Abstract
Purpose
Cyclophosphamide, epirubicin, and fluorouracil (CEF) and doxorubicin and cyclophosphamide (AC) followed by paclitaxel (T) are commonly used adjuvant regimens in women with early breast cancer. In a previous trial in women with locally advanced breast cancer, 3 months of high-dose epirubicin and cyclophosphamide (EC) administered every 2 weeks (dose-dense) was equivalent to 6 months of CEF. We hypothesized that 3 months of paclitaxel after dose-dense EC (EC/T) would be superior to CEF or AC/T.
Methods
After lumpectomy or mastectomy, women 60 years of age or younger with axillary node-positive or high-risk node-negative breast cancer were randomly assigned to receive CEF, EC/T, or AC/T for 6 months. This article reports the interim analysis for recurrence-free survival (RFS), which was planned after 227 recurrences.
Results
A total of 2,104 patients were enrolled. The median follow-up is 30.4 months. Hazard ratios for recurrence are as follows: AC/T versus CEF, 1.49 (95% CI, 1.12 to 1.99), P = .005; AC/T versus EC/T, 1.68 (95% CI, 1.25 to 2.27), P = .0006; and EC/T versus CEF, 0.89 (95% CI, 0.64 to 1.22), P = .46. Three-year RFS rates for CEF, EC/T, and AC/T are 90.1%, 89.5%, and 85.0%, respectively. There was more febrile neutropenia with CEF (22.3%) and EC/T (16.4%) compared with AC/T (4.8%), but more neuropathy with the last two regimens.
Conclusion
Three-weekly AC/T is significantly inferior to CEF or EC/T in terms of RFS. It is too early to detect any difference between CEF and dose-dense EC/T.
INTRODUCTION
In the last three decades, incremental benefits in breast cancer disease-free survival (DFS) and overall survival (OS) have occurred with the introduction of anthracycline and taxane adjuvant chemotherapy regimens and with the exploration of higher doses (dose intense) and more frequent administration of these drugs (dose dense).1–3 In the National Cancer Institute of Canada (NCIC) Clinical Trials Group (CTG) MA5 trial, 6 months of cyclophosphamide, epirubicin, and fluorouracil (CEF) improved 5-year DFS and OS compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil (CMF).4 Subsequently, CEF was compared with a 12-week regimen of epirubicin and cyclophosphamide (EC) administered with higher doses and more frequent dosing in locally advanced breast cancer.5 No difference was detected between regimens in progression-free survival at 34 months. Meanwhile, the results of the Cancer and Leukemia Group B (CALGB) 9344 trial showed an improvement in 3-year DFS with the addition of four cycles of 3-weekly paclitaxel (T), after a 12-week regimen of doxorubicin and cyclophosphamide (AC),6 which had been shown to be equivalent to CMF.7
Our intention in the MA21 trial was to further improve results associated with the CEF regimen by incorporating a taxane. Because 12 weeks of dose-dense EC was equivalent to 6 months of CEF,5 we hypothesized that the addition of four cycles of 3-weekly paclitaxel after EC (EC/T) would improve outcome, without increasing the duration of treatment. We also postulated that EC/T would improve outcome over AC/T. The strategy was to compare the experimental arm (EC/T) with two existing standard regimens (CEF and AC/T) and to compare the two standard regimens that were the same duration but differed in anthracycline and presence of a taxane.
METHODS
Patient Population
Women were eligible if they were premenopausal or postmenopausal, 60 years of age or younger, had axillary node-positive or high-risk node-negative breast cancer, and had undergone complete resection of all known disease by total or partial mastectomy, including axillary node clearance. Node-negative patients were eligible if the tumor was ≥ 1 cm and one or more of the following were present: histologic grade 3, estrogen receptor (ER) negativity, or lymphovascular invasion. Sentinel node biopsy was allowed; when positive, patients underwent axillary dissection. Microscopic residual invasive disease present at partial mastectomy margins led to a recommendation for further excision. However, patients were still eligible without further excision if administered breast irradiation with a boost to the tumor bed.
