi: 10.1158/1078-0432.CCR-06-2883. [DOI] [PubMed] [Google Scholar]
- 28.Baselga J, Zambetti M, Llombart-Cussac A, et al. Phase II genomics study of ixabepilone as neoadjuvant treatment for breast cancer. J Clin Oncol. 2009;27:526–534. doi: 10.1200/JCO.2007.14.2646. [DOI] [PubMed] [Google Scholar]
- 29.Sissung TM, Mross K, Steinberg SM, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42:2893–2896. doi: 10.1016/j.ejca.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson RO. New Tubulin targeting agents currently in clinical development. Expert Opin Investig Drugs. 2008;17:707–722. doi: 10.1517/13543784.17.5.707. [DOI] [PubMed] [Google Scholar]
- 31.Harrison M, Swanton C. Epothilones and new analogues of the microtubule modulators in taxane-resistant disease. Expert Opin Investig Drugs. 2008;17:523–546. doi: 10.1517/13543784.17.4.523. [DOI] [PubMed] [Google Scholar]
- 32.Smit WM, Sufliarsky J, Spanik S, et al. Phase I/II dose-escalation trial of patupilone every 3 weeks in patients with resistant/refractory ovarian cancer. Eur J Cancer. 2005;3:261. abstr 909. [Google Scholar]