Abstract
Purpose
To determine the potential efficacy of combining cetuximab with chemotherapy in patients with advanced nodal disease, we conducted a phase II trial with induction chemotherapy (ICT) consisting of six weekly cycles of paclitaxel 135 mg/m2 and carboplatin (area under the curve = 2) with cetuximab 400 mg/m2 in week 1 and then 250 mg/m2 (PCC).
Patients and Methods
Forty-seven previously untreated patients (41 with oropharynx primaries; 33 men, 14 women; median age, 53 years; performance status of 0 or 1) with squamous cell carcinoma of the head and neck (SCCHN; T1-4, N2b/c/3) were treated and evaluated for clinical and radiographic response. After ICT, patients underwent risk-based local therapy, which consisted of either radiation, concomitant chemoradiotherapy, or surgery, based on tumor stage and site at diagnosis.
Results
After induction PCC, nine patients (19%) achieved a complete response, and 36 patients (77%) achieved a partial response. The most common grade 3 or 4 toxicity was skin rash (45%), followed by neutropenia (21%) without fever. At a median follow-up time of 33 months, locoregional or systemic disease progression was observed in six patients. The 3-year progression-free survival (PFS) and overall survival (OS) rates were 87% (95% CI, 78% to 97%) and 91% (95% CI, 84% to 99%), respectively. Human papillomavirus (HPV) 16, found in 12 (46%) of 26 biopsies, was associated with improved PFS (P = .012) and OS (P = .046).
Conclusion
ICT with weekly PCC followed by risk-based local therapy seems to be feasible, effective, and well tolerated. PFS is promising, and this sequential treatment strategy should be further investigated. Patients with HPV-positive tumors have an excellent prognosis.
INTRODUCTION
A majority of patients with squamous cell carcinoma of the head and neck (SCCHN) present with stage III or IV M0 disease, and a main therapeutic aim is locoregional disease control. However, risk of distant metastases tends to correlate with site and the extent of nodal involvement at presentation. As highly effective concomitant chemoradiotherapy programs improve local control, there may be a relative increase to as high as 30% to 40% in the risk of distant disease recurrence, especially among patients with N2/3 staging.1–4
Induction chemotherapy (ICT) as a component of primary treatment has been shown in several studies and meta-analyses5,6 to decrease the emergence of metastatic disease. Moreover, the addition of a taxane to platinum and fluorouracil ICT has recently been reported to be superior to platinum and fluorouracil in randomized phase III studies, with increased tumor responses7 and overall survival (OS)8,9 observed for the three-drug schedule. The efficacy of ICT, followed by radiotherapy with concomitant chemotherapy, is currently under study in prospective randomized trials.10
Prior study of paclitaxel and carboplatin administered in a 6-week course before chemoradiotherapy demonstrated that this regimen is feasible and resulted in a high complete response rate (CR) of 35% and overall response rate of 87% before subsequent chemoradiotherapy, with an OS rate of 70% at 3 years.11 The addition of epidermal growth factor receptor (EGFR) –targeted therapy with cetuximab seems to augment local tumor control and OS in patients treated with radiotherapy12 and OS in patients with recurrent or metastatic disease receiving platinum-fluorouracil chemotherapy.13
We hypothesized that the addition of cetuximab to the weekly paclitaxel-carboplatin ICT regimen (PCC) would be an effective and well-tolerated regimen for the treatment of previously untreated patients with multiple cervical nodal metastases at risk for distant metastases. The study was also designed for risk-based variation with respect to the intensity of definitive locoregional treatment in an attempt to achieve local and regional tumor control with acceptable long-term adverse effects.
PATIENTS AND METHODS
Eligibility Criteria and Baseline Staging
From February 2005 to December 2005, previously untreated patients with histologically proven, stage IVA or IVB SCCHN and nodal staging of N2b/c or N3 (oral cavity, oropharynx, larynx, hypopharynx, and nasopharynx) were entered. Patients have been observed through August 2008. Normal hematopoietic, hepatic, and renal functions were required. Patients rendered disease free by initial surgical resection were not eligible. Staging procedures consisted of physical examination, panendoscopy, and head and neck computed tomography scan. The protocol and the informed consent were approved by The University of Texas M. D. Anderson Cancer Center Institutional Review Board.