Patients were excluded if they had evidence of metastasis, documented history of cardiac disease or previous cancer (except treated basal cell and squamous cell carcinoma of the skin or any other cancer except invasive breast cancer treated > 5 years before study entry, and presumed cured), inadequate renal function (serum creatinine level >1.5× the upper limit of normal) or elevated bilirubin (> 1.5× the upper limit of normal), a serious underlying medical or psychiatric illness, inflammatory or locally advanced breast cancer before surgery, microscopic evidence of residual tumor at the resection margins of the total mastectomy, gross tumor remaining in the axilla postsurgery, previous radiation therapy or chemotherapy for breast cancer, or were more than 12 weeks from definitive surgery for breast cancer.
Potentially eligible patients underwent bone scan (if indicated by abnormal alkaline phosphatase), chest radiograph, abdominal ultrasound or computed tomography (if indicated by abnormal liver function tests), and radionuclide cardiac scan before being randomly assigned to treatment. Informed consent was obtained from eligible patients before assignment to treatment. The study protocol was approved by the institutional review board of each participating center.
Treatment Regimens
Patients were assigned using a minimization procedure to one of three regimens by the NCIC CTG central office. Stratification was by number of positive nodes (0, one to three, four to 10, and > 10), type of surgery (total v partial mastectomy), and ER status (ER positive v ER negative). Chemotherapy regimens are shown in Table 1. The results of the CALGB 9741 trial were presented in December 2002.8 At a median follow-up of 36 months, AC/T administered every 2 weeks with filgrastim was associated with improved DFS and OS compared with administration every 3 weeks. The MA21 Steering Committee decided to continue with the AC/T arm as designed because it was felt that AC/T every 3 weeks would still continue to be a widely used standard.
Table 1.
Treatment Regimens
| CEF | EC/T | AC/T |
|---|---|---|
| Cyclophosphamide 75 mg/m2 orally, days 1-14 | Epirubicin 120 mg/m2 IV, day 1 | Doxorubicin 60 mg/m2 IV, day 1 |
| Epirubicin 60 mg/m2 IV, days 1 and 8 | Cyclophosphamide 830 mg/m2 IV, day 1 | Cyclophosphamide 600 mg/m2 IV, day 1 |
| Fluorouracil 500 mg/m2 IV, days 1 and 8 | EC administered every 14 days for 6 cycles | AC administered every 21 days for 4 cycles |
| Cotrimoxazole 2 tablets orally bid or ciprofloxacin 500 mg orally bid for duration of chemotherapy | Paclitaxel 175 mg/m2 IV, every 21 days for 4 cycles | Paclitaxel 175 mg/m2 IV, every 21 days for 4 cycles |
| Duration = six 28-day cycles | Filgrastim 5 μg/kg subcutaneously, days 2-13 | Filgrastim and epoetin permitted |
| Filgrastim and epoetin permitted | Epoetin 40,000 U subcutaneously weekly |
NOTE. A complete blood count with differential and platelet count was performed at the beginning of each cycle of chemotherapy. Dose modifications were performed according to predefined guidelines based on hematologic and non-hematologic toxicity.
Abbreviations: CEF, cyclophosphamide, epirubicin, and fluorouracil; EC/T, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; AC/T, doxorubicin and cyclophosphamide followed by paclitaxel; IV, intravenously; bid, twice per day.
All patients who underwent lumpectomy received local radiation to the breast after completion of chemotherapy. Radiation to the chest wall and regional nodes after total mastectomy was permitted and given according to local institutional practice.
The hormone receptor assays were performed locally. A positive test was defined by ≥ 10 fmol/mg or by local determination of positive on immunohistochemistry. Patients with ER-positive tumors received tamoxifen after completion of chemotherapy. After October 2004, an aromatase inhibitor was allowed. After June 2005, trastuzumab for 1 year was allowed for patients with human epidermal growth factor receptor (HER2) –positive disease.
