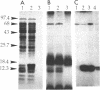
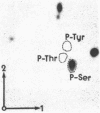
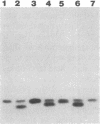
Abstract
An analysis of the biochemical basis for the lack of phosphoenolpyruvate:glycose phosphotransferase activity in heterofermentative lactobacilli was carried out. Extracts of Lactobacillus brevis and Lactobacillus buchneri failed to reconstitute phosphotransferase activity of extracts of Staphylococcus aureus mutants impaired in the phosphotransferase system due to the absence of enzyme I, enzyme IILac, or enzyme IIILac activity, suggesting that these lactobacilli lack those phosphotransferase system components. In contrast, complementation tests with an extract of a S. aureus mutant deficient in heat-stable protein (HPr) indicated the presence of HPr activity in heterofermentative lactobacilli. The HPr of L. brevis was purified and shown to have properties similar to those of a typical HPr. In addition, L. brevis possesses an ATP-dependent protein kinase that phosphorylates a serine residue of the endogenous HPr as well as other HPrs of Gram-positive origin. The kinase activity is markedly stimulated by phosphorylated compounds related to sugar metabolism and is negatively modulated by orthophosphate, pyrophosphate, or arsenate and by a low molecular weight endogenous factor. In keeping with the idea of a regulatory role for the phosphorylation of HPr in lactobacilli, a HPr[Ser(P)] phosphatase activity in L. brevis was also demonstrated. On the basis of the finding of HPr and a system for its reversible covalent modification in an organism devoid of a functional phosphotransferase system we propose that, in lactobacilli, HPr has a role in the regulation of pathways other than the phosphotransferase system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert C. A., Frank R., Stüber K., Deutscher J., Hengstenberg W. Phosphoenolpyruvate-dependent protein kinase enzyme I of Streptococcus faecalis: purification and properties of the enzyme and characterization of its active center. Biochemistry. 1985 Feb 12;24(4):959–964. doi: 10.1021/bi00325a023. [DOI] [PubMed] [Google Scholar]
- Baumann P., Baumann L. Catabolism of D-fructose and D-ribose by Pseudomonas doudoroffii. I. Physiological studies and mutant analysis. Arch Microbiol. 1975 Nov 7;105(3):225–240. doi: 10.1007/BF00447141. [DOI] [PubMed] [Google Scholar]
- Beyreuther K., Raufuss H., Schrecker O., Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 1. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977 May 2;75(1):275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- Breidt F., Jr, Hengstenberg W., Finkeldei U., Stewart G. C. Identification of the genes for the lactose-specific components of the phosphotransferase system in the lac operon of Staphylococcus aureus. J Biol Chem. 1987 Dec 5;262(34):16444–16449. [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMOSS R. D., BARD R. C., GUNSALUS I. C. The mechanism of the heterolactic fermentation; a new route of ethanol formation. J Bacteriol. 1951 Oct;62(4):499–511. doi: 10.1128/jb.62.4.499-511.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Beyreuther K., Sobek H. M., Stüber K., Hengstenberg W. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: factor IIIlac, a trimeric phospho-carrier protein that also acts as a phase transfer catalyst. Biochemistry. 1982 Sep 28;21(20):4867–4873. doi: 10.1021/bi00263a006. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Kessler U., Hengstenberg W. Streptococcal phosphoenolpyruvate: sugar phosphotransferase system: purification and characterization of a phosphoprotein phosphatase which hydrolyzes the phosphoryl bond in seryl-phosphorylated histidine-containing protein. J Bacteriol. 1985 Sep;163(3):1203–1209. doi: 10.1128/jb.163.3.1203-1209.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A., Koshland D. E., Jr Energy expenditure in the control of biochemical systems by covalent modification. J Biol Chem. 1987 Apr 5;262(10):4460–4471. [PubMed] [Google Scholar]
- Gupta K. D., Ghosh S. Identification of a phosphoenolpyruvate:fructose 1-phosphotransferase system in Azospirillum brasilense. J Bacteriol. 1984 Dec;160(3):1204–1206. doi: 10.1128/jb.160.3.1204-1206.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Phosphotransferase system of Staphylococcus aureus: its requirement for the accumulation and metabolism of galactosides. J Bacteriol. 1969 Aug;99(2):383–388. doi: 10.1128/jb.99.2.383-388.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mimura C. S., Poy F., Jacobson G. R. ATP-dependent protein kinase activities in the oral pathogen Streptococcus mutans. J Cell Biochem. 1987 Mar;33(3):161–171. doi: 10.1002/jcb.240330303. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Grossowicz N. Properties of alpha-aminoisobutyric acid transport in a thermophilic microorganism. J Bacteriol. 1974 May;118(2):414–424. doi: 10.1128/jb.118.2.414-424.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Hengstenberg W., Saier M. H., Jr Properties of ATP-dependent protein kinase from Streptococcus pyogenes that phosphorylates a seryl residue in HPr, a phosphocarrier protein of the phosphotransferase system. J Bacteriol. 1984 Oct;160(1):333–340. doi: 10.1128/jb.160.1.333-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Stuiver I., Saier M. H., Jr Regulation of glycerol uptake by the phosphoenolpyruvate-sugar phosphotransferase system in Bacillus subtilis. J Bacteriol. 1984 Jul;159(1):243–250. doi: 10.1128/jb.159.1.243-250.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Brino G., Peterkofsky A., Reizer J. Regulation of beta-galactoside transport and accumulation in heterofermentative lactic acid bacteria. J Bacteriol. 1987 Dec;169(12):5589–5596. doi: 10.1128/jb.169.12.5589-5596.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Trifone J. D., Brustolon M. Distribution of the phosphoenolpyruvate:glucose phosphotransferase system in fermentative bacteria. J Bacteriol. 1979 Jul;139(1):93–97. doi: 10.1128/jb.139.1.93-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]