Treatment
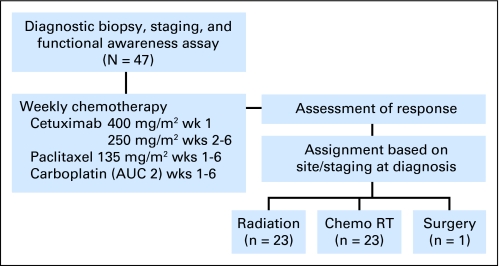
On the basis of the presentation at diagnosis, the protocol guideline was for patients to receive PCC and then proceed to definitive local therapy with radiation as a single modality if T1-2, concomitant chemotherapy and radiation if T3-4, or surgery if an oral cavity was the primary site (Fig 1). The definitive treatment assignment was not to be determined by the response to ICT, but flexibility was permitted, dependent on chemotherapy-associated toxicity and physician judgment. For example, if a patient presented with a large T2 base of tongue primary tumor with an endophytic growth pattern, the attending physician had an option to recommend concomitant chemoradiotherapy.
Fig 1.
CONSORT diagram. AUC, area under the curve; chemo RT, chemoradiotherapy.
ICT.
A loading dose of cetuximab 400 mg/m2 intravenously and then weekly infusions of cetuximab 250 mg/m2 and paclitaxel 135 mg/m2 followed by carboplatin area under the curve (AUC) 2 were administered for 6 consecutive weeks, with usual premedications. Treatment cycles were repeated if the absolute neutrophil count (ANC) was ≥ 1,500 cells/mL and the platelet count was ≥ 100,000 cells/mL. Midweek use of filgrastim (5 μg/kg subcutaneously on days 3 and 4) was permissible. If febrile neutropenia occurred or if blood counts had not recovered to ANC more than 1,500/mL and platelets more than 100,000/mL after a 2-week delay, chemotherapy was discontinued. The paclitaxel dose was decreased to 90 mg/m2 for grade 2 peripheral neuropathy.
Dose modifications of cetuximab infusions to levels −1 and −2 (200 and 150 mg/m2, respectively) were instituted in case of severe (grade 3) rash. If there was no recovery over 2 weeks, the drug was discontinued. Cetuximab was administered even during chemotherapy delays but was discontinued at the same time as chemotherapy.
Radiotherapy.
Radiotherapy was started 2 to 3 weeks after ICT. Target volumes were based on tumor site and extent at diagnosis. Treatment and target volume definitions have been described in detail elsewhere.14 Patients could be treated with either three-dimensional conformal radiation or intensity-modulated radiation therapy. Gross disease and margin were administered a dose of 66 Gy in 30 fractions for T1 disease and 72 Gy in 40 to 42 fractions with a concomitant boost fractionation schedule15 for patients with T2-4 tumors. All radiation schedules were planned for 6 weeks of therapy.
Chemotherapy administered with radiation.
Protocol guidelines were for cisplatin (100 mg/m2) to be administered intravenously on days 1 and 22, with appropriate antiemetics and intravenous hydration. Treatment was held for ANC less than 1,000/mL or platelet count less than 75,000/mL until recovery to more than 1,000/mL and more than 75,000/mL, respectively, and then full doses were administered. Cisplatin was reduced to 60 mg/m2 for grade 2 neurotoxicity, discontinued for grade 3 neurotoxicity, and reduced for renal insufficiency. Weekly cisplatin 30 mg/m2 was considered a suitable option, at the discretion of the attending physicians. For patients unable to tolerate cisplatin or with grade 2 peripheral neuropathy after PCC, weekly carboplatin (AUC 2) was administered.
Surgery.
Surgery was recommended for residual disease at the primary site or neck after completion of chemoradiotherapy.
Toxicity
Adverse events were coded according to Common Terminology Criteria of Adverse Events version 3. Infusion reactions were graded according to allergic reaction/hypersensitivity.
Correlative Studies
In consenting patients, optional tumor biopsy specimens for genomic profiling and other correlative studies were obtained at baseline. Blood samples for serum proteomics (10 mL each) were drawn at baseline, at week 1, during the postinduction response assessment period, and 3 months after the completion of all therapy, including local therapy such as radiation or surgery. Additional correlative studies are described in a separate report.
Functional Assessment
Functional outcomes were prospectively assessed at baseline and at 6, 12, and 24 months, including a modified barium swallow (MBS) study conducted in standard format as previously described.16 Aspiration was rated according to the Penetration-Aspiration Scale17 on 10-mL trials of thin liquid barium (Penetration-Aspiration Scale score ≥ 6 was coded as aspiration). The Performance Status Scale for Head and Neck Cancer Patients18 was administered at each MBS test interval and was mailed to patients who missed their MBS study.
Human Papillomavirus and EGFR Testing
Tumor specimens from 26 of 47 patients were available for HPV-16 DNA detection. Formalin-fixed, paraffin-embedded tissue sections were evaluated by in situ hybridization for human papillomavirus (HPV) nucleic acids using the automated BenchMark (Ventana, Tucson, AZ), per manufacturer recommended protocol. The Ventana INFORM HPV III Family 16 Probe containing labeled HPV genomic probes for high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56 58, and 66) was used. Tumor cells were evaluated for positive nuclear expression with concurrent positive and negative controls reviewed. Immunostaining for EGFR expression was performed and evaluated semiquantitatively based on the intensity and localization of stains in tumor cells.