Toxicity evaluations by National Cancer Institute (NCI) Common Toxicity Criteria Version 2.0 were performed on day 1 of each cycle of chemotherapy. Quality of life (QOL) was assessed by the Breast Cancer Chemotherapy Questionnaire and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30.9,10 These assessments were performed within 7 days before random assignment and afterwards. The QOL results will be the subject of another article.
Follow-Up Studies
After completion of chemotherapy, patients were seen every 3 months until the end of the first year, every 4 months in Year 2, then every 6 months until the end of 5 years and yearly thereafter. Patients underwent a history and physical examination at each follow-up, along with CBC count, platelet count, and liver function tests.
A mammogram was performed yearly and a radionuclide cardiac scan at 12, 24, 36, 48, and 60 months after random assignment.
Outcome Measures
The primary end point of this study was relapse-free survival (RFS), which was defined as the time from random assignment to the time of recurrence of the primary breast cancer. Local breast recurrence (including ductal carcinoma in situ), nodal recurrence, and metastatic disease were considered a recurrence. Patients who had contralateral breast cancer, a second malignancy, or non–disease-related death were censored. Secondary outcome measures consisted of OS, toxicity as assessed by the NCI Common Toxicity Criteria and QOL. The interim analysis for OS was scheduled to take place after the final RFS analysis, so will be reported after 453 deaths.
Statistical Design
Originally, the trial was to have enrolled approximately 500 patients per group to detect a hazard ratio (HR) for RFS between CEF or AC/T, and EC/T of 1.43, which corresponded to a 10% improvement in 5-year RFS from 60% to 70%. To have 80% power to detect this HR using a two-sided 5% α level test, 453 recurrences would be needed. It was postulated that 1,500 patients would be entered over 3 years, with further follow-up of at least 2.45 years before the final analysis.
As a result of the CALGB 9344 trial results, the MA21 sample size was re-examined.11 The HR of 1.43 was maintained between CEF or AC/T, and EC/T, and hence the requirement for 453 events remained. We considered reasonable RFS rates to then be 70% for AC/T and CEF and 78% for EC/T. Thus 2,100 patients were to be recruited over approximately 4.2 years, along with 2.7 years of additional follow-up.
Statistical Analysis
One interim analysis was planned after observation of about half the events to allow early disclosure of the study outcomes if the results were extreme. By intention-to-treat, all patients accrued to the trial were to be included in the primary analyses; randomization values of the stratification factors were used for the primary analyses.
The comparison of the three treatment groups was based on the least significant difference approach,12 in which the sample size is determined by the maximum HR for any pairwise comparison of the trial arms. The method accounts for multiple comparisons of global and pairwise testing.
A two-sided stratified log-rank test, adjusting for the stratification factors, was the primary method to compare RFS between the three groups using the least significant difference approach. This involved first performing a global stratified test to see whether there were differences between the three treatment groups. If the global test was significant at the nominal level, .005 for the interim analysis (.048 in the final analysis), then three stratified pairwise comparisons were to be performed at the same nominal level. The nominal level was based on O'Brien-Fleming type boundaries as proposed by Lan and DeMets,13 such that the overall significance level at final analysis was maintained at 5%. The RFS experiences of the three treatment groups are reported here with Cox survivor plots, adjusted by the stratification factors.
As an exploratory analysis, a Cox proportional hazards model was used in a stepwise model to assess and adjust for factors significantly related to RFS. Factors examined in the stepwise adjusted Cox modeling were age at allocation (missing/< 40/40 to 49 v 50 to 60 years), race (white v other), performance status (missing/unkown/0 v 1/2/3), T status (missing/TX/T1 v T2/T3/T4), N status (missing/N0 v N1/N2), menopausal status (missing/postmenopausal v premenopausal), and HER2/neu status (missing/unknown/0/1/2+ or negative v 3+ or positive).
All patients who received at least one dose of study treatment for whom we had on-treatment data at the time of analysis were included in the safety analysis.
RESULTS
Patient Population
A total of 2,104 patients were recruited from Canadian and US centers, between December 2000 and May 2005. The prespecified interim analysis was performed on a database that was locked October 18, 2006 when there were 261 events. Median follow-up was 30.4 months.
A total of 701 women were allocated to CEF, 701 women were assigned to EC/T, and 702 women were assigned to AC/T. See Figure 1 for CONSORT description. Of the 2,104 patients who were randomly assigned, 21 patients never received their protocol-specified treatment. All 2,104 patients randomly assigned to the study are included in the efficacy analysis and in Table 2 describing the baseline characteristics. The number of patients in each arm who switched from tamoxifen to an aromatase inhibitor before recurrence were 74 (CEF), 81 (EC/T), and 84 (AC/T). A small number of women received trastuzumab: 29 (CEF), 27 (EC/T), and 26 (AC/T). Baseline characteristics were similar among treatments (Table 2).
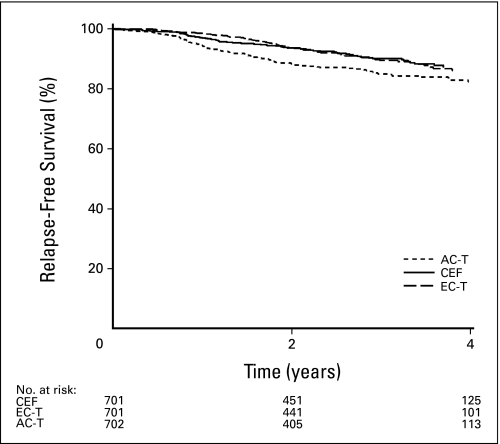
Fig 1.
Three-year adjusted relapse-free survival. CEF, cyclophosphamide, epirubicin, and fluorouracil; AC-T, doxorubicin and cyclophosphamide followed by paclitaxel; EC-T, dose-dense epirubicin and cyclophosphamide followed by paclitaxel.
Table 2.
Baseline Characteristics
| Factor | CEF (n = 701) |
EC/T (n = 701) |
AC/T (n = 702) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| ≤ 39 | 97 | 13.8 | 130 | 18.5 | 97 | 13.8 |
| 40-49 | 322 | 45.9 | 297 | 42.4 | 341 | 48.6 |
| 50+ | 282 | 40.2 | 274 | 39.1 | 264 | 37.6 |
| Surgery | ||||||
| Lumpectomy | 355 | 50.6 | 350 | 49.9 | 354 | 50.4 |
| Mastectomy | 346 | 49.4 | 351 | 50.1 | 348 | 49.6 |
| Nodes positive | ||||||
| 0 | 196 | 28.0 | 198 | 28.2 | 195 | 27.8 |
| 1-3 | 303 | 43.2 | 303 | 43.2 | 306 | 43.6 |
| 4-10 | 155 | 22.1 | 156 | 22.3 | 158 | 22.5 |
| > 10 | 47 | 6.7 | 44 | 6.3 | 43 | 6.1 |
| Tumor stage | ||||||
| T1 | 240 | 34.2 | 240 | 34.2 | 255 | 36.3 |
| T2 | 391 | 55.8 | 377 | 53.8 | 383 | 54.6 |
| T3 | 62 | 8.8 | 68 | 9.7 | 57 | 8.1 |
| T4 | 8 | 1.1 | 12 | 1.7 | 7 | 1.0 |
| Tx | 0 | 0.0 | 4 | 0.6 | 0 | 0.0 |
| ER status | ||||||
| Negative | 283 | 40.4 | 288 | 41.1 | 289 | 41.2 |
| Positive | 418 | 59.6 | 413 | 58.9 | 413 | 58.8 |
| HER2/neu* | ||||||
| Negative | 492 | 70.2 | 494 | 70.5 | 481 | 68.5 |
| Positive | 90 | 12.8 | 74 | 10.6 | 78 | 11.1 |
| Unknown | 119 | 17.0 | 133 | 19.0 | 143 | 20.4 |
Abbreviations: CEF, cyclophosphamide, epirubicin, and fluorouracil; EC/T, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; AC/T, doxorubicin and cyclophosphamide followed by paclitaxel; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
HER2 not performed routinely when MA21 started.