Treatment Evaluation and Statistical Analysis
An intent-to-treat analysis was performed. Primary objectives were to increase the overall clinical/radiographic CR rate after ICT from 30% (expected with paclitaxel/carboplatin) to 50% with PCC and to determine toxicity. A clinical and radiographic response evaluation was performed 1 week after ICT and 6 weeks after local therapy. Response Evaluation Criteria in Solid Tumors (RECIST) were used as previously described.19 The response to both ICT and local therapy was defined as the lesser of clinical and radiographic response. A Bayesian predictive probability design was implemented. To test for a 20% improvement in the CR rate, with a 10% type I error rate and 90% power, a maximum sample size of 46 patients is required.
PFS was measured from the first day of therapy until first disease progression. OS was measured from the date of study entry until date of last follow-up or death.
Descriptive statistics including mean, standard deviation, median, range, and percentage were used to describe patient demographic, pathologic, and clinical characteristics. The χ2 test or Fisher's exact test was used to test differences in categoric variables, and the Wilcoxon rank sum test or Kruskal-Wallis test was used to detect differences for continuous variables. Exact binomial test was used to test the significance of observed CR rate. The distributions of PFS and OS were estimated using the Kaplan-Meier method. The log-rank test was performed to test differences in survival between patients with different characteristics.
RESULTS
Patients
Forty-seven patients were enrolled. Table 1 lists clinicopathologic characteristics of the study population. Median age was 53 years (range, 21 to 78 years). A large majority of tumors originated from the oropharynx (87%). Eighteen patients (38%) were never smokers, 16 patients (34%) were former smokers, and 13 patients (28%) were considered current smokers. Median follow-up time was 33 months (range, 25 to 40 months).
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Sex | ||
| Male | 33 | 70 |
| Female | 14 | 30 |
| Race | ||
| White | 42 | 89 |
| Black | 2 | 4 |
| Hispanic | 2 | 4 |
| Asian | 1 | 2 |
| Staging | ||
| T1N2b | 9 | |
| T1N2c | 6 | |
| T1N3 | 1 | |
| T2N2b | 11 | |
| T2N2c | 1 | |
| T2N3 | 2 | |
| T3Nx | 1 | |
| T3N2b | 8 | |
| T3N2c | 3 | |
| T4N2b | 2 | |
| T4N2c | 3 | |
| Differentiation | ||
| Poorly | 18 | 38 |
| Moderately | 14 | 30 |
| Not defined (FNA) | 9 | 19 |
| Poorly/moderately | 3 | 6 |
| Moderately/well | 2 | 4 |
| Well | 1 | 2 |
| Site of primary tumor | ||
| Oral cavity (oral tongue) | 1 | 2 |
| Oropharynx | 41 | 87 |
| Base of tongue | 26 | 55 |
| Tonsil | 14 | 30 |
| Lateral or posterior wall | 1 | 2 |
| Larynx (supraglottic/AE fold) | 2 | 4 |
| Hypopharynx | 2 | 4 |
| Pyriform sinus | 1 | 2 |
| Hypopharyngeal wall | 1 | 2 |
| Nasopharynx | 1 | 2 |
Abbreviations: FNA, fine-needle aspiration; AE, aryepiglottic.
ICT Administered
ICT was delivered over a median period of 43 days (range, 26 to 57 days). A majority of the patients received the full course of induction treatment planned per protocol, in terms of number of chemotherapy doses and dose-intensity (Table 2). Thirty patients (64%) received granulocyte colony-stimulating factor support.
Table 2.
Treatment Administered
| Parameter | Paclitaxel | Carboplatin | Cetuximab |
|---|---|---|---|
| No. of doses | |||
| Median | 6 | 6 | 6 |
| Range | 4-6 | 4-6 | 1-8 |
| Dose-intensity* | |||
| Median | 810 | 12 | 1,650 |
| Range | 450-810 | 7-12 | 400-2,150 |
| Patients with ≥ 1 dose held for ≥ 7 days | |||
| No. of patients | 28 | 28 | 29 |
| % | 60 | 60 | 62 |
| Patients with dose reductions | |||
| No dose reduction | |||
| No. of patients | 43 | 45 | 46 |
| % | 91 | 96 | 98 |
| Dose level −1† | |||
| No. of patients | 4 | 2 | 1 |
| % | 9 | 4 | 2 |
| Dose density‡ | |||
| Median | 135 | 2 | 275 |
| Range | 75-135 | 1.2-2 | 67-358 |
Abbreviation: AUC, area under the curve.
Total dose delivered during the induction program. Units of measure are as follows: carboplatin, AUC; paclitaxel, mg/m2; and cetuximab, mg/m2.
Dose level −1 was as follows: carboplatin AUC 1.5/wk, paclitaxel 90 mg/m2/wk, and cetuximab 200 mg/m2/wk.
Dose delivered per week during the induction program, accounting for treatment delays and dose reductions. Units of measure are as follows: carboplatin, AUC/wk; paclitaxel, mg/m2/wk; and cetuximab, mg/m2/wk. The loading dose of cetuximab (ie, 400 mg/m2) was included in the calculation of dose density.
Response to ICT
All 47 patients had evaluable disease before treatment (Table 3). Four patients were not evaluable for response in the primary site and one patient was not evaluable in the neck as a result of surgical resection performed before ICT. The response designation represents a combined clinical and radiographic assessment. Overall, nine (19%; 95% CI, 9% to 33%) of the 47 patients achieved CR; 36 patients (77%; 95% CI, 62% to 88%) had a partial response, and two patients (4%) had stable disease. Never smokers were more likely (38.9%) to achieve a CR to ICT than former (12.5%) or current smokers (0%; P = .02).
Table 3.
Responses to Induction Chemotherapy
| Clinicoradiographic Assessment After PCC | Patients |
|
|---|---|---|
| No. | % | |
| Primary tumor | ||
| CR | 30 | 70 |
| PR | 13 | 30 |
| Stable disease | 0 | 0 |
| Neck | ||
| CR | 10 | 22 |
| PR | 34 | 74 |
| Stable disease | 2 | 4 |
| Overall response (primary tumor and neck) | ||
| CR | 9 | 19 |
| PR | 36 | 77 |
| Stable disease | 2 | 4 |
Abbreviations: PCC, paclitaxel, carboplatin, and cetuximab; CR, complete response; PR, partial response.
Toxicity
During ICT, the most common nonhematologic toxicity was rash/folliculitis (grades 2 and 3 in 38% and 45% of patients, respectively), followed by fatigue, diarrhea, and sensory neuropathy (Table 4). The most common grade 2 to 4 hematologic toxicity was neutropenia (grades 2, 3, and 4 in 23%, 19%, and 2% of patients, respectively). There were no instances of febrile neutropenia. Dose reductions were needed in one patient for cetuximab and in four and two patients for paclitaxel and carboplatin, respectively. Treatment delays of ≥ 7 days occurred in 60% of patients.
Table 4.
Selected Acute Grade 2 to 4 Toxicities (highest grade per patient)
| Toxicity | Grade 2 |
Grade 3 |
Grade 4 |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Induction chemotherapy | ||||||
| Nonhematologic | ||||||
| Arthralgia/myalgia | 6 | 13 | ||||
| Anorexia | 4 | 9 | 1 | 2 | ||
| Diarrhea | 4 | 9 | 4 | 9 | ||
| Fatigue | 19 | 40 | 1 | 2 | ||
| Nausea/vomiting | 6 | 13 | ||||
| Pruritus | 3 | 6 | ||||
| Rash/folliculitis | 18 | 38 | 21 | 45 | ||
| Sensory neuropathy | 7 | 15 | 1 | 2 | ||
| Allergic reaction | 2 | 4 | ||||
| Hematologic | ||||||
| Neutropenia | 11 | 23 | 9 | 19 | 1 | 2 |
| Anemia | 4 | 9 | ||||
| Thrombocytopenia | — | — | — | |||
| Definitive therapy* | ||||||
| Anorexia | 4 | 9 | 3 | 6 | ||
| Dry mouth | 14 | 30 | 2 | 4 | ||
| Taste alteration | 15 | 32 | 2 | 4 | ||
| Fatigue | 7 | 15 | 2 | 4 | ||
| Dehydration | 3 | 6 | ||||
| Mucositis | 7 | 15 | 36 | 77 | 1 | 2 |
| Nausea/vomiting | 2 | 4 | 3 | 6 | ||
| Rash/desquamation | 6 | 13 | ||||
| Tinnitus | 3 | 6 | ||||
| Sensory neuropathy | 2 | 4 | ||||
| Febrile neutropenia | 1 | 2 | ||||
NOTE. The attribution to induction or definitive treatment was according to the time of onset.
Includes patients treated with radiation alone (n = 23), chemoradiation with various regimens (n = 23), and surgery followed by adjuvant radiation (n = 1).