Ninety-one percent of CEF patients received six cycles of treatment, 50.6% of whom received 100% of their anthracycline dose. Eighty-three percent of EC/T patients received 10 cycles of chemotherapy, 65.7% and 59.8% of whom received 100% of intended anthracycline and paclitaxel dose, respectively. Finally, 90% of the AC/T arm received eight cycles of chemotherapy, with 100% of anthracycline and paclitaxel dose delivered to 94.2% and 80.2% of patients, respectively.
RFS
The 3-year adjusted RFS rates for CEF, EC/T, and AC/T were 90.1%, 89.5%, and 85% (P = .001; Fig 1). The pairwise comparisons of the three treatment groups are shown in Table 3. HRs for recurrence are as follows: AC/T versus CEF, 1.49 (95% CI, 1.12 to 1.99), P = .005; AC/T versus EC/T, 1.68 (95% CI, 1.25 to 2.27), P = .0006; and EC/T versus CEF, 0.89 (95% CI, 0.64 to 1.22), P = .46. The comparison of the treatment groups by nodal status and in ER-positive and -negative patients is shown in Table 3. In the Cox model, the only significant factor included in addition to treatment was age. Older women had better RFS than younger: HR = 0.7 (95% CI, 0.54 to 0.91), P = .01.
Table 3.
3-Year Relapse-Free Survival
| Group | Treatment (%) |
||
|---|---|---|---|
| CEF (n = 701) | EC/T (n = 701) | AC/T (n = 702) | |
| All patients | 90.1 | 89.5 | 85.0 |
| Node status, No. of positive nodes | |||
| 0 | 92.3 | 91.5 | 87.8 |
| 1-3 | 92.2 | 90.6 | 86.1 |
| 4+ | 76.2 | 80.8 | 70.3 |
| ER status* | |||
| Positive | 91.5 | 90.4 | 88.0 |
| Negative | 82.0 | 84.7 | 73.2 |
Abbreviations: CEF, cyclophosphamide, epirubicin, and fluorouracil; EC/T, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; AC/T, doxorubicin and cyclophosphamide followed by paclitaxel; ER, estrogen receptor.
There was no significant interaction between ER status and treatment (P = .23).
Survival
Fifty patients on the CEF arm have died, compared with 47 patients on the EC/T arm and 65 patients on the AC/T arm.
Toxicity
The worst toxicity levels experienced by each patient according to the NCI Common Toxicity Criteria are listed in Table 4. Forty-one patients from one center with unverifiable toxicity reports on audit were excluded from Table 4; results were similar with inclusion of these patients. One patient who received study therapy did not have toxicity reports available at the time of analysis. The rates of febrile neutropenia were 22.3% and 16.4% in the CEF and EC/T patients compared with 4.8% in AC/T (P < .001). None of the febrile neutropenic events were fatal. The rates of erythrocyte transfusion in the three treatment arms were 23.8%, 39.9%, and 1.6%, respectively (P < .001). There was more grade 3 and 4 cardiac toxicity after completion of the chemotherapy in the CEF patients compared with the other groups: 2.1%, 0.7%, and 0.3%, respectively (P < .001). Four patients (0.57%) in the CEF group and four patients (0.57%) in the EC/T group experienced acute leukemia compared with none in the AC/T group. There was more peripheral neuropathy in the EC/T and AC/T patients.
Table 4.