Locoregional Therapy After ICT
The definitive locoregional therapy used was radiotherapy in 46 patients; radiotherapy was used as a single modality in 23 patients (22 T1/2 patients and one T3 patient) or with concomitant chemotherapy in 23 patients (seven T1/2 patients and 16 T3/4 patients). Concomitant chemotherapy regimens included cisplatin 100 mg/m2 every 3 weeks (11 patients); weekly cisplatin 25 to 30 mg/m2/wk (three patients); and weekly carboplatin AUC 1.5 to 2.0 per week (nine patients). Of the latter 12 patients, five had developed grade 2 peripheral neuropathy during ICT. Forty-one patients (87%) received intensity-modulated radiation therapy, and 18 patients (38%) had altered fractionation delivered with concomitant boost. Surgery was performed in 11 patients. A single patient with an oral tongue cancer had transoral glossectomy and ipsilateral neck dissection followed by postoperative radiation. One patient had a modified radical neck dissection before radiation. Nine patients with residual neck masses had selective neck dissections after either radiation alone (four patients) or chemoradiotherapy (five patients) without evidence of viable disease or regional tumor recurrence.
Response and Toxicity After Locoregional Therapy
The overall CR rate after completion of radiotherapy, with or without chemotherapy, was 70% (95% CI, 55% to 83%), with partial responses in 26% of patients. The most common grade 2 to 4 toxicities attributed to definitive radiotherapy or chemoradiotherapy were mucositis, taste alteration, dry mouth/xerostomia, and fatigue (Table 4). One patient experienced grade 3 febrile neutropenia, which occurred after the first dose of weekly carboplatin (AUC 1.5).
Survival, Disease Control, and Patterns of Failure
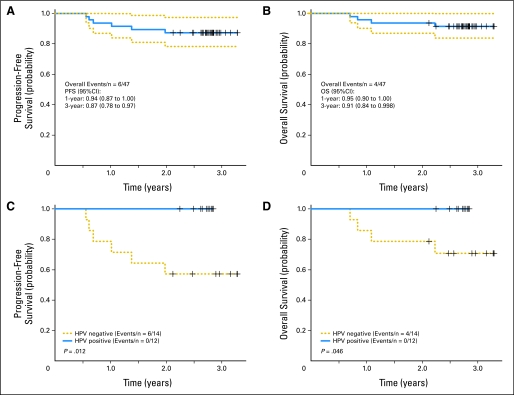
OS rates at 1 and 3 years were 95% (95% CI, 90% to 100%) and 91% (95% CI, 84% to 99%), respectively. None of the patients developed a second or metachronous primary tumor. PFS rates at 1 and 3 years were 94% (95% CI, 87% to 100%) and 87% (95% CI, 78% to 97%), respectively (Fig 2). Six patients had recurrences (two local, one local and distant, two regional and distant, and one distant). Notably, three of five patients with T4 staging had tumor recurrences (one local, one regional and distant, and one distant). Of the 23 patients receiving primary radiotherapy without concomitant chemotherapy, one patient experienced regional and distant tumor recurrence. None had tumor recurrence at the primary site. Of the six patients with tumor recurrence, three had salvage surgery, and all patients went on to individualized therapy.
Fig 2.
(A) Progression-free survival (PFS) and (B) overall survival (OS) for the entire study population. (C) PFS and (D) OS by human papillomavirus (HPV) status.
Functional Outcomes
A gastrostomy tube was placed in 33 patients (70.2%) during radiotherapy (Table 5). Thirty-one tubes (93.9%) were removed at a median of 3.6 months (range, 1.3 to 19.3 months). Two patients were tube dependent at last follow-up; one patient is alive at 30 months with dysphagia, and one patient died of disease progression 6 months after chemoradiotherapy. Thirty (83%) of 36 patients consumed a full diet with or without the use of a liquid assist (Performance Status Scale for Head and Neck Cancer Patients score = 90 or 100) at 24 months, and less than 10% of patients aspirated liquids on MBS during each functional assessment interval. One patient who was tracheostomy tube dependent before treatment was decannulated 32 months after treatment completion.
Table 5.
Functional Outcomes
| Outcome | Baseline |
6 Months |
12 Months |
24 Months |
||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| No. with MBS* | 47 | 36 | 29 | 26 | ||||
| No. who answered questionnaire† | 47 | 39 | 34 | 36 | ||||
| Follow-up time, months | ||||||||
| Mean | 6.4 | 12.3 | 24.7 | |||||
| Range | 4.3-9.0 | 9.6-14.6 | 21.8-31.2 | |||||
| Aspiration | ||||||||
| No | 46 | 98 | 34 | 94 | 28 | 97 | 24 | 92 |
| Yes | 1 | 2 | 2 | 6 | 1 | 3 | 2 | 8 |
| Feeding tube | ||||||||
| No | 43 | 91 | 33 | 85 | 31 | 91 | 35 | 97 |
| Yes | 4 | 9 | 6 | 15 | 3 | 9 | 1 | 3 |
| PSS-HN‡ Normalcy of Diet Scale | ||||||||
| Full diet (no restriction) | 35 | 75 | 10 | 26 | 4 | 12 | 10 | 28 |
| Full diet (liquid assist) | 2 | 4 | 8 | 21 | 19 | 56 | 20 | 56 |
| All meat | 2 | 5 | ||||||
| Raw carrots, celery | 3 | 8 | 1 | 3 | ||||
| Dry bread and crackers | 1 | 2 | 1 | 3 | 3 | 9 | ||
| Soft, chewable foods | 4 | 9 | 12 | 31 | 5 | 15 | 5 | 14 |
| Soft, nonchewable foods | 3 | 6 | 1 | 3 | 1 | 3 | ||
| Pureed foods | 1 | 3 | ||||||
| Warm liquids | ||||||||
| Cold liquids | 2 | 5 | ||||||
| Nonoral feeding (NPO) | 2 | 4 | 1 | 3 | ||||
Abbreviations: MBS, modified barium swallow; PSS-HN, Performance Status Scale for Head and Neck Cancer Patients; NPO, nothing by mouth.
One patient who underwent total laryngectomy 4 months after chemoradiotherapy was excluded from subsequent functional assessment.
Questionnaires were mailed to patients who missed MBS.
Performance Status Scale for Head and Neck Cancer.
HPV Status and EGFR Expression
Of the 26 diagnostic biopsies available for study, genomic DNA of an oncogenic HPV type was detected in tumor nuclei in 12 biopsies. HPV positivity was associated with male sex (12 [71%] of 17 men and zero of nine women), never (six [60%] of 10 patients) rather than former (three [43%] of seven patients) or current smokers (three [33%] of nine patients), and T1-2 stage (eight [67%] of 12 patients). All 12 HPV-positive tumors were of the oropharynx (four tonsil and eight base of tongue tumors). HPV-negative sites were oral cavity (n = 1), oropharynx (n = 10), larynx (n = 1), hypopharynx (n = 1), and nasopharynx (n = 1). An overall CR to ICT was observed in three (25%) of 12 HPV-positive patients compared with three (21%) of 14 HPV-negative patients (P = 1.0). Tumor recurrence was not detected in the 12 patients with HPV-positive tumors. Both PFS and OS for patients with HPV-positive tumors were superior to PFS and OS in patients with HPV-negative tumors (P = .012 and .046, respectively, log-rank test). Membrane and/or cytoplasmic EGFR expression was observed in 37 of 39 available biopsies and did not impact response to therapy or clinical outcome.
DISCUSSION
In this phase II study, cetuximab was added to an intensive 6-week paclitaxel and carboplatin induction regimen. We have observed distant metastases in four of 47 patients, a quite favorable result, with moderate treatment-related toxicity and 3-year OS rate of 91%. Our sequential treatment strategy also provided for individualization of definitive locoregional therapy.
The primary objective of increasing the CR rate by 20% was not achieved, but we did observe a high tumor response rate, similar to others20,21 (70% CR rate and 30% partial response rate at the primary sites and 22% CR rate and 74% partial response rate at nodal metastases), despite the relatively short treatment period. Vokes et al11 had reported a CR rate of 35% with the weekly paclitaxel and carboplatin regimen. However, eligibility criteria differed because our study required N2b or greater nodal staging, in contrast to 67% of patients with N2b/c or N3 staging in the Chicago report. A higher percentage of our patients had oropharynx primary tumors (87% v 44%, respectively). Moreover, there may also have been differences in imaging techniques. We observed grade 2 peripheral neuropathy in 15% of patients and grade 3 neuropathy in one patient during the induction period. Because the use of ICT will affect the administration of definitive locoregional treatment, in part because of drug toxicity, our view is that therapeutic efficacy, as reflected ultimately by PFS, OS, and functional outcomes, is a result of the entire sequence of therapy.
Encouraging functional outcomes were observed, with a 3% gastrostomy tube dependence rate (in a single living patient) and 8% aspiration rate, comparing favorably with existing literature, with reported rates of 15% to 30%22–24 and up to 69%,25,26 respectively. The basis for these favorable functional outcomes is unclear, and further studies are needed to determine the effects of sequential therapy, newer radiotherapy techniques, and use of a risk-based local therapy model on swallowing abilities.