Incidence of Worst Ever Toxicity
| Toxicity and Grade | CEF |
EC/T |
AC/T |
P* | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Patients with toxicity | 680 | 100 | 688 | 100 | 674 | 100 | |
| Nausea, grade 3/4 | 34 | 5.0 | 98 | 14.2 | 37 | 5.5 | < .001 |
| Vomiting, grade 3/4 | 38 | 5.6 | 103 | 15.0 | 42 | 6.2 | < .001 |
| Diarrhea, grade 3/4 | 18 | 2.7 | 25 | 3.6 | 8 | 1.2 | < .001 |
| Stomatitis, grade 3/4 | 61 | 9.0 | 68 | 9.9 | 5 | 0.7 | < .001 |
| Granulocytes, grade 3/4† | 412 | 60.9 | 379 | 55.3 | 287 | 42.6 | < .001 |
| Platelets, grade 3/4† | 96 | 14.1 | 165 | 24.1 | 10 | 1.5 | < .001 |
| Thrombosis, grade 3/4 | 22 | 3.2 | 18 | 2.6 | 3 | 0.5 | < .001 |
| Sensory neuropathy, grade 3/4 | 2 | 0.3 | 41 | 6.0 | 37 | 5.5 | < .001 |
| Motor neuropathy, grade 3/4 | 2 | 0.3 | 10 | 1.5 | 2 | 0.3 | < .001 |
| Febrile neutropenia, grade 3/4 | 153 | 22.5 | 111 | 16.1 | 32 | 4.8 | < .001 |
| Decreased LVEF (acute), grade 3/4 | 3 | 0.4 | 2 | 0.3 | 2 | 0.3 | .02 |
| Decreased LVEF (delayed), grade 3/4 | 14 | 2.1 | 5 | 0.7 | 2 | 0.3 | < .001 |
| Hemoglobin, grade 3/4† | 112 | 16.5 | 199 | 29.0 | 7 | 1.0 | < .001 |
| Acute leukemia | 4 | 0.57 | 4 | 0.57 | 0 | 0.0 | .14 |
Abbreviations: CEF, cyclophosphamide, epirubicin, and fluorouracil; EC/T, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; AC/T, doxorubicin and cyclophosphamide followed by paclitaxel; LVEF, left ventricular ejection fraction.
P value based on Fisher's exact test to compare toxicities between the three arms.
Respectively, N for granulocytes are 677, 686, and 674; for platelets, 680, 686, and 674; for delayed decreased LVEF, 670, 682, and 659; for hemoglobin, 680, 687, and 674.
DISCUSSION
The outcomes of women with early breast cancer have improved incrementally through trials that have evaluated new chemotherapy drugs and different doses and schedules of drugs postoperatively. Although much progress has been made, women still develop recurrences.
The results of our trial showed that AC/T was inferior to both CEF and high-dose EC/T in terms of RFS. There are several possible reasons to explain the superiority of CEF over AC/T. The anthracycline was different in the regimens. On an equimolar basis, epirubicin is less myelosuppressive and less cardiotoxic than doxorubicin, with no loss of antitumor efficacy.14 Thus the total planned cumulative dose and duration of anthracycline in CEF was greater than in AC/T. The two regimens also differed in the total cumulative dose, dose-intensity, and duration of cyclophosphamide. However, previous trials found no advantage with high-dose cyclophosphamide and dose-intensified cyclophosphamide compared with standard-dose cyclophosphamide.15,16
In the EC/T and the AC/T arms, the taxane dose and schedule and the duration of anthracycline were the same. However, the anthracyclines were different, and their total planned cumulative dose in EC/T was greater than in AC/T. Moreover, the EC component of the experimental arm was more dose-dense than the AC component of AC/T. As the planned taxane dose and schedule were the same for the two regimens, this provides further evidence for the benefits of an optimized anthracycline regimen.
Currently, no difference in RFS has been detected between CEF and EC/T; however, there are insufficient events for an appropriate comparison. All patients in the EC/T arm received erythropoietin. Recently, there has been much discussion on the potential for erythropoietin to stimulate tumor growth.17 In our trial, EC/T efficacy at this follow-up was still superior to AC/T.
In our trial, there was more nausea and vomiting with the EC/T regimen compared with the other two arms. The rate of febrile neutropenia was highest in the CEF arm. Cardiac toxicity as reflected by symptomatic congestive heart failure was low, but was highest in the CEF arm. To date, the rate of leukemia/myelodysplastic syndrome is low and in keeping with the results of other adjuvant chemotherapy trials. As expected, there was more neuropathy with the taxane-containing regimens.
This first analysis of the accumulating data was preplanned. The number of events required for the survival analysis has not been reached, so no formal statistical comparison has been performed. However, the observed number of deaths in each arm is consistent with the RFS.