In analyzing the low observed number of failure events, there was no clear association with sex, site of tumor origin, EGFR expression, or response to ICT, but this phase II study has insufficient numbers for a meaningful regression analysis. Of the six patients with tumor recurrence, three had presented with T4 disease (a total of five T4 patients were entered onto the study). With 41 patients having oropharynx primary tumors, the influence of HPV positivity on outcomes was explored. HPV has a role in the pathogenesis of a subset of oropharynx cancers,20,27 and prognosis is favorable,28–31 irrespective of the fundamental treatment approach, in comparison to tobacco-related squamous cancers. Of 22 oropharynx tumors tested, 12 were positive for high-risk HPV subtypes with no tumor recurrences in this group. Six of eight HPV-positive patients with T1/2 primaries received single-modality radiotherapy. Notably, the risk of distant metastases for HPV-positive or -negative tumors in patients receiving concomitant chemoradiotherapy in Radiation Therapy Oncology Group Protocol 0129 was observed to be similar,28 affecting 9.7% and 12.9% of patients, respectively. Thus, a sequential treatment strategy with ICT and then radiotherapy as a single modality may be particularly applicable to patients with HPV-positive tumors with staging of T0-2N2/3 and should be further tested.
Traditionally, the objective of ICT has been to reduce risk of distant disease recurrence. The rationale for a risk-based approach to definitive local therapy is to administer effective comprehensive treatment individualized at diagnosis (and not after assessment of response to ICT), avoiding unnecessary and potentially toxic concomitant chemoradiotherapy for selected patients. Of the 23 patients receiving radiotherapy without chemotherapy, one developed regional tumor recurrence. With only five of 47 patients observed to have local or regional tumor recurrences, our data are consistent with the observations of Posner et al9 that effective sequential therapy affects local tumor control. Modification of definitive primary therapy based on a favorable response to ICT requires prospective investigation. Current plans are to continue study of ICT and risk-based local therapy to confirm the favorable preliminary results and to integrate biomarker data in protocol design as we attempt to advance sequential treatment strategies.
Acknowledgment
We thank Ganene Steinhaus, RN, for research nursing contributions and Gloria M. Riojas for secretarial support.
Footnotes
See accompanying editorial on page 1
Supported by peer-reviewed funding from Specialized Program of Research Excellence in Head and Neck Cancer Grant No. P50 CA97007 from the National Cancer Institute and the “Clinician Investigator Program in Translational Research” Grant No. K12 CA88084 (F.C.H.). Additional support was provided by the The University of Texas M. D. Anderson Cancer Center Support Grant No. CA 16672, Bristol-Myers Squibb Oncology Investigator Initiated Trials program and Grant No. CS 2004-00011435 WC from Imclone Systems.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00301028.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Merrill S. Kies, ImClone Systems (C); Floyd Christopher Holsinger, Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Floyd Christopher Holsinger, Bristol-Myers Squibb; Katharine A. Gillaspy, Bristol-Myers Squibb Research Funding: Merrill S. Kies, Bristol-Myers Squibb, ImClone Systems; Bonnie S. Glisson, ImClone Systems Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Merrill S. Kies, Floyd Christopher Holsinger, J. Jack Lee, Waun K. Hong, Adam S. Garden
Financial support: Merrill S. Kies, Floyd Christopher Holsinger, Waun K. Hong
Administrative support: Merrill S. Kies, Floyd Christopher Holsinger, Scott M. Lippman
Provision of study materials or patients: Merrill S. Kies, Floyd Christopher Holsinger, Bonnie S. Glisson, Katharine A. Gillaspy, Erminia Massarelli, Scott M. Lippman, Adel K. El-Naggar, Adam S. Garden
Collection and assembly of data: Merrill S. Kies, Floyd Christopher Holsinger, William N. William Jr, Katharine A. Gillaspy, Erminia Massarelli, Waun K. Hong, Adel K. El-Naggar, Vassiliki Papadimitrakopoulou
Data analysis and interpretation: Merrill S. Kies, Floyd Christopher Holsinger, J. Jack Lee, William N. William Jr, Bonnie S. Glisson, Heather Y. Lin, Jan S. Lewin, Lawrence E. Ginsberg, Erminia Massarelli, Lauren Byers, Scott M. Lippman, Waun K. Hong, Adel K. El-Naggar, Vassiliki Papadimitrakopoulou
Manuscript writing: Merrill S. Kies, Floyd Christopher Holsinger, J. Jack Lee, William N. William Jr, Bonnie S. Glisson, Heather Y. Lin, Jan S. Lewin, Lauren Byers, Scott M. Lippman, Adel K. El-Naggar, Adam S. Garden, Vassiliki Papadimitrakopoulou