To date MA21 shows that CEF and EC/T are superior to AC/T in terms of RFS (HR = 1.49 and 1.68, respectively). In the CALGB 9741 trial at a median follow-up of 36 months, the RFS of dose-dense AC/T was superior to 3-weekly AC/T (HR = 1.35).5 There are limitations to cross-study comparisons. Nonetheless, using the common comparator of AC/T, the RFS of dose-dense AC/T does not appear superior to that associated with either CEF or EC/T. However, dose-dense AC/T does appear to be less toxic than the two epirubicin regimens in MA21. In the 9741 trial at 69 months median follow-up, the improvement in RFS with dose-dense AC/T compared with 3-weekly AC/T persisted and was statistically significantly, but there was no difference in OS.18 In a recent Spanish trial in women with node-positive disease, four cycles of fluorouracil, epirubicin, and cyclophosphamide (epirubicin 90 mg/m2) every 3 weeks followed by weekly paclitaxel for 8 weeks was superior to six cycles of fluorouracil, epirubicin, and cyclophosphamide (HR for DFS = 1.3).19 The cumulative dose of epirubicin was higher in our trial than in the Spanish trial. In the not too distant future, MA21 will have sufficient events to examine whether adding a taxane to a dose-dense epirubicin-containing regimen improves RFS over CEF.
We have previously reported that expression or amplification of HER2 predicted responsiveness to epirubicin-based adjuvant chemotherapy.20 Recently, in a retrospective analysis of the CALGB 9344 trial, HER2 status predicted benefit from the addition of paclitaxel after AC,21 but this was not the case in the recent Spanish study.19 The results of future correlative studies should help optimize anthracycline and taxane regimens by tailoring them to the individual patient.
Currently a number of different chemotherapy regimens with different drugs and schedules are being studied in trials. Dose-dense AC/T is commonly used in practice in the United States and Canada.8 The results of our trial support the notion that AC/T administered every 3 weeks is less than optimal. However, it could be argued that the question of the scheduling of drugs in the AC/T regimen is still open. In a recently published trial, women with early breast cancers received four cycles of AC every 3 weeks postoperatively and then in a factorial design were allocated to weekly taxane versus 3-weekly taxane and paclitaxel versus docetaxel.22 The arms that received weekly paclitaxel and 3-weekly docetaxel were associated with improved DFS compared with 3-weekly paclitaxel, and the DFS of the weekly docetaxel arm was similar to 3-weekly paclitaxel. The weekly paclitaxel arm was also associated with improved survival compared with 3-weekly paclitaxel.
In conclusion, AC/T administered every 3 weeks is significantly inferior to CEF or EC/T in terms of RFS in patients with high-risk operable breast cancer.
Acknowledgment
We thank the Clinical Trials Group and the Breast Intergroup with endorsements by Cancer and Leukemia Group B, Southwest Oncology Group, and North Central Cancer Treatment Group.
Footnotes
The MA21 study is supported in part by the Canadian Cancer Society, National Institutes of Health, and by the following companies: Pfizer, Bristol-Myers Squibb, Amgen, Janssen Ortho, and Ortho Biotech. The National Cancer Institute of Canada Clinical Trials Group is funded by the Canadian Cancer Society and is based at Queen's University in Kingston, Ontario.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00014222.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Karen Gelmon, Pfizer (C), Amgen (C); Kathleen Pritchard, Pfizer (C), Novartis (C), AstraZeneca (C) Stock Ownership: None Honoraria: Karen Gelmon, Pfizer, Amgen; Kathleen Pritchard, Pfizer, Novartis, AstraZeneca Research Funding: Hope Rugo, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Margot Burnell, Mark N. Levine, Vivien Bramwell, Barbara Walley, Kathy S. Albain, Edith A. Perez, Hope Rugo, Lois E. Shepherd
Administrative support: Patti O'Brien
Provision of study materials or patients: Margot Burnell, Mark N. Levine, Vivien Bramwell, Karen Gelmon, Barbara Walley, Ted Vandenberg, Haji Chalchal, Kathy S. Albain, Edith A. Perez, Hope Rugo, Kathleen Pritchard
Collection and assembly of data: Judith-Anne W. Chapman, Patti O'Brien, Lois E. Shepherd
Data analysis and interpretation: Margot Burnell, Mark N. Levine, Judith-Anne W. Chapman, Karen Gelmon, Kathy S. Albain, Kathleen Pritchard, Lois E. Shepherd
Manuscript writing: Margot Burnell, Mark N. Levine, Judith-Anne W. Chapman, Vivien Bramwell, Karen Gelmon, Kathy S. Albain, Edith A. Perez, Kathleen Pritchard, Lois E. Shepherd
Final approval of manuscript: Margot Burnell, Mark N. Levine, Judith-Anne W. Chapman, Vivien Bramwell, Karen Gelmon, Barbara Walley, Ted Vandenberg, Kathy S. Albain, Edith A. Perez, Hope Rugo, Lois E. Shepherd
REFERENCES
- 1.Levine MN, Whelan T. Adjuvant chemotherapy for breast cancer: 30 years later. N Engl J Med. 2006;355:1920–1922. doi: 10.1056/NEJMe068204. [DOI] [PubMed] [Google Scholar]
- 2.Levine MN, Eisen A. Anthracycline adjuvant chemotherapy: How much is enough? J Clin Oncol. 2001;19:599–601. doi: 10.1200/JCO.2001.19.3.599. [DOI] [PubMed] [Google Scholar]
- 3.Dang C, Hudis C. Adjuvant taxanes in the treatment of breast cancer: No longer at the tip of the iceberg. Clin Breast Cancer. 2006;7:51–58. doi: 10.3816/CBC.2006.n.013. [DOI] [PubMed] [Google Scholar]
- 4.Levine MN, Bramwell VH, Pritchard KI, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16:2651–2658. doi: 10.1200/JCO.1998.16.8.2651. [DOI] [PubMed] [Google Scholar]
- 5.Therasse P, Mauriac L, Welnicka-Jaskiewicz M, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: An EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 2003;21:843–850. doi: 10.1200/JCO.2003.05.135. [DOI] [PubMed] [Google Scholar]
- 6.Henderson IC, Berry D, Demetri G, et al. Improved disease-free (DFS) and overall survival (OS) from the addition of sequential paclitaxel (T) but not from the escalation of doxorubicin (A) dose level in the adjuvant chemotherapy of patients (PTS) with node-positive primary breast cancer (BC) Proc Am Soc Clin Oncol. 1998;17:101a. abstr 390. [Google Scholar]
- 7.Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: Results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 8.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 9.Levine MN, Guyatt GH, Gent M, et al. Quality of life in stage II breast cancer: An instrument for clinical trials. J Clin Oncol. 1988;6:1798–1810. doi: 10.1200/JCO.1988.6.12.1798. [DOI] [PubMed] [Google Scholar]
- 10.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 11.Henderson IC, Berry DA, Demetri G, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 12.Makuch RW, Simon RM. Sample size requirements for comparing time-to-failure among k treatment groups. J Chron Dis. 1982;35:861–867. doi: 10.1016/0021-9681(82)90051-0. [DOI] [PubMed] [Google Scholar]
- 13.Lan G, DeMets D. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 14.Coukell AJ, Faulds D. Epirubicin: An updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of breast cancer. Drugs. 1997;53:453–482. doi: 10.2165/00003495-199753030-00008. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol. 1997;15:1858–1869. doi: 10.1200/JCO.1997.15.5.1858. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Anderson S, DeCillis A, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol. 1999;17:3374–3388. doi: 10.1200/JCO.1999.17.11.3374. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 18.Hudis C, Citron M, Berry D, et al. Five year follow-up of INT C9741: Dose-dense (DD) chemotherapy (CRx) is safe and effective. Presented at the 28th Annual San Antonio Breast Cancer Symposium; December 8–11, 2005; San Antonio, TX. abstr 41. [Google Scholar]
- 19.Martín M, Rodriguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard KI, Shepherd LE, O'Malley FP, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 21.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 22.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]