Final approval of manuscript: Merrill S. Kies, Floyd Christopher Holsinger, J. Jack Lee, William N. William Jr, Bonnie S. Glisson, Jan S. Lewin, Lawrence E. Ginsberg, Lauren Byers, Scott M. Lippman, Adel K. El-Naggar, Adam S. Garden, Vassiliki Papadimitrakopoulou
REFERENCES
- 1.Adelstein DJ, Leblanc M. Does induction chemotherapy have a role in the management of locoregionally advanced squamous cell head and neck cancer? J Clin Oncol. 2006;24:2624–2628. doi: 10.1200/JCO.2005.05.3629. [DOI] [PubMed] [Google Scholar]
- 2.Brockstein B, Haraf DJ, Rademaker AW, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: A 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15:1179–1186. doi: 10.1093/annonc/mdh308. [DOI] [PubMed] [Google Scholar]
- 3.Garden AS. Where are the at-risk cervical nodes? Int J Radiat Oncol Biol Phys. 2004;58:1–2. doi: 10.1016/s0360-3016(03)01451-2. [DOI] [PubMed] [Google Scholar]
- 4.Garden AS, Asper JA, Morrison WH, et al. Is concurrent chemoradiation the treatment of choice for all patients with Stage III or IV head and neck carcinoma? Cancer. 2004;100:1171–1178. doi: 10.1002/cncr.20069. [DOI] [PubMed] [Google Scholar]
- 5.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data—Meta-Analysis of Chemotherapy on Head and Neck Cancer Collaborative Group. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 6.Monnerat C, Faivre S, Temam S, et al. End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol. 2002;13:995–1006. doi: 10.1093/annonc/mdf172. [DOI] [PubMed] [Google Scholar]
- 7.Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23:8636–8645. doi: 10.1200/JCO.2004.00.1990. [DOI] [PubMed] [Google Scholar]
- 8.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 9.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 10.Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol. 2004;22:1743–1752. doi: 10.1200/JCO.2004.06.147. [DOI] [PubMed] [Google Scholar]
- 11.Vokes EE, Stenson K, Rosen FR, et al. Weekly carboplatin and paclitaxel followed by concomitant paclitaxel, fluorouracil, and hydroxyurea chemoradiotherapy: Curative and organ-preserving therapy for advanced head and neck cancer. J Clin Oncol. 2003;21:320–326. doi: 10.1200/JCO.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 13.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 14.Ang KK, Garden AS. ed 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. Radiotherapy for Head and Neck Cancers Indications and Techniques. [Google Scholar]
- 15.Ang KK, Peters LJ, Weber RS, et al. Concomitant boost radiotherapy schedules in the treatment of carcinoma of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 1990;19:1339–1345. doi: 10.1016/0360-3016(90)90341-g. [DOI] [PubMed] [Google Scholar]
- 16.Logemann J. ed 2. Austin, TX: Pro-Ed; 1998. Evaluation and Treatment of Swallowing Disorders; pp. 168–180. [Google Scholar]
- 17.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 18.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66:564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 21.Wanebo HJ, Ghebremichael M, Burtness B, et al. Phase II evaluation of cetuximab (C225) combined with induction paclitaxel and carboplatin followed by C225, paclitaxel, carboplatin, and radiation for stage III/IV operable squamous cancer of the head and neck (ECOG, E2303) J Clin Oncol. 2007;25(suppl):302s. abstr 6015. [Google Scholar]
- 22.Ackerstaff AH, Balm AJ, Rasch CR, et al. First-year quality of life assessment of an intra-arterial (RADPLAT) versus intravenous chemoradiation phase III trial. Head Neck. 2009;31:77–84. doi: 10.1002/hed.20937. [DOI] [PubMed] [Google Scholar]
- 23.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiley SG, Hargunani CA, Skoner JM, et al. Swallowing function after chemoradiation for advanced stage oropharyngeal cancer. Otolaryngol Head Neck Surg. 2006;134:455–459. doi: 10.1016/j.otohns.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 25.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 26.Langerman A, Maccracken E, Kasza K, et al. Aspiration in chemoradiated patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1289–1295. doi: 10.1001/archotol.133.12.1289. [DOI] [PubMed] [Google Scholar]
- 27.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 28.Gillison ML, Harris J, Westra W, et al. Survival outcomes by tumor human papillomavirus (HPV) status in stage III-IV oropharyngeal cancer (OPC) in RTOG 0129. J Clin Oncol. 2009;27(suppl):301s. abstr 6003. [Google Scholar]
- 29.Kumar B, Cordell KG, Lee JS, et al. Response to therapy and outcomes in oropharyngeal cancer are associated with biomarkers including human papillomavirus, epidermal growth factor receptor, gender, and smoking. Int J Radiat Oncol Biol Phys. 2007;69(suppl):S109–S111. doi: 10.1016/j.ijrobp.2007.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 31.